Xiaohong Wand, W. Z. Li*, D. Kong1 and Yu Duan
Department of Pharmacy, Weifang Medical University, Weifang, Shandong, 261 053, People's Republic of China.
1Department of Basic Medicine, Weifang Medical University, Weifang, Shandong, 261 053, People's Republic of China.
- *Corresponding Author:
- W. Z. Li
Department of Pharmacy
Weifang Medical University
Weifang, Shandong, 261 053
People's Republic of China
E-mail: wanzhongli@hotmail.com
Received Date: 16 Sep 2015; Revised Date: 21 May 2016; Accepted Date: 26 May 2016
Abstract
Toona sinensis (A. Juss.) Roem has long been used in traditional Chinese medicine for the treatment of diabetic mellitus. The aim of this study was to investigate the effect of ethanol extracts of T. sinensis seeds on diabetic peripheral neuropathy and the preliminary mechanism. Male wistar rats were used for inducing diabetic peripheral neuropathy model, the variation of body weight; blood glucose, general status, thermal perception thresholds and sciatic nerve conduction velocity were used to confirm the diagnosis of diabetic peripheral neuropathy. After the treatment, the sciatic nerve conduction velocity was accelerated, the tailflick latency was reduced, the hyperglycemia and oxidative stress injury in sciatic nerve were ameliorated, contents of serum nerve growth factor β were up-regulated and tumor necrosis factor α, interleukin-6 were down-regulated significantly (P<0.05) compared with the model rats. This research demonstrates that ethanol extracts of T. sinensis seeds offers potential for intervening diabetic peripheral neuropathy and the protective mechanisms may be alleviating hyperglycemia, inhibiting oxidative stress and regulating growth factor and inflammatory cytokines.
Keywords
Toona sinensis, diabetic peripheral neuropathy, oxidative stress, NGF-β, TNF-α
Diabetic peripheral neuropathy (DPN) is one of the most common chronic complications in patients with diabetes mellitus (DM)[1]. Nearly 60% of the patients suffer from peripheral neuropathy[2]. The pathogenesis of DPN includes many factors such as metabolic, vascular, autoimmune, oxidative stress and neurohormonal growth factor deficiency[3,4]. Among them, oxidative stress[5,6] plays an important role and a number of neurohormonal cytokines were involved such as interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), transforming growth factor beta 1 (TGF-β1) and nerve growth factor beta (NGF-β)[7-9]. Thus, the pathophysiology and therapeutic development of DPN are active areas of research.
Toona sinensis (A. Juss.) Roem is widely used as a delicious vegetable in China. T. sinensis is also a well-known Chinese herb and all parts of it, including root, bark, petioles, leaves, fruits, and seeds, have been used for medicinal purposes. The aqueous extracts of T. sinensis leaves have revealed anticancer, antioxidant, antidiabetic and antivirus activity[10-12]. The chemical compositions include polyphenol, saponin, sesquiterpene, alkaloid and volatile oil, etc[13,14]. In the present research, ethanol extracts from T. sinensis seeds (ETS) were used to observe the protective effects on peripheral neuropathy in rats with type 2 DM by inhibiting oxidative stress and regulating growth factor in an attempt to investigate the protective mechanism and provide evidence for the clinical treatment of DPN.
Materials and Methods
T. sinensis seeds were purchased from Jinan Shengke Technology Development Co., Ltd. China, and authenticated at Department of Pharmacognosy of Weifang Medical University. The powdered seeds (10 kg) were extracted with 85% ethanol and 75% ethanol (2×100 l, each 3 h), respectively. The combined ethanol extracts were concentrated to yield crude extract (1064 g) under reduced pressure. Male Wistar albino rats weighing between 180-220 g were provided by Weifang Medical Experimental Animal Center and bred in standard animal facility. The animals were kept in controlled conditions and fed with standard pellet diet and water ad libitum. The current study protocol was approved by Ethics Committee of Weifang Medical University for animal studies.
STZ (Sigma Co., USA); DPPH (Sigma Co., USA); Rat NGF-β (1909871108), TNF-α (R131029-12b) and IL-6(R130926-06b) ELISA kit were purchased from NEO Bioscience technology, Beijing, China; total antioxidant capability (T-AOC, 20130726), malondialdehyde (MDA, 20130726), superoxide dismutase (SOD, 20131218), glutathione peroxidase (GSH-PX, 20130723) assay kits were purchased from Jiancheng Bioengineering Institute, Nanjing, China. All other chemicals and reagents used were of analytical grade.
Antioxidant activities in vitro:
The total antioxidant capability (T-AOC) kit and microplates were used for quantitative assay. The reaction principle is antioxidants reduce Fe3+ to be Fe2+, while Fe2+ and Fehling's substances form stable complex compounds. The ETS were dissolved with 80% alcohol; with the concentration (mg/ml) as horizontal coordinate and the absorbance as vertical coordinate, a reducing power curve was drawn. Ascorbic acid was used for positive control.
