- *Corresponding Author:
- K. Byrappa
Center for Research & Innovations, BGSIT, Adichunchanagiri University, B.G. Nagara 571448, Karnataka, India
E-mail: kbyrappa@gmail.com
| Date of Received | 16 March 2020 |
| Date of Revision | 22 August 2022 |
| Date of Acceptance | 26 November 2022 |
| Indian J Pharm Sci 2022;84(6):1444-1452 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Vranaharin, an ayurvedic oil manufactured by MMCA Remedies Pharmaceuticals, Karnataka, India, is a red-colored drug, prepared from several traditionally used plant ingredients and is used in postoperative dressings, bedsores, burns, cuts, wounds, diabetic ulcers, abscesses, skin infections, nail fungus, herpes and so on. This research aims to investigate Vranaharin oil, to understand its side-effects such as antioxidant enzymes alteration in the cerebral cortex of the brain and liver of developing chick embryos, pathological effect/antimicrobial nature, antioxidant ability and hemolytic activity. This study is designed to give an overall toxicological analysis of the selected drug. The oil which is used for topical applications of necrotic wounds is observed to cause angioneogenesis in necrotic wound bed within a short span of application. The oil has been investigated for the first time to assess the systemic effect of local application in terms of possible genotoxicity and embryonic toxicity. The study concludes that the oil can be effectively applied over the necrotized wound bed for a short period and its long-term application needs to be further investigated. Setting of maximum dose needs to be standardized as it caused notable fluctuations to enzyme equilibrium and variations in superoxide dismutase, catalase, glutathione peroxidase and glutathione- S-transferases levels when higher concentrations were used in chick embryos. Its topical application is highly recommended, especially in necrotizing wounds due to its excellent antioxidant, and antimicrobial potential. The angiogenic potential of the oil in avascular wounds is a very interesting phenomenon that is being investigated in our laboratory.
Keywords
Antioxidant enzyme, cerebral cortex, liver, antimicrobial, haemolysis, ayurvedic drug
It has been proven by several researchers that Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX) play a major role as the first line of the antioxidant defence system in the body. The most powerful antioxidant enzyme in a cell is SOD. It also acts as a primary detoxification enzyme. The deficiency of SOD causes cerebral vascular hypertrophy, vascular dysfunction in hyper-homocysteinemia, myocardial injury, accelerated aging process, weak immune response, neurodegenerative disorders and several other issues[1]. Most of the living tissue that utilizes oxygen contains CAT, which is a common antioxidant enzyme. Another important intracellular enzyme is GPX, which helps in the breakdown of Hydrogen peroxides (H2O2) into Water (H2O). Deficiency in CAT and GPX causes mental disorders, predisposition to type 2 diabetes mellitus, Deoxyribonucleic Acid (DNA) oxidative damage, cardiovascular disorders, impaired antioxidant protection, oxidative damage of fatty acids and functional proteins and so on. The study of alterations in these enzymes upon administration of drugs is an integral part of drug toxicology[2,3].
A wide variety of secondary metabolites are produced by plants, like phenols, alkaloids, terpenoids, coumarins, glycosides, saponins and so on. These secondary metabolites act as plant derived antimicrobial substances. These plant extracts effectively treat bacterial and fungal infections[4]. India has a huge variety of medicinal plant species that are studied even from ancient times of Ayurveda. Even with the availability of Western medicines, a huge fraction of the Indians still uses herbal medicines in preference to orthodox medicines (Table 1)[5].
| S. no. | Constituents scientific name | Vernacular name | Common name |
|---|---|---|---|
| 1 | Ventilago maderaspatana | Rakthavalli | Red Creaper |
| 2 | Cinnamomum camphora | Karpoor | Camphor |
| 3 | Carum capiticum | Yavani carum | Ajwain |
| 4 | - | Navaneetam | Butter |
| 5 | Pongamia pinnata | Karanjataila | Kranja oil |
| 6 | Azardirachta indica | Nimbataila | Neem oil |
| 7 | Sesomum indica | Tila taila | Gingelly oil |
| 8 | Cocos nucifera | Narikela taila | Coconut oil |
Table 1: Variety of Medicinal Plant Species
One such ayurvedic oil is “Vranaharin”, manufactured by MMCA Remedies Pharmaceuticals, Karnataka, India. It is used in postoperative dressings, bedsores, burns, cuts, wounds, diabetic ulcers, abscesses, skin infections, nail fungus, herpes and so on.
