- *Corresponding Author:
- V. K. Agnithotri
Dietetics and Nutrition Technology Division, Council of Scientific and Industrial Research (CSIR)-Institute of Himalayan Bioresource Technology, Palampur, Himachal Pradesh 176061, India.
E-mail: kantvijai@yahoo.com
| Date of Received | 23 December 2020 |
| Date of Revision | 21 September 2021 |
| Date of Acceptance | 15 June 2022 |
| Indian J Pharm Sci 2022;84(3):783-790 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this study was to examine essential oil composition, biological activities and safety evaluation of Hedychium spicatum seeds. The phytochemical constituents of essential oil were investigated using gas chromatography-flame ionization detector and gas chromatography-mass spectrometry. Twenty three components were characterized, representing 97.8 % of the total essential oil. Major components of the essential oil were found as beta-pinene (64.0 %), alpha-pinene (17.0 %), alpha-humulene (5.4 %) and E-caryophyllene (4.2 %). The obtained essential oil was evaluated for anticancer activity against human skin carcinoma A431, human lung carcinoma A549 and human cervical cancer SiHa cells, which showed half maximal inhibitory concentration values 71.2, 96.9 and 171.7 μg/ml, respectively. The toxicity of the essential oil was assessed against mouse splenocytes using (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide) assay. Also, the oil was tested against a series of gram-positive and gramnegative bacteria. Bacillus subtilis MTCC121 was the most susceptible to the essential oil with minimum inhibitory concentration at 1 % v/v. The observed findings indicated that essential oil possess good cytotoxicity as well as antibacterial properties.
Keywords
Zingiberaceae, Hedychium spicatum, essential oil, chemical composition, cytotoxicity, antibacterial activity
Over the time, cancer has emerged as one of the most life-threatening diseases globally. The main feature of cancer is its ability to uncontrolled proliferation and invasion of abnormal cells to different tissues leading to the generation of tumor. This abnormal proliferation process can be retarded by activities such as apoptosis, cytotoxicity and antiproliferative. Among various types of cancer, lung cancer has highest rate of occurrence and mortality (11.6 % and 18.4 %), followed by female breast cancer (11.6 % and 6.6 %). At present, natural chemotherapeutic drugs including natural product based formulations, Essential Oil (EO) and their chemical constituents have been studied and reported to exhibit anticancer properties[1-4].
The overuse of antibiotics has led to the prevalence of bacterial resistance, which has necessitates the discovery and development of new antibacterial agents from various natural sources. Hence, attention has been paid on investigation of natural resources like (plant extract and secondary metabolites isolated from plants), fungi, bacteria, seaweed or obtained semi-synthetically as a potential antibacterial agent[5,6]. Bacteria such as Staphylococcus aureus (S. aureus) are the major cause of food spoilage and may even result in food poisoning and foodborne diseases. Synthetic preservatives such as sodium benzoate and nitrate have been commonly used for the control of pathogenic bacteria to reduce economic loss and foodborne illnesses. Recently, extracted EOs from plants have attracted great interest among the researchers for their tremendous potential as a safer bio preservative in food industry[7,8].
Hedychium (family Zingiberaceae) is the genus of perennial rhizomatous herb accounting approximately 80 species[9], distributed at an altitude ranging from 1000-3000 m in India, Nepal, Bhutan and China[10,11]. Hedychium spicatum (H. spicatum) Ham. ex. Smith is one of the most appreciated herb, popularly known as kapur kachri, ginger lily and shati (Ayurvedic classics) [11-13]. It is a small hardy rhizomatous herb with green leaves and large orange whitish flowers[14]. Its rhizomes are used as an ingredient in several oriental medicine formulations[13-16]. EOs extracted from this herb has a significant role in different areas, particularly in perfumery, pharmaceutical industries and as a food preservative[17-19]. A brief literature survey revealed that its rhizomes EO has antimicrobial, hypotensive, antispasmodic, CNS depressant, analgesic, antiinflammatory, antioxidant, cytotoxic and anthelmintic activities[13,20,21]. Also, rhizomes EO is used to check seed-borne diseases of crops[22]. In Himachal Pradesh, leaves are used in making mats for the home, combined with wheat straw, enhancing the durability of the product[9]. The phytochemical screening of the plant recorded the presence of diverse bioactive metabolites such as starch, regins, organic acids, glycosides, albumen and furanoid diterpenes[11]. In continuation to our different work programs on Western Himalayan bioresources[23-28], this study will add more benefits for biological applications and possible use as a natural medicinal agent.
Materials and Methods
Chemicals and reagents:
The following chemicals were used for the experiments; mixture of standard n-alkane ranging from (C9-C24) and solvents (deuterated chloroform, Concanavalin A (Con A) and dichloromethane (Gas Chromatography (GC) grade) used, were commercially available and purchased from Sigma Aldrich, India and Merck Life Science Pvt. Ltd, Mumbai, India. Also, Dimethyl Sulfoxide (DMSO), Mueller-Hinton agar and ampicillin (10 μg/disc) from Himedia, India. Cancer cell lines were procured from National Centre for Cell Sciences (NCCS), Pune, India.