DPPH was dissolved and diluted with absolute alcohol[15] to attain a concentration of 0.2 mmol/l. The ETS were dissolved with 80% alcohol at different concentrations and the same volume of DPPH solution was added, and the mixtures were placed away from light for 15 min. The absorbance was immediately detected at 515 nm wavelength with ascorbic acid as positive control.
Fresh liver was separated from healthy Wistar rat and 10% homogenate was made in ice bath. Different concentrations of ETS were added into 1.5 ml of homogenate, then 0.1 ml of H2O2 was added and the mixture was reacted in 37° for 30 min. MDA contents were assessed using thiobarbituric acid reactive substances assay by measuring the absorbance value at wavelength of 532 nm.
Induction of experimental DPN and treatments:
20 Wistar rats were selected as the control group (group 1). The remaining rats were fed with a high-fat diet (HFD) for 6 w and were injected intraperitoneally with a dose of 35 mg/kg streptozotocin (STZ) in citrate buffer (pH=4.5). Blood was drawn from the tail vein after 72 h and the rats with blood glucose greater than 16.7 mmol/l were treated as DM rats and were used for further experiments. 6 w later, tail-flick tests were conducted and eight control rats and eight DM rats were selected randomly for detection of DPN by means of sciatic nerve conduction velocity (SNCV) and determination of NGF-β. The abnormalities of these parameters were used to confirm the diagnosis of DPN. The DPN rats were then divided into four groups according to their blood glucose gradient, each 12 as follows: group 2 (DPN model); groups 3 to 5 (DPN rats treated with ETS 0.10, 0.15, 0.2 g/kg/day, respectively). The rats in groups 1 and 2 were treated with an equal volume of distilled water. Each group was administered by gavage once a day for 8w. General status and food intake were observed every day, the values of body weight and blood glucose were determined regularly.
Tail-flick tests:
Tail-flick tests were conducted before and after administration at the 6th, 12th and 20th weeks. Tailflick tests were assessed by tail-flick latency evoked by a noxious heat stimulus by tail-flick instrument (YLS-12A, Yiyan Technology Development Co., Ltd., Shandong, China.). In the test, radiant heat was focused on the distal part of animal’s tail. The cut-off time for the tail-flick reaction was set to 16 s to avoid damage to the tail[16]. Prior to any treatment, the rats were allowed to acclimatize to the test procedure and apparatus for at least 20 min. The test was repeated three times for each rat and mean of three measures was reported. The interval of three measurements was 5 min.
SNCV detection and biomarker analysis:
The experiment animals were anesthetized with 10% chloral hydrate (0.4 ml/100 g) after overnight fasting. With the subjects prone, right sciatic nerves were dissected with a glass needle. The stimulating and recording electrodes were placed directly under the sciatic nerve and the distance between the stimulation electrode and recording electrode was recorded. Then the nerve conductions were made and recorded using the Functional Experiment System (BL-420s, Taimeng, Sichuan, China). SNCV was calculated using the following equations: SNCV: s/t (m/s)=distance between stimulating and recording electrode/ latency[17]. After SNCV was detected, blood samples from heart were collected and the serum biomarkers including NGF-β, TNF-α and IL-6 were determined by commercial ELISA kits using a BioTek microplate reader (Gene Co., Ltd. USA.). The left sciatic nerve was immediately made into 10% homogenate (tissue gram/saline volume in milliliter=1:9) used for analysis of MDA, SOD, and GSP-PX using the commercial kits.
Statistical analysis:
The experimental results were subjected to analysis of variance using SPSS 16.0 and expressed as mean±SD.
Results and Discussion
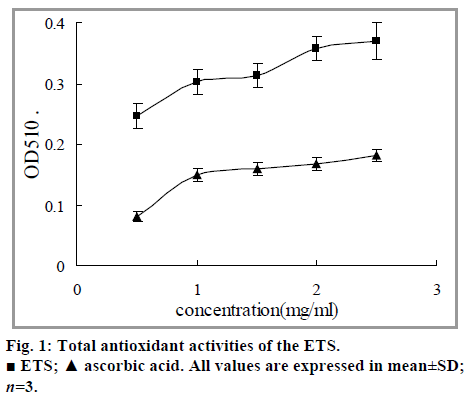
Antioxidant activities in vitro are shown in fig. 1. The absorbance at 510 nm showed the antioxidant capability of ETS in spite of the inferiority compared with ascorbic acid. The IC50 values of ETS and positive control drug on DPPH radical and lipid peroxidation were detected and the results are listed in Table 1. They indicate that the extracts could well scavenge DPPH radicals and inhibited lipid peroxidation.
| ETS (mg/ml) | Ascorbic acid (μg/ml) | |
|---|---|---|
| DPPH | 0.33±0.02 | 9.51±0.75 |
| Lipid peroxidation | 8.19 ±0.71 | 61.50±4.19 |
All values are expressed in mean±SD.