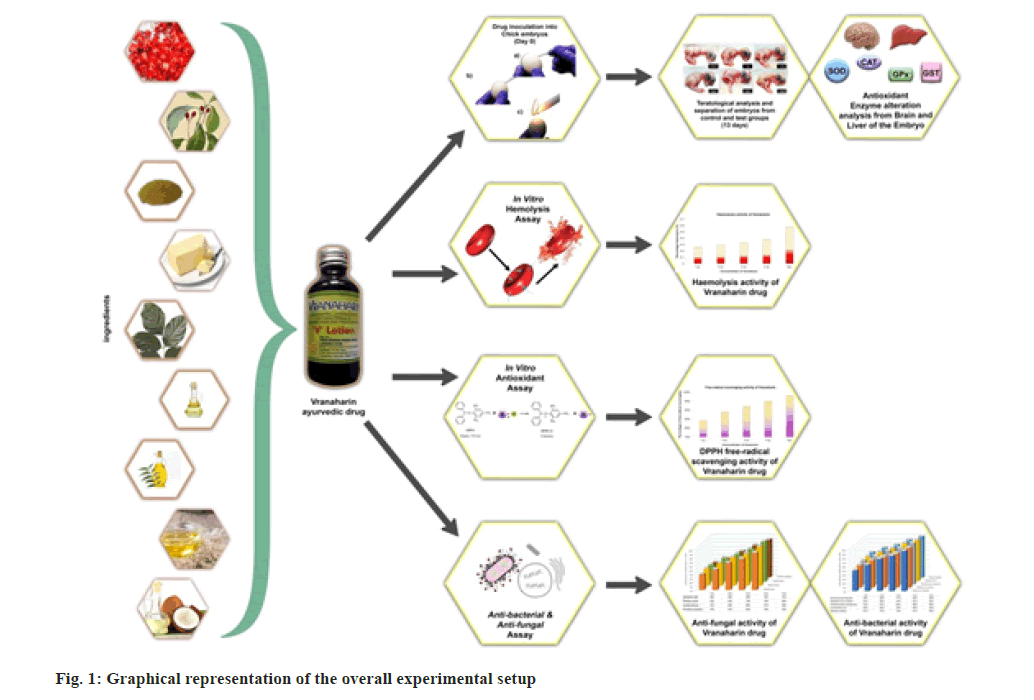
In the present research, over-the-counter ayurvedic oil named “Vranaharin” has been selected for studying its effect on antioxidant enzymes in developing chick embryos, antimicrobial nature, and hemolytic activity. This study is designed to give an overall toxicological analysis of the selected drug. A graphical representation of this research is shown in fig. 1.
Materials and Methods
Enzyme alteration analysis:
Freshly laid fertilized eggs (0 d) of Babcock strain were obtained from National Hatcheries, (Gundlupete, Karnataka, India). Incubation was done in an egg incubator (Lead® instruments (P) Ltd) by placing the eggs horizontally at a temperature of about 37.5±0.5° and relative humidity of 65 % for 8 d. The embryos were treated with 1, 2, 3, 4 and 5 μl of Vranaharin oil as a single dose on the 8th d and incubated for 72 h. The tissues were collected from treated and untreated embryos after 72 h of incubation and kept at -20° up until further use[6].
Tissue processing for assay of antioxidant enzymes: Liver and brain tissues from normal and treated chick embryos were removed from the freezer and thawed slowly, minced with scissors and homogenized in 50 mM Tris-Hydrochloride (Tris- HCl) buffer (pH 8) comprising 0.25 M sucrose and 1 mM Phenylmethylsulfonyl Fluoride (PMSF), using a glass homogenizer. The homogenates were filtered using double-layered cheesecloth to eliminate fat and the filtrates were centrifuged at 12 000 rpm on a refrigerated centrifuge (Eppendorf 5810R Centrifuge) for 30 min. The resulted clear liquid was used as an enzyme basis. The above procedures were conducted at 4°.
Enzyme analysis: Standard Lowry’s method was used for determining protein in all the enzyme preparations. Bovine Serum Albumin (BSA, Sigma) was used as standard[7].
SOD assay: SOD activity was evaluated as per the method determined by Misra et al.[8]. The test mixture of 3 ml was prepared using 50 mM sodium carbonate/bicarbonate buffer (pH 9.8), 0.1 mM Disodium Edetate (EDTA), 0.6 mM epinephrine and enzyme. Towards the end, Epinephrine was added. In the next 4 min, the formation of adrenochrome was recorded at 475 nm wavelength using an Ultraviolet- Visible (UV-Vis) spectrophotometer. The amount of required enzyme to cause 50 % epinephrine oxidation inhibition under the experimental conditions is considered as one unit of SOD activity.
CAT assay: A similar procedure was followed for CAT assay with few modifications. The homogenization of tissues was done with a 1:10 weight-to-volume ratio in 50 mmol/l potassium phosphate buffer (pH 7.4). The homogenates were centrifuged for 30 min at 40 000 xg. In a cuvette 2.95 μl of 19 mmol/l H2O2 solutions made using potassium phosphate buffer and about 50 μl of supernatant was added. The desertion of H2O2 was observed at 240 nm wavelength at every 1 min interval for 5 min. The precise activity of the enzyme was articulated as mmol of H2O2 consumed per mg protein[9,10].