Plant material:
The cultivated dried seeds of H. spicatum were collected from Council of Scientific and Industrial Research (CSIR)-Institute of Himalayan Bioresource Technology (IHBT), Palampur (Himachal Pradesh, India) (32°06’05”N and 76°34’10”E), in January 2017, at lower altitudes of 1325 m Above Sea Level (ASL). Authentication of the plant was done by the taxonomist of CSIR-IHBT, Palampur, India. A voucher specimen (voucher #PLP 1109) was deposited in the herbarium of CSIR-IHBT.
Extraction of the EO:
Dried seeds (100 g) of H. spicatum were crushed and transferred to a 2000 ml round bottomed boiling flask as the distilling apparatus. 500 ml distilled water was added to the boiling flask. Sample was hydrodistilled for 3 h using Clevenger-type apparatus. The extracted EO was dried over anhydrous sodium sulphate and preserved in sterilized glass vial at low temperature until chemical and biological evaluation.
Gas Chromatography-Mass Spectrometry (GCMS)/ GC analyses:
Characterization of EO components was carried out with the help of GC/GC-MS. GC-MS measurements were conducted on a Shimadzu QP 2010 coupled with AOC-5000 auto-injector using a Zebron™ ZB-5MS (Phenomenex, USA) capillary column (30 m×0.25 mm ID, 0.25 μm thickness). The typical linear temperature programming was from 70° for 4 min and then continuously increased to 220°, at a rate of 4°/min and held for 5 min, the carrier gas was helium. Injector and interface temperatures were maintained at 240° and 250° respectively. Other parameters were adopted as acquisition mass range, 40-800 m/z; ionization energy, 70 eV and injection mode split (10:1)[29].
The GC oven temperature was programmed as follows, 70° held for 4 min and then at 4°/min to 220° and held for 5 min, injector temperature, 240°, detector temperature, 260°, injection mode, split (10:1). Nitrogen was used as carrier gas at a flow rate of 1.05 ml/min (65.3 kPa).
Identification of EO components:
The individual EO components were identified by comparing their GC Arithmetic index (AI) relative to standard of n-alkanes chain (C9-C24, linear hydrocarbons) on ZB-5MS capillary column and using National Institute of Standards and Technology (NIST) database[30]. Further characterization was performed on matching their AI value with literature data[31]. The reconfirmation of major chemical constituents in a mixture was explored by comparison of characteristic peaks of Carbon-13 (13C) Nuclear Magnetic Resonance (NMR) spectra with previously reported data[29,32,33] .
Cytotoxic activity
Cell lines and cell culture: Human skin carcinoma A431, human lung carcinoma A549 and human cervical cancer SiHa cells were procured from NCCS, Pune, India. All the cells (A431, A549 and SiHa) were grown in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen Biosciences, India) supplemented with 10 % fetal bovine serum (Invitrogen Biosciences, India) and 1 % antibiotic-antimycotic solution (Invitrogen Biosciences, India). Cells were incubated at 37° in a Carbon dioxide (CO2) incubator[27,34].
Sulphorhodamine B (SRB) assay: Cytotoxicity potential of EO from seeds of H. spicatum was assessed using SRB assay as described previously[27,34]. Briefly, all the cells (A431, A549 and SiHa) distributed at a density of 13 000 to 18 000 cells/well into 96-well plates. The cells were exposed with four concentrations (25, 50, 100, 200 μg/ml) of EO from seeds of H. spicatum. Vinblastine (1 μM) was used as positive control, whereas alone cells were used as negative control. Plates were incubated at 37° for 48 h in CO2 incubator. After 48 h, 50 μl of 50 % Trichloroacetic acid (TCA) was added to the wells and plates were incubated for 1 h at 4°. The plates were washed five times with water and then air-dried. After that 100 μl of SRB solution was added and incubated for 30 min at room temperature. Subsequently, plates were washed six times with 1 % acetic acid. 100 μl of 10 mM tris base (Sigma Aldrich, India), was added to the wells. The absorbance was recorded using microplate reader (BioTeK Synergy H1 Hybrid Reader) at 540 nm.
Isolation of splenocytes: Splenocytes were isolated from the mice spleen. Briefly, spleen was macerated using a rubber syringe plunger and centrifuge at 400 xg for 10 min. Subsequently, 2 ml of Red Blood Cell (RBC) lysis buffer was added to 1 ml of cell suspension for 15 min followed by centrifugation at 400 xg for 5 min at room temperature. The resultant supernatant was removed and again 1 ml of RBC lysis buffer was added to 1 ml of cell suspension for 5 min and centrifuged at 400 xg for 5 min at room temperature. The pellet was again suspended in 5 ml of complete Roswell Park Memorial Institute (RPMI)-1640 medium and viable cells were counted using haemocytometer[35].