GPX assay: In a cuvette the following ingredients were added; 75 mm phosphate buffer of pH 7.0 (2.4 ml), 60 mm Reduced Glutathione (GSH, 50 μl), 1 Unit of glutathione reductase (10 μl), 120 mM sodium azide (50 μl), 15 mM sodium EDTA (100 μl), Nicotinamide Adenine Dinucleotide Phosphate (NADPH) (100 μl), enzyme (100 μl) and the quantity was made up to 2.9 μl using ultrapure water. By the addition of 100 μl of the substrate, H2O2/CHP, the reaction was initiated. Expression of the activity of GPX was as Nano moles of reduced NADPH oxidized to NADP per minute per milligram protein[11].
Glutathione-S-Transferases (GSTs) assay: The activity of GST was evaluated by the technique determined by Habig et al.,[12]. This technique is done spectrophotometrically to degree the rate of conjugation of GSH to a standard substrate 1-Chloro- 2,4-Dinitrobenzene (CDNB). The following ingredients were added to the cuvettes; 1 mm CDNB and 5 mm GSH in 0.1 M sodium phosphate buffer of pH 6.5 (final volume of 3.0 ml). Reagents, substrate and aliquots were freshly prepared before the assay. The rates of the reaction were identified at 340 nm for every 5 min. Calculations were done in mmol CDNB conjugated/min/mg for determining GST activity per individual, using the published extinction coefficient of 9.6 and 8.5 for CDNB. The precise activity was determined by correcting for protein content.
Statistical analysis: Statistical analyses were done using Microsoft Excel 2010 statistical package and Statistical Package for the Social Sciences (SPSS) software. The results from each experiment done in triplicates were expressed as mean±Standard Deviation (SD). Oneway Analysis of Variance (ANOVA) was done where applicable in the data and a two-tailed t-test was used to determine differences between samples, after Bonferroni error correction of the predictive value. p<0.05 were considered statistically significant[13].
Enzyme actions were stated in terms of micromoles of epinephrine unit/mg protein. Statistical differences between the control embryos and the ones treated with Vranaharin were assessed by the independent samples t-test. This is a parametric test that compares the means of two independent groups, say control embryos and test embryos, to determine whether there is a significant difference between the two. p>0.01 means not significant; p≤0.01 means significant; p≤0.001 means more significant and p≤0.0001 means extremely significant
High-Performance Liquid Chromatography (HPLC) characterization of Vranaharin: Chemical characterization of Vranaharin was done using the HPLC technique. HPLC (Shimadzu) with UV-Florescence detector was used for this analysis (JSS College of Pharmacy, Mysore). The setup consists of system controller CBM-20A, solvent delivery unit LC-20A, auto-sampler SIL-20A, column oven CTO-20A, UV-VIS detector SPD- 20A, photo-diode array detector SPD-M20A, online degassing unit DGU-20A, low-pressure gradient unit and a high-pressure switching valve. Solvent A consists of 0.5 % acetic acid in water (mobile phase) and solvent B consists of acetonitrile containing 0.5 % acetic acid. Filtration of samples and mobile phase was done using a membrane filter (0.45 μm) in solvent filtration apparatus (Millipore). The process of gradient elution was initiated through 5 % B (flow rate 1.0 ml/min). There was a constant increase in the percentage of B, i.e., 15 % at 10 min, 85 % at 45 min, 95 % at 50 min and it was reduced to 15 % at 55 min. By the end of 60 min, initial conditions were achieved. The wavelength of the detector was set at 254 nm and the injection volume was set at 20 μl. Data collected was carried out at 190-400 nm range and possible coelution in drug solution was observed. Wavelength 254 nm was selected based on maximum chromatographic signal response for fingerprint.
Free-radical scavenging analysis:
1, 1-Diphenyl 2-Picrylhydrazyl (DPPH) was used to evaluate the antioxidant property of Vranaharin. The free radical scavenging ability of the selected drug was assayed[14]. The experiment was conducted in a darkroom where DPPH was mixed with Vranaharin at different concentrations (1, 2, 3, 4 and 5 μl/ml), and its degradation through colour change was observed (violet to pale yellow). After the incubation period of 20 min, the mixtures were centrifuged, and the supernatant was measured at 517 nm using a UV-Vis spectrophotometer (ELICO® SA 165, India). The following formula was used to determine the percentage of DPPH-free radical scavenging activity:
((Ac-As)/As)×100)
Where, (Ac)=Absorbance of the control and (As)=Absorbance of the sample
Haemolysis assay:
Change in the isotonicity of the solution causes lysis of Red Blood Cells (RBC)[15]. This concept was used in haemolysis assay to study the lysis of RBC due to the addition of Vranaharin. Experiments were conducted with fresh chicken blood. Heparin was used to prevent blood from clotting. Dulbecco’s Phosphate Buffer Saline (DPBS) was used to prepare the stock solution to ensure the presence of ~5×108 RBC/ml in the mixture. It was then subjected to different concentrations of the title drug for 3 h. Contents were centrifuged and the absorbance of the supernatant was measured at 541 nm using a UV-Vis spectrometer. The following formula was used to determine the percentage haemolysis:
Percentage haemolysis=((As-An)/(Ap-An))×100
Where, (As)=Absorbance of the sample; (An)=Absorbance of the negative control and (Ap)=Absorbance of the positive control.
Antimicrobial assay:
Six strains of bacteria and four strains of fungi were selected to determine the antimicrobial activity of Vranaharin. The following bacteria were acquired from (Microbial Type Culture Collection (MTCC) and Gene Bank, Chandigarh, India): Enterococcus faecalis (439), Streptococcus mutans (497), Pseudomonas aeroginosa (4673), Escherichia coli (1698), Bacillus subtilus (121) and Staphylococcus aureus (6908). The following fungi were procured: Aspergillus niger (9687), Rhizopus oryzae (6584), Candida albicans (1637) and Penicillium aculeatum (1984).
Experiments were conducted using 96-well micro plates, in which, respective nutrient media and different concentrations of Vranaharin oil were added[16-18]. Labelled plates were placed in an incubator-shaker for 24 h. After the incubation duration, plates were scanned using a micro plate reader at 600-650 nm. The absorbance readings were noted and calculations were done using the following formula:
Pg=((S-Sb)/(Cp-Cn))×100
Where, Pg=Percentage growth; S=Absorbance of the sample with inoculum; Sb=Absorbance of the sample blank; Cp=Absorbance of the positive control and Cn=Absorbance of the negative control
Percentage inhibition (Pi)=100-Pg
Results and Discussion
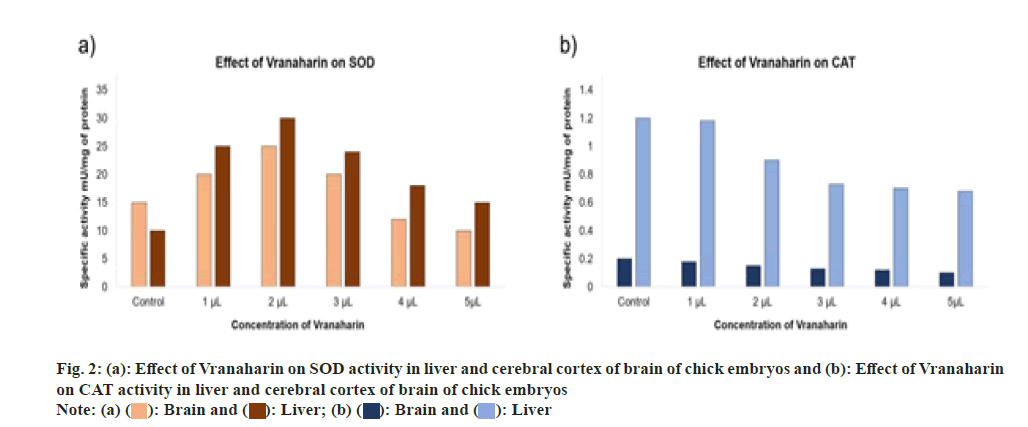
SOD activities were calculated and the results were graphed as shown in fig. 2a. Analysis of the liver and cerebral cortex showed a reduced activity of SOD with an increase in Vranaharin dosage (p<0.0001). SOD activity initially started to escalate in the liver and brain, in comparison with control samples, upon administration of 1 and 2 μl of Vranaharin to chick embryos respectively. Nevertheless, a decrease in SOD activity was noticed in both the tissues with an increase in the quantity of Vranaharin to 5 μl.
CAT activities were calculated, and the results were graphed as shown in fig. 2b. Analysis confirms a significant decrease in CAT activity upon administration of Vranaharin oil when compared to the control group. Moreover, its activity was further reduced as the drug concentrations were amplified. The lowest activity was recorded when 5 μl Vranaharin was administered (p<0.0001).
Graph plotted in fig. 3a. indicates the fluctuations in GPX activity in the liver and cerebral cortex of the brain of control and treated chick embryos. Results suggest a constant reduction in GPX activity with an increase in Vranaharin dosage. The least activity was noted upon administration of 5 μl Vranaharin to the embryos, in comparison to the control (p<0.0001). Particularly when it comes to the cerebral cortex of the brain, although there is a decrease in GPX activities, there is no significant difference (p<0.32) when 2 μl of Vranaharin was given to embryos and there is a significant difference (p<0.04, 0.002) when 3, 4 and 5 μl was administered respectively.