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay: Splenocytes were seeded at a density of 0.35×106 cells per well in 100 μl of complete RPMI-1640 medium. The viability of cells was assessed by MTT assay in 96 well plates. The cells were treated with different concentration (25, 50, 100 and 200 μg/ ml) of EO from seeds of H. spicatum for 48 h. After incubation period 20 μl of 5 mg/ml MTT was added to the wells and plates were incubated in dark at 37º for 4 h. After 4 h supernatant was removed and 100 μl of DMSO was added to plates and incubated for 30 min in the dark at 37°. The optical density was recorded at 570 nm using microplate reader.
Proliferation assay: Splenocyte proliferation assay was performed as described with slight modification. Briefly, splenocytes were isolated and cultured on 96 well plates at a density of 0.35×106. Con A, a mitogen was added to each well at a concentration of 5 μg/ml and further co-cultured with different concentrations (25, 50, 100 and 200 μg/ml) of EO from seeds of H. spicatum for 48 h. After incubation, proliferation was assessed using MTT assay. Cell proliferation rate was expressed as stimulation index (Optical Density (OD) 570 nm with Con A/OD 570 nm without Con A)[35].
Antibacterial activity:
Micro-organisms preparation: EO extracted from H. spicatum seeds was tested for antibacterial activity following disc-diffusion method, using 6 mm sterile filter discs (Whatman No. 1)[36]. Antibacterial assay was performed against three gram-positive bacteria viz. Bacillus subtilis (B. subtilis) MTCC121, Micrococcus luteus (M. luteus) MTCC2470 and S. aureus MTCC96, and three Gram-negative bacteria viz. Klebsiella pneumoniae (K. pneumoniae) MTCC109, Escherichia coli (E. coli) MTCC43 and Salmonella typhi (S. typhi) MTCC733. All test bacteria were grown at 37° and inoculums were adjusted to 1.0×105 CFU/ ml. A suspension of 100 μl was spread on the surface of Mueller-Hinton agar (Himedia, India) plates with a sterile cotton swab and allowed to dry. The discs impregnated with 20 μl of EO (diluted with dimethyl sulfoxide in 1:2 ratio) were aseptically placed on the surface of agar plates. Plates were first incubated at 4° for 2 h under aerobic conditions in order to allow the diffusion of EO and then incubated at 37° for 24 h. The formation of clear zones around wells indicated anti-bacterial activity. Inhibition zone diameter around each disc was measured and recorded in mm. A disc containing 20 μl DMSO was used as a negative control and ampicillin (10 mcg/disc) (Himedia, India) was used as a reference (positive control). All experiments were performed in triplicates.
Determination of Minimum Inhibitory Concentration (MIC): MIC of the EO was determined by broth microdilution assay in a 96-well microtitre plate[37-43]. Overnight bacterial culture was adjusted to 0.5 McFarland standard. EO was diluted in Mueller-Hinton broth containing 0.5 % (v/v) Tween 20 as emulsifier and two-fold serial dilutions were made to obtain the desired concentration ranges starting from 8 %. The prepared bacterial suspension (50 μl) was added to each well. The microplate was then incubated for 24 h at 37° and 30 μl of resazurin (0.015 %) was added to all wells. After, further incubation for 2-4 h, a change in color from blue to pink was visually observed. The MIC was defined as the lowest concentration showing no visible color change. Broth medium (100 μl) in one column represented control for sterility check.
Results and Discussion
The obtained EO possess colorless, spicy with mild acrid fragrance (2.8 ml; yield 2.8 % (v/w); moisture free basis (mfb) 3.1 %). EO was found as a complex mixture having major concentration of mono-terpene (86.4 %) and sesqui-terpene (9.7 %) hydrocarbons. 23 components were characterized representing 97.8 % of the total oil and grouped into mono-, sesquiterpene hydrocarbons (beta (β)-pinene 64.0 %; alpha (α)-pinene 17.0 %; limonene 2.1 %; sabinene,1.5 %; E-caryophyllene 4.2 % and α-humulene 5.4 %) and oxygenated monoterpene (1,8-cineole, 1.2 %) as shown in Table 1. EO from the rhizomes part of this plant is well reported in literature. The fragrance and EO composition of seeds part was found to be different from the rhizomes[13]. The characteristic major compounds of H. spicatum rhizomes EO (10-epi-gamma (γ)-eudesmol, β-eudesmol, elemol, linalool, α-terpineol, β-farnesene and hinesol) were absent in this EO. However, nine metabolites (α-pinene, camphene, sabinene, β-pinene, β-myrcene, 1,8-cineole, 4-terpineol, α-humulene and delat (δ)-cadinene) from seeds were common to rhizomes EO. Quality of seeds EO extracted from the same area indicated significant variations in chemical composition and therefore seeds can be utilized as a good source along with its rhizomes. Phytochemical profiling of the EO showed the presence of biologically active monoterpene hydrocarbons α-pinene and β-pinene, as major components. α-Pinene was favored for the exceptional pharmaceutical applications and due to its pleasant aroma, it is extensively used in perfumery industry[29]. α-Pinene is well known for its diverse bioactivities such as cytotoxicity, antiosteoarthritis, antiulcerogenic and gastroprotective effects while β-pinene exhibited antibacterial and cytotoxic activity[23,44-46]. α-Pinene alone and with the synergy of other pinene is well reported for their antibacterial, antifungal, insecticidal, sedative and anticarcinogenic activities[13,23]. Other major molecules (α-humulene and E-caryophyllene) of this oil have also very good anti-inflammatory effects[47,48].
| S. No. | Compounds | RIa | RIb | Area % | Mode of identification |
|---|---|---|---|---|---|
| 1 | α-Thujene | 925 | 924 | 0.2±0.00 | AI, MS |
| 2 | α-Pinene | 933 | 932 | 17.0±0.03 | AI, MS, 13C NMR |
| 3 | Camphene | 950 | 946 | 0.2±0.00 | AI, MS |
| 4 | Sabinene | 977 | 969 | 1.5±0.00 | AI, MS |
| 5 | β-Pinene | 979 | 974 | 64.0±0.12 | AI, MS, 13C NMR |
| 6 | β-Myrcene | 988 | 988 | 0.7±0.01 | AI, MS |
| 7 | α-Phellandrene | 1008 | 1002 | 0.1±0.00 | AI, MS |
| 8 | α-Terpinene | 1018 | 1014 | 0.1±0.00 | AI, MS |
| 9 | Limonene | 1030 | 1024 | 2.1±0.01 | AI, MS |
| 10 | 1,8-Cineole | 1033 | 1026 | 1.2±0.01 | AI, MS |
| 11 | E-β-Ocimene | 1045 | 1044 | 0.2±0.00 | AI, MS |
| 12 | γ-Terpinene | 1058 | 1054 | 0.2±0.01 | AI, MS |
| 13 | α-Terpinolene | 1086 | 1086 | 0.1±0.00 | AI, MS |
| 14 | Nopinone | 1141 | 1135 | 0.1±0.01 | AI, MS |
| 15 | Pinocarvone | 1164 | 1160 | 0.1±0.00 | AI, MS |
| 16 | 4-Terpineol | 1185 | 1177 | t | AI, MS |
| 17 | Myrtenal | 1198 | 1195 | t | AI, MS |
| 18 | cis-α-Bergamotene | 1414 | 1411 | t | AI, MS |
| 19 | E-Caryophyllene | 1422 | 1417 | 4.2±0.02 | AI, MS |
| 20 | α-Humulene | 1459 | 1452 | 5.4±0.01 | AI, MS |
| 21 | δ-Cadinene | 1521 | 1522 | 0.1±0.03 | AI, MS |
| 22 | Caryophyllene oxide | 1587 | 1582 | 0.1±0.02 | AI, MS |
| 23 | Humulene epoxide II | 1615 | 1608 | 0.2±0.04 | AI, MS |
| Total identified | 97.8 | ||||
| Monoterpene hydrocarbons | 86.4 | ||||
| Oxygenated monoterpenes | 1.4 | ||||
| Sesquiterpene hydrocarbons | 9.7 | ||||
| Oxygenated sesquiterpenes | 0.3 |
Note: aAI experimentally determined relative to n-alkanes (C9-C24) on the ZB-5MS GC column; bAI value of compounds in literature data and (t): Detected in trace amount
Table 1: EO composition of the cultivated H. spicatum seeds
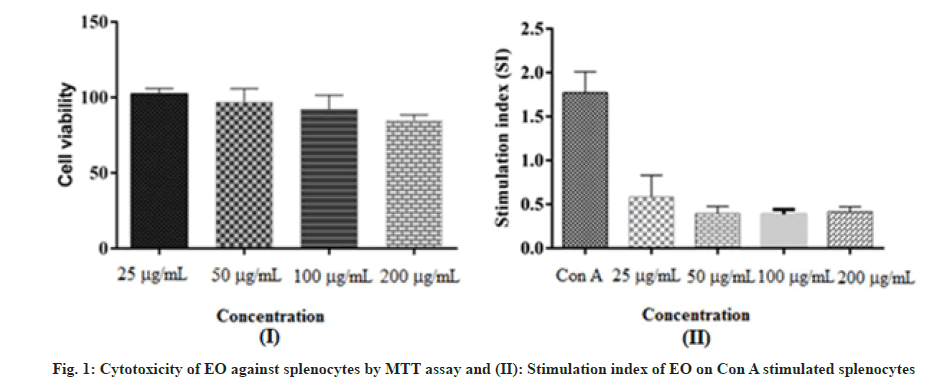
In the present study, EO from seeds of H. spicatum showed the highest activity (59.5, 76.1 and 69.7) at a concentration of 200 μg/ml on A431, A549 and SiHa cells respectively. The Half-maximal inhibitory concentration (IC50) values of EO from seeds of H. spicatum against A431, A549 and SiHa cells ranged from 71.2 to 171.7 μg/ml as shown in Table 2. Our results in line with a study performed by Mishra et al. 2016 where in authors reported cytotoxic attributes of rhizomes EO (MHS-4) as promising cytotoxic activity against human cell lines such as, the lung (A549), colon (DLD-1, SW620), breast (MCF-7, MDA-MB-231), head and neck (FaDu) and cervix (HeLa) with IC50 values ranging from 26.77 to 94.33 μg/ml. However, other EOs samples (MHS-1, MHS-2 and MHS-3) exhibited different level of cytotoxic properties[21]. In