Fig. 3b. represents GST activities in the liver and cerebral cortex of the brain, in control and test chick embryos. GST activity was noted to escalate greatly in the liver and brain, in comparison with the control group, upon administration of 1 and 2 μl of Vranaharin (p<0.0022). Nonetheless, GST activity significantly decreased in both the tissues with an increase in the quantity of Vranaharin to 3, 4 and 5 μl.
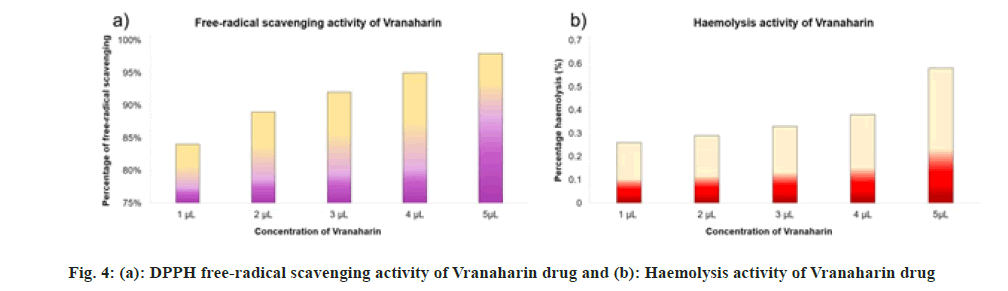
Free-radical scavenging properties of the title drug were assessed using DPPH as the free-radical source and the results are plotted in fig. 4a. These test results suggest a noteworthy antioxidant activity of Vranaharin, which specifies the role of secondary metabolites, namely phenols, alkaloids, terpenoids, coumarins, glycosides, saponins and so on, in elimination free radicals. DPPH radical solution is normally purple in color and its maximum absorbance is at 517 nm. Upon exposure to the title drug, the solution turned into pale yellow color. This suggests a strong antioxidant ability of Vranaharin. Freeradical scavenging activity amplified with increment in concentration from 1 to 5 μl/ml and the percentage of inhibition ranged from (84±1) % to (98±1) %. Excellent inhibition percentage of (84±1) % was achieved even at its lowest concentration of 1 μl/ ml, which is in parallel to that of 5 μl/ml Vranaharin which achieved (98±1) %.
Haemato-compatibility of Vranaharin was tested through haemolysis of RBC analysis and the results were plotted in fig. 4b. The results obtained say the title drug caused a negligible amount of damage to about 0.26 % at 1 μl/ml concentration. At 5 μl/ml percentage of RBC lysis was only 0.58 %, which is also very trivial. We can infer by this that Vranaharin ayurvedic oil is haemato-compatible.
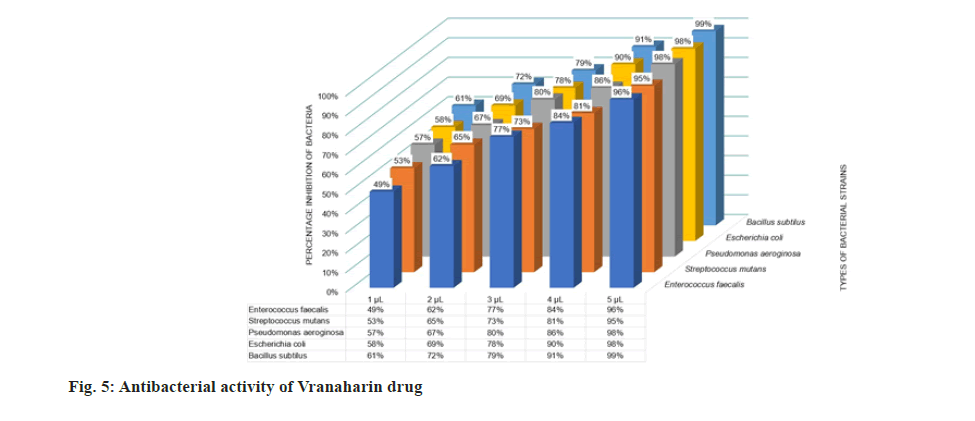
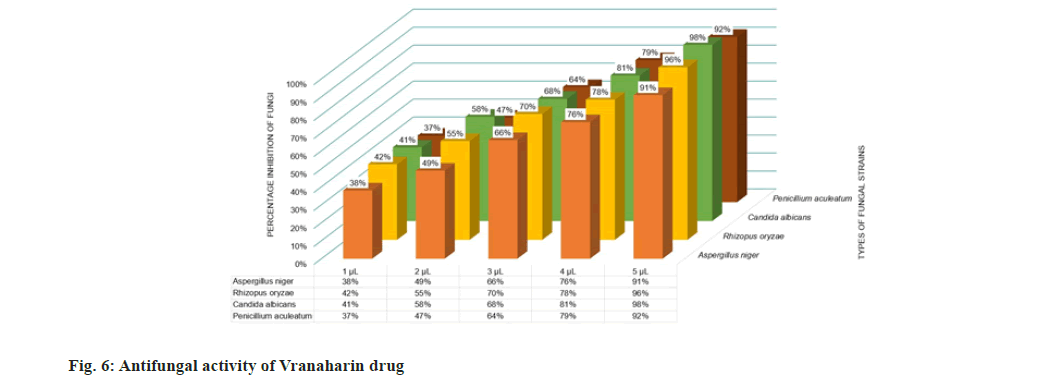
Antimicrobial experiments were conducted thrice to confirm the results and the mean values were plotted in fig. 5 and fig. 6. The results obtained show that the title drug, Vranaharin, possesses tremendous antimicrobial properties. The results show that ayurvedic medicine acted as an excellent bactericide and fungicide. At 5 μl of Vranaharin dosage, percentage growth inhibition of about (96±1) % of Enterococcus faecalis, (95±1) % Streptococcus mutants, (98±1) % Pseudomonas aeroginosa, (98±1) % Escherichia coli, (99±1) % Bacillus subtilus, (95±1) % Staphylococcus aureus, (91±1) % Aspergillus niger, (96±1) % Rhizopus oryzae, (98±1) % Candida albicans and (92±1) % Penicillium aculeatum, were noted from the results.