addition, Rawat et al. 2019 also reported moderate to potent cytotoxicity of EOs against A431 (epidermal) and MCF (lung) cell lines at different concentrations[49]. Cytocompatibility of EO from seeds of H. spicatum was also determined against normal mice splenocytes by the MTT assay. In this test, percentage of splenocytes survival was quantified by determination of the formation of formazan crystals from exogenous MTT as shown in fig. 1. Results revealed that EO was safe even at a higher concentration i.e. 200 μg/ml as presented in fig. 1 indicate its non-lethal nature for normal immune cells. Further, Splenocyte Stimulation Index (SSI) was used to demonstrate immunomodulatory role of EO. Con-A was used as mitogen that specifically stimulates T cells and also act as an antigen[50]. Enhanced proliferation of splenocytes in presence of Con-A was reported as one characteristic features of persistent inflammation. Here, in this study, results revealed that proliferation index was enhanced upto 1.75 in Con-A treated cells in comparison to control. However, EO reduced proliferation index by 0.54, 0.38, 0.38 and 0.41 at 25, 50, 100 and 200 μg/ml respectively (fig. 1). It has been established that Con A acts as a mitogen that specifically stimulates T cells. Here, we found that EO from seeds of H. spicatum decreases the proliferation of T cells[51]. These in vitro findings demonstrated that the EO from H. spicatum seeds is cytotoxic against A431, A549 and SiHa cells and safe on mice splenocytes. Hence, further studies to reach at molecular level can be proceeded to develop anticancer agents.
| EO | Concentration µg/ml | A431 | A549 | SiHa |
|---|---|---|---|---|
| 25 | 0±0.27 | 0±0.5 | 0±4.2 | |
| H. spicatum oil | 50 | 24.4±3.6 | 31.5±0.9 | 0±2.1 |
| 100 | 50.2±3.6 | 75.2±1.6 | 0±1.9 | |
| 200 | 59.5±4.3 | 76.1±0.7 | 69.7±1.8 | |
| Vinblastin | 1 µM | 51.4±3.2 | 75.8±0.3 | 69.6±0.2 |
| IC50 value in µg/ml | 96.9 | 71.2 | 171.7 |
Table 2: In vitro cytotoxicity against A431, A549 AND SiHa cells by SRB assay
The in vitro analysis of antibacterial activity of EO of H. spicatum seeds revealed activity against the tested bacteria as displayed in Table 3. EO showed inhibition against all the tested gram-positive bacteria viz. B. subtilis MTCC121 (6.6 mm), S. aureus MTCC96 (3.3 mm) and M. luteus MTCC2470 (2.0 mm). Among the gram-negative bacteria, EO showed inhibition against E. coli MTCC43 (8.3 mm) and S. typhi MTCC733 (5.7 mm), while K. pneumoniae MTCC109 was found resistant. The maximum activity of the EO was elucidated against E. coli MTCC43, followed by B. subtilis MTCC 121 and S. typhi MTCC733.
| Bacterial strains | Diameter of inhibition zone (mm) | MIC (%v/v) |
|---|---|---|
| Gram-positive | ||
| B. subtilis MTCC121 | 6.6±0.5 | 1 |
| S. aureus MTCC96 | 3.3±0.5 | 2 |
| M. luteus MTCC2470 | 2.0±0.0 | 2 |
| Gram-negative | ||
| S. typhi MTCC733 | 5.7±0.5 | 2 |
| E. coli MTCC43 | 8.3±0.5 | 2 |
| K. pneumoniae MTCC109 | - | - |
Table 3: Antibacterial activity and MIC (% v/v) of H. spicatum seeds EO
The antibacterial activity of extracted EO was quantitatively assessed by MIC against the bacterial strains that exhibited positive result in disc diffusion assay. MIC was observed at a range between two and one percent (v/v). The lowest MIC i.e. most susceptibility was observed with the strain B. subtilis MTCC121 at 1 % (v/v) as shown in Table 3. However, MIC for all other strains was observed at 2 % (v/v). A previously published article demonstrated that rhizomes EO exhibited a promising antibacterial activity against Salmonella enterica enterica with zone of inhibition (10-15 mm)[20]. Moreover, fungitoxic property of EO has also been reported[9]. A similar inhibitory effect was noticed for EO of H. spicatum seeds. Current study has shown that the EO is capable of restricting growth of all tested gram-positive bacteria. Regarding gram-negative bacteria, E. coli and S. typhi could be inhibited while K. pneumoniae was found resistant. The cause for bacterial inhibition by EO may be credited to its hydrophobicity that helps in its partitioning with lipids of the cell membrane and mitochondria leading to more membrane permeability. This leads to leakage of essential molecules, eventually causing cell death[29,50,52,53].
In conclusion, the chemical composition, biological activities and safety evaluation of H. spicatum seeds were checked in this study. From current research findings it has been revealed that EO exhibited cytotoxic activity against different cell lines and safe for cell growth on mouse splenocytes at lower concentration. In recent years, natural components and extracts had been utilized frequently for the prevention of cancer. Also presence of bioactive metabolites in air will make a healthy environment[24]. Additionally, evaluation of antibacterial activity suggested that H. spicatum seeds EO possessed bacterial inhibition activity. As discussed above based on the results obtained, it could be concluded that H. spicatum seeds EO needs to be evaluated for detailed in vitro and in vivo anticancer and antibacterial activities to develop it as natural medicinal agent and also possesses scope of utilization in the field of healthcare and food as well.
Acknowledgements:
The authors are grateful to Dr. Sanjay Kumar, Director, CSIR-IHBT Palampur (Himachal Pradesh), India, for continuous encouragement and providing the needed facilities during the course of study. The authors are appreciatively acknowledging Mr. Shiv Kumar for providing GC/GC-MS and NMR data. We are also grateful to CSIR, New Delhi for financial support of these projects (BSC-0209, MLP-0142 and HCP-0007) under which the work was completed.
Funding:
CSIR, New Delhi sponsored BSC-0209, MLP-0142 and HCP-0007 projects.
Conflict of interests:
The authors declare that there is no conflict of interests in this paper.
References
- Al?Qudah MA, Saleh AM, Alhawsawi NL, Al?Jaber HI, Rizvi SA, Afifi FU. Composition, antioxidant and cytotoxic activities of the essential oils from fresh and air?dried aerial parts of Pallenis spinosa. Chem Biodivers 2017;14(8):e1700146.
[Crossref] [Google Scholar] [Pub Med]
- Palma CE, Cruz PS, Cruz DT, Bugayong AM, Castillo AL. Chemical composition and cytotoxicity of Philippine calamansi essential oil. Ind Crops Prod 2019;128:108-14.
- Jaradat N, Al-Maharik N, Abdallah S, Shawahna R, Mousa A, Qtishat A. Nepeta curviflora essential oil: Phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities. Ind Crops Prod 2020;158:112946.
- Sharma S, Chhimwal J, Kumar S, Padwad Y, Kumar D. Cytotoxic steroidal saponins from Polygonatum verticillatum Linn. Phytochem Lett 2021;45:30-6.
- Pinheiro PF, Menini LA, Bernardes PC, Saraiva SH, Carneiro JW, Costa AV, et al. Semisynthetic phenol derivatives obtained from natural phenols: Antimicrobial activity and molecular properties. J Agric Food Chem 2018;66(1):323-30.
[Crossref] [Google Scholar] [Pub Med]
- Sharma S, Patial V, Singh D, Sharma U, Kumar D. Antimicrobial homoisoflavonoids from the rhizomes of Polygonatum verticillatum. Chem Biodivers 2018;15(12):e1800430.
[Crossref] [Google Scholar] [Pub Med]
- Deng J, He B, He D, Chen Z. A potential biopreservative: Chemical composition, antibacterial and hemolytic activities of leaves essential oil from Alpinia guinanensis. Ind Crops Prod 2016;94:281-7.
- Kacem N, Roumy V, Duhal N, Merouane F, Neut C, Christen P, et al. Chemical composition of the essential oil from Algerian Genista quadriflora Munby and determination of its antibacterial and antifungal activities. Ind Crops Prod 2016;90:87-93.
- Rawat S, Jugran AK, Bhatt ID, Rawal RS. Hedychium spicatum: A systematic review on traditional uses, phytochemistry, pharmacology and future prospectus. J Pharm Pharmacol 2018;70(6):687-712.