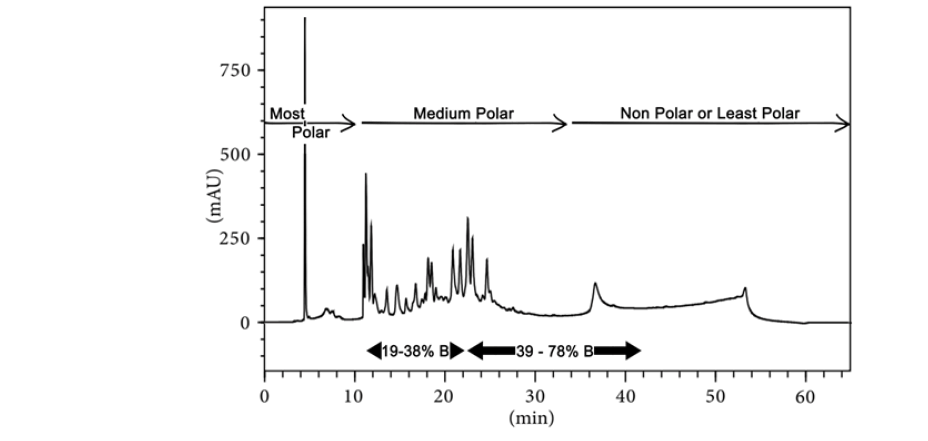
For obtaining a consistent chromatographic fingerprint (quality control) of Vranaharin oil solution, the Photometric Diode Array (PDA) HPLC fingerprint analysis method was adopted using the parameters such as retention time and peak area. The experiment was designed such that the phytochemicals are classified into three categories based on their polarity and their concentration was determined. The categories are most polar, medium polar, and nonpolar or least polar compounds. Several characteristic peaks were not identified due to the lack of reference material. A significant presence of flavonoids and alkaloids in Vranaharin in the medium polar region has been reported. Chromatographic representation is as shown in fig. 7. Phytocompounds separated in the experimental conditions, with chromophore groups have been reported in the chromatogram.
Reactive oxygen species formation and generation of oxygen-derived radicals occur due to several reasons in cells and tissues. The body naturally produces oxygen radicals under oxidative stress. But this formation causes damage to DNA, lipids, proteins, tissues, and so on[19]. Hence to prevent this damage, cell in turn produce enzymatic and non-enzymatic antioxidant components[20]. Among these are SOD, CAT, GPX and GST, which stand at the frontline of defense. SOD often helps in the rapid conversion of superoxide anions (O2-) to H2O2. GPX and CAT further help in splitting hydrogen peroxide into water[21].
In the present study, it was confirmed that administration of Vranaharin oil to chick embryos caused significant variations in antioxidant enzyme activities and hence caused a disturbance in metabolic equilibrium. SOD activity increased gradually upon administration of 1, 2 and 3 μl of Vranaharin to chick embryos, when compared to the control groups. This suggests that oxidative stress was initiated due to presence of non-native compounds from Vranaharin[22]. But it was also noted that the SOD activity was reduced upon a further increase in the drug concentration. Similar results were observed in case of CAT, GPX and GST. This can be explained in two ways. There are two possibilities. One is that the oxidative stress was persistent at very high levels, that the protein damage became profound and caused a decrease in antioxidant enzyme levels[23]. This can be either by direct depletion of enzymatic protein or by damaging the genes responsible for its expression.