[Crossref] [Google Scholar] [Pub Med]
- Rawat S, Bhatt ND, Rawal RS, Nandi HK. Effect of developmental stage on total phenolics composition and anti-oxidant activities in Hedychium spicatum Buch.-Ham. ex. D. Don. J Hortic Sci Biotechnol 2014;89(5):557-63.
- Ghildiyal S, Gautam MK, Joshi VK, Goel RK. Pharmacognostical study of Hedychium spicatum (Ham-Ex-Smith) rhizome. Asian Pac J Trop Dis 2012;2:S148-53.
- Hartati R, Suganda AG, Fidrianny I. Botanical, phytochemical and pharmacological properties of Hedychium (Zingiberaceae)–A review. Procedia Chem 2014;13:150-63.
- Koundal R, Rawat K, Agnihotri VK, Meena RL, Gopichand, Singh RD, et al. Temporal and spatial variation in quality of essential oil of Hedychium spicatum and evaluation of its antioxidant activity. J Essent Oil Res 2015;27(3):217-24.
- Rasool S, Maqbool M. An overview about Hedychium spicatum: A review. J Drug Deliv Ther 2019;9(1):476-80.
- Rawat S, Bhatt ID, Rawal RS. Total phenolic compounds and antioxidant potential of Hedychium spicatum Buch. Ham. ex D. Don in west Himalaya, India. J Food Comp Anal 2011;24(4-5):574-9.
- Rao GV, Mukhopadhyay T, Madhavi MS, Lavakumar S. Chemical examination and hair growth studies on the rhizomes of Hedychium spicatum Buch.-Ham. Pharmacog Commun 2011;1:90-3.
- Prakash B, Singh P, Kedia A, Dubey NK. Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Food Res Int 2012;49(1):201-8.
- Jadhav V, Kore A, Kadam VJ. In vitro pediculicidal activity of Hedychium spicatum essential oil. Fitoterapia 2007;78(7-8):470-3.
[Crossref] [Google Scholar] [Pub Med]
- Solanki DJ, Chavda H, Makim, R. A review on vulnerable plant Shati-Hedychium spicatum. Int J Ayurveda Altern Med 2016;4:2348-017320.
- Joshi S, Chanotiya CS, Agarwal G, Prakash O, Pant AK, Mathela CS. Terpenoid compositions and antioxidant and antimicrobial properties of the rhizome essential oils of different Hedychium species. Chem Biodivers 2008;5(2):299-309.
[Crossref] [Google Scholar] [Pub Med]
- Mishra T, Pal M, Meena S, Datta D, Dixit P, Kumar A, et al. Composition and in vitro cytotoxic activities of essential oil of Hedychium spicatum from different geographical regions of western Himalaya by principal components analysis. Nat Prod Res 2016;30(10):1224-7.
[Crossref] [Google Scholar] [Pub Med]
- Sravani T, Paarakh PM. Hedychium spicatum Buch. Ham.-An overview. Pharmacologyonline 2011;2:633-42.
- Koundal R, Kumar D, Walia M, Kumar A, Thakur S, Chand G, et al. Chemical and in vitro cytotoxicity evaluation of essential oil from Eucalyptus citriodora fruits growing in the Northwestern Himalaya, India. Flavour Fragr J 2016;31(2):158-62.
- Agnihotri VK. Anabaena flos-aquae. Crit Rev Env Sci Tech 2014;44:1995-2037.
- Kumar A, Maurya AK, Chand G, Agnihotri VK. Comparative metabolic profiling of Costus speciosus leaves and rhizomes using NMR, GC-MS and UPLC/ESI-MS/MS. Nat Prod Res 2018;32(7):826-33.
[Crossref] [Google Scholar] [Pub Med]
- Maurya AK, Kumar A, Agnihotri VK. New iridoids from the roots of Valeriana jatamansi Jones. Nat Prod Res 2020:1-8.
[Crossref] [Google Scholar] [Pub Med]
- Maurya AK, Sharma A, Kumar K, Chander R, Kumar A, Kumar D, et al. Comparative studies of essential oils composition and cytotoxic activity of Valeriana jatamansi Jones. J Essent Oil Res 2021;33(6):584-91.
- Vashisath S, Maurya AK, Agnihotri VK. Comparative chemical profiling of Zanthoxylum armatum DC. from western Himalayan bioresource. J Essent Oil Res 2021;33(6):592-600.
- Maurya AK, Devi R, Kumar A, Koundal R, Thakur S, Sharma A, et al. Chemical composition, cytotoxic and antibacterial activities of essential oils of cultivated clones of Juniperus communis and wild Juniperus species. Chem Biodivers 2018;15(9):e1800183.
[Crossref] [Google Scholar] [Pub Med]
- Stein SE. Mass spectral database and software, version 3.02. Gaithersburg, MD: National Institute of Standards and Technology; 2005.