The second reason could be that the administered drug reduced oxidative stress by uptake of freeradicals. It is a well-established fact that plant extracts possess high antioxidant properties. This claim is further supported experimentally by DPPH free-radical scavenging activity test on Vranaharin oil. The title drug showed an excellent antioxidant property of (84±1) % at its least concentration of 1 μl/ml and (98±1) % at 5 μl/ml concentration. Since the administered drug itself acted as an antioxidant agent, the activity of native antioxidant enzymes seems to have reduced[24,25].
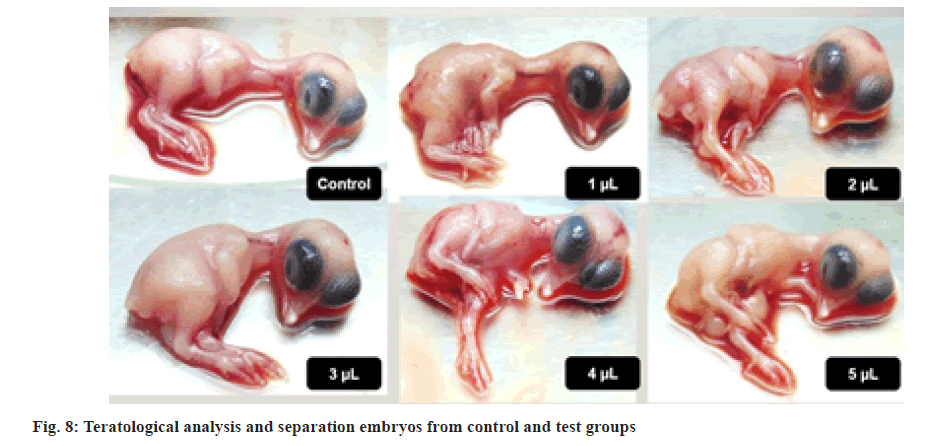
These claims were further supported by teratological analysis of chick embryos[26]. All the chick embryos that were collected on the 11th d for dissection were teratologically well developed and completely normal in comparison with the control group. Some of the photographs are displayed in fig. 8. Further support was taken from haemolysis assay. Results show that the title drug did not cause any significant damage to RBC of chicken blood and hence concluding that it is a haemato-compatible drug.
Vranaharin is said to be used for postoperative dressings, bedsores, abscesses, skin infections, nail fungus, herpes and so on. Hence, an antibacterial and antifungal test has been conducted to understand its activity toward microbes. The results show that the drug certainly stands up to its claims. Inhibition of all the selected bacteria and fungi was observed on an average of 90 % and above. Certain differences in fungicidal and bactericidal effects were noted. This can be due to the differences in their cell wall structures, surface functional groups, and so on[27]. Since Vranaharin contains several plant oils and butter as its base, it can also act in preventing cellular respiration in bacteria and fungi[28].
In this study, the authors conclude that Vranaharin is a very good antibacterial and antifungal agent while being completely biocompatible. The drug showed complete compatibility with the blood and did not cause haemolysis. However, ingestion or chronic administration and high dosage of the drug is not recommended, as it caused notable fluctuations to enzyme equilibrium and variations in SOD, CAT, GPX and GST levels. It can be used for topical applications as it possesses outstanding antioxidant properties and helps in wound healing.
Acknowledgement
The authors thankfully acknowledge the funding given by University with Potential for Excellence project grants from UGC, India. The authors recognize the help received from the research scholars of our group, Dr. Kashinath Lellela, Ms. Mina Zare, Mr Abdo Hezam and Mr. Abishad.
Conflict of Interests
The authors declared no conflict of interests.
References
- Niki E. Antioxidant defenses in eukariotic cells: An overview. Free radicals: From basic science to medicine 1993:365-73.
- Fridovich I. Superoxide radical and superoxide dismutases. Ann Rev Biochem 1995;64(1):97-112.
[Crossref] [Google Scholar] [PubMed]
- Krishnamurthy P, Wadhwani A. Antioxidant enzymes and human health. Antioxidant enzyme. 2012;1:3-18.
- Srivastava J, Chandra H, Nautiyal AR, Kalra SJ. Antimicrobial resistance (AMR) and plant-derived antimicrobials (PDAms) as an alternative drug line to control infections. 3 Biotech 2014;4(5):451-60.
[Crossref] [Google Scholar] [PubMed]
- Eloff JN. On expressing the antibacterial activity of plant extracts--a small first step in applying scientific knowledge to rural primary health care. South African J Sci 2000;96(3):116-8.
- Ruxana Begum SK, Kedam TR. Effect of acrylamide on chick embryo liver GSTs. Med J Nutr Met 2010;3(1):31-8.
- Lowry OH. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193(1):265-75.