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream: Allured Publishing Corporation; 2007.
- Kubeczka KH, Formá?ek V. Essential oils analysis by capillary gas chromatography and carbon-13 NMR spectroscopy. John Wiley & Sons Ltd; 2002.
- Ferreira MJP, Emerenciano VP, Linia GAR, Romoff P, Macari PAT, Rodirigues GV. 13C NMR spectroscopy of monoterpenoids. Prog Nucl Magn Reson Spectrosc 1998;33:152-06.
- Kumar D, Sukapaka M, Babu GK, Padwad Y. Chemical composition and in vitro cytotoxicity of essential oils from leaves and flowers of Callistemon citrinus from Western Himalayas. PloS one 2015;10(8):e0133823.
[Crossref] [Google Scholar] [Pub Med]
- Rana S, Kumar S, Rana A, Sharma V, Katoch P, Padwad Y, et al. Phenolic constituents from apple tree leaves and their in vitro biological activity. Ind Crops Prod 2016;90:118-25.
- Sabulal B, George V, Dan M, Pradeep NS. Chemical composition and antimicrobial activities of the essential oils from the rhizomes of four Hedychium species from south India. J Essent Oil Res 2007;19:93-7.
- Elshikh M, Ahmed S, Funston S, Dunlop P, McGaw M, Marchant R, et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett 2016;38(6):1015-9.
[Crossref] [Google Scholar] [Pub Med]
- Adukwu EC, Allen SC, Phillips CA. The anti?biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (Citrus paradisi) essential oils against five strains of Staphylococcus aureus. J Appl Microbiol 2012;113(5):1217-27.
[Crossref] [Google Scholar] [Pub Med]
- Pauli A, Kubeczka KH. Evaluation of inhibitory data of essential oil constituents obtained with different microbiological testing methods. Essent Oil Basic Appl Res 1996:33-6.
- Janssen AM, Scheffer JJ, Svendsen AB. Antimicrobial activity of essential oils: A 1976-1986 literature review. Aspects of the test methods. Planta Med 1987;53(5):395-8.
[Crossref] [Google Scholar] [Pub Med]
- Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem 2003;10(10):813-29.
[Crossref] [Google Scholar] [Pub Med]
- Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol 2006;106(3):290-302.
[Crossref] [Google Scholar] [Pub Med]
- Rios JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol 2005;100(1-2):80-4.
[Crossref] [Google Scholar] [Pub Med]
- Rufino AT, Ribeiro M, Judas F, Salgueiro L, Lopes MC, Cavaleiro C, et al. Anti-inflammatory and chondroprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J Nat Prod 2014;77(2):264-9.
[Crossref] [Google Scholar] [Pub Med]
- Rivas da Silva AC, Lopes PM, Barros de Azevedo MM, Costa DC, Alviano CS, Alviano DS. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012;17(6):6305-16.
[Crossref] [Google Scholar] [Pub Med]
- Magalhães RM, Torres DM, Cavalcante RC, Mota FS, EM OC, Moreira HP, et al. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. Pharmacogn Mag 2015;11(41):123-30.
[Crossref] [Google Scholar] [Pub Med]
- Fernandes ES, Passos GF, Medeiros R, da Cunha FM, Ferreira J, Campos MM, et al. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol 2007;569(3):228-36.
[Crossref] [Google Scholar] [Pub Med]
- Rogerio AP, Andrade EL, Leite DF, Figueiredo CP, Calixto JB. Preventive and therapeutic anti?inflammatory properties of the sesquiterpene α?humulene in experimental airways allergic inflammation . Br J Pharmacol 2009;158(4):1074-87.
[Crossref] [Google Scholar] [Pub Med]
- Rawat A, Thapa P, Prakash O, Kumar R, Pant AK, Srivastava RM, et al. Chemical composition, herbicidal, antifeedant and cytotoxic activity of Hedychium spicatum Sm.: A Zingiberaceae herb. Trends Phytochem. Res 2019;3(2):123-36.
- Salvadori F, Tournefier A. Activation by mitogens and superantigens of axolotl lymphocytes: Functional characterization and ontogenic study. Immunology 1996;88(4):586-92.
- Malaczewska J. The splenocyte proliferative response and cytokine secretion in mice after 28 d oral administration of silver nanocolloid. Pol J Vet Sci 2014;17(1):27-35.
[Crossref] [Google Scholar] [Pub Med]
- Dob T, Dahmane D, Benabdelkader T, Chelghoum C. Studies on the essential oil composition and antimicrobial activity of Thymus algeriensis Boiss. et Reut. Inter J Aromather 2006;16(2):95-100.
- Solórzano-Santos F, Miranda-Novales MG. Essential oils from aromatic herbs as antimicrobial agents. Curr Opin Biotechnol 2012;23(2):136-41.
[Crossref] [Google Scholar] [Pub Med]