[Google Scholar] [PubMed]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170-5.
[Crossref] [Google Scholar] [PubMed]
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133-40.
[Google Scholar] [PubMed]
- Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121-6.
[Crossref] [Google Scholar] [PubMed]
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 1967 Jul 1;70(1):158-69.
[Google Scholar] [PubMed]
- Habig WH. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249(22):7130-9.
[Crossref] [Google Scholar] [PubMed]
- Krousel-Wood MA, Chambers RB, Muntner P. Clinicians' guide to statistics for medical practice and research: Part I. Ochsner J 2006;6(2):68-83.
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol 2011;48(4):412-22.
[Crossref] [Google Scholar] [PubMed]
- Vicas CS, Namratha K, Byrappa K, Yathirajan HS. Comprehensive risk assessment of Ni-Cu ferrite nanoparticles and their action against dental caries and lung infections causing bacteria. J Chem Pharm Res 2015;7(7):1114-24.
- Riesselman MH, Hazen KC, Cutler JE. Determination of antifungal MICs by a rapid susceptibility assay. J Clin Microbiol 2000;38(1):333-40.
[Crossref] [Google Scholar] [PubMed]
- Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007;42(4):321-4.
[Crossref] [Google Scholar] [PubMed]
- Rufián-Henares JA, Morales FJ. Microtiter plate-based assay for screening antimicrobial activity of melanoidins against E. coli and S. aureus. Food Chem 2008;111(4):1069-74.
- ??eri ÖD, Körpe DA, Yurtcu E, Sahin FI, Haberal M. Copper-induced oxidative damage, antioxidant response and genotoxicity in Lycopersicum esculentum Mill. and Cucumis sativus L. Plant Cell Rep 2011;30(9):1713-21.
[Crossref] [Google Scholar] [PubMed]
- Mallepogu SV, Kurumala D, Chittoor P, Kedam T. Assessment of alterations in antioxidant enzymes and histology of liver and cerebral cortex of developing chick embryo in acrylamide toxicity. IJAR 2013;1(5):256-64.
- Fatima RA, Ahmad M. Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci Total Env 2005;346(1-3):256-73.
[Crossref] [Google Scholar] [PubMed]
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clinical Interv Aging 2007;2(2):219-36.
[Google Scholar] [PubMed]
- Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci 2009;46(5-6):241-81.
[Crossref] [Google Scholar] [PubMed]
- Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, Biyani MK, et al. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry 2003;63(1):97-104.
[Crossref] [Google Scholar] [PubMed]
- Sruthi CV, Sindhu A. A comparison of the antioxidant property of five Ayurvedic formulations commonly used in the management of vata vyadhis. J Ayurveda and Integr Med 2012;3(1):29.
[Google Scholar] [PubMed]
- Henshel DS, DeWitt J, Troutman A. Using chicken embryos for teratology studies. Curr Protoc Toxicol 2002;14(1):13-4.
[Crossref] [Google Scholar] [PubMed]
- Ambrogi V, Artini D, De Carneri I, Castellino S, Dradi E, Logemann W, et al. Studies on the antibacterial and antifungal properties of 1, 4-naphthoquinones. Br J Pharmacol 1970;40(4):871.
[Crossref] [Google Scholar] [PubMed]
- Suman A, Chauhan S, Lata S, Sharma RK. Antifungal activity of various plant oils against yeast isolates from ICU patients. Int J Res Med Sci 2017;5(7):3227.
 ): Brain and (
): Brain and ( ): Liver; (b) (
): Liver; (b) ( ): Brain and (
): Brain and ( ): Liver
): Liver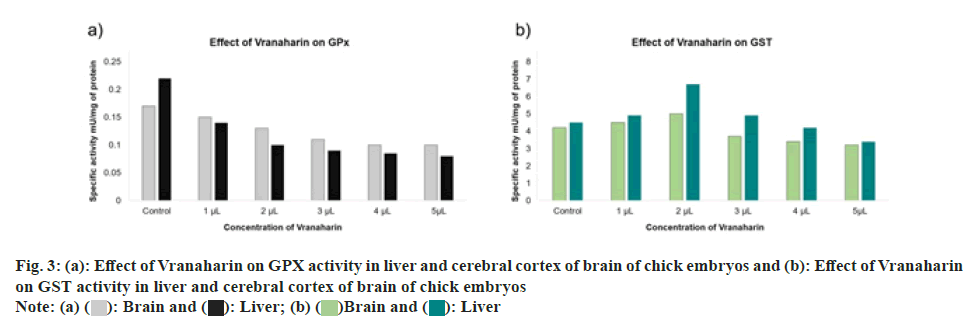
 ): Brain and (
): Brain and ( ): Liver; (b) (
): Liver; (b) ( )Brain and (
)Brain and ( ): Liver
): Liver