TABLE 1: IC50 OF POLYPHENOLS ON REACTIVE OXYGEN RADICALS (n=5)
During the experiment period, 4 rats died from intubations problems during drug administration including 2 in group 2, 1 in group 3 and 1 in group 5. The animals were immediately necropsied after death, and no abnormality was observed in gross examination. Rats in DPN group showed depressive spirit, less activity, increase of feeding, drinking, urinating and other signs of DM. Treatment groups improved these general features. The blood glucose and body weight of the experimental rats are shown in Table 2. The body weight of normal rats markedly increased from 200±20 g at the baseline to 358±32 g at the end of the experiment (P<0.05). By contrast, the values of DPN rats were decreased after a period of increase caused by the HFD. Treated groups prevented the body weight loss to varying degrees. The blood glucose levels of DPN rats were significantly higher than that of normal rats in group 1 (P<0.05); however, the blood glucose levels of rats in groups 3 to 5 were significantly decreased at the end.
| Group | n | Body weight (g) | Blood glucose (mM) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 w | 6w | 12w | 20w | 0 w | 6w | 12w | 20w | ||
| 1 | 12 | 200±20 | 251±23b | 284±23b | 358±32b | 3.3±0.3 | 3.4±0.4b | 3.2±0.3b | 3.8±0.6b |
| 2 | 10 | 200±20 | 438±36a | 508±49a | 434±41a | 3.3±0.3 | 20.9±3.5a | 21.3±3.7a | 23.1±5.0a |
| 3 | 11 | 200±20 | 441±38a | 471±40a | 481±39a | 3.3±0.3 | 21.2±2.2a | 21.9±2.9a | 9.5±1.6b |
| 4 | 12 | 200±20 | 439±32a | 462±36a | 499±37a | 3.3±0.3 | 19.5±2.9a | 20.6±2.6a | 10.3±0.9b |
| 5 | 11 | 200±20 | 424±33a | 453±37a | 484±40a | 3.3±0.3 | 20.7±2.9a | 19.3±2.9a | 8.4±1.2b |
All values are expressed in mean±SD. aP<0.05 compared with the normal control group; bP<0.05 compared with the DPN group.
TABLE 2: EFFECTS ON BODY WEIGHT AND BLOOD GLUCOSE
The results of effects on oxidative stress in sciatic nerve of DPN rats are listed in Table 3. Compared with that of rats in normal control, the MDA contents in sciatic nerve of rats in DPN group were significantly increased while the SOD and GSH–PX activities were significantly decreased (P<0.05). The groups treated ETS apparently prevented these abnormities (P<0.05).
| Group | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| n | 12 | 10 | 11 | 12 | 11 |
| SOD (U/mgprot) | 95.14±8.33b | 68.47±6.90a | 76.13±7.41b | 84.01±8.44b | 82.37±8.05b |
| MDA (nmol/mgprot) | 2.82±0.28b | 7.93±0.68a | 6.03±0.59b | 4.96±0.45b | 4.52±0.42b |
| GSH?PX (U) | 129.11±10.34b | 79.13±7.83a | 89.76±8.71b | 96.45±9.23b | 101.72±9.55b |
All values are expressed in mean±SD. aP<0.05 compared with the normal control group; bP<0.05 compared with the DPN group.
TABLE 3: EFFECTS ON OXIDATIVE STRESS INDICATORS IN SCIATIC NERVE OF DPN RATS
DPN, one of the most common chronic complications of DM, is a heterogeneous group of disorders with various pathologies and hyperglycemia must be the initiator. To diabetic, affected by the hyperglycemia in vivo, carbohydrate oxidation increases and a series of pathways are activated, which lead to the unbalanced oxidation and anti-oxidization of organisms and the excessive active oxygen radicals, causing the damage of tissues and cells, finally resulting in many DM complications. Oxygen free radical damage is the main contributor to DPN and is regarded as the direct or indirect common pathway in which DPN progresses[18]. Biomarkers for oxidative damage such as MDA were demonstrated to increase in diabetes. Oxidative stress also diminishes endogenous antioxidant enzyme defenses such as SOD and GSH-PX activities[19], which are very important to the regulation of oxidative status in diabetes. The research shows that the ETS possesses certain antioxidant activity in vitro and in vivo. The changes before and after administration suggest that one of the mechanisms of the protective effect may be anti-oxidative stress.
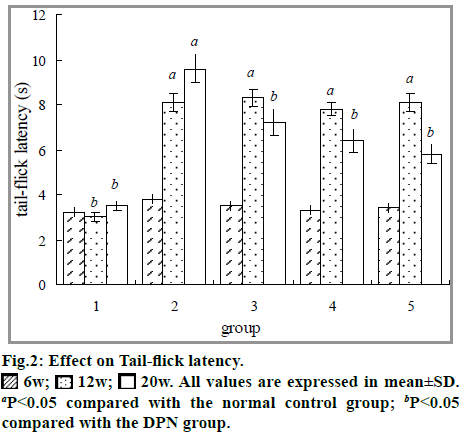
Tail-flick is one of the standard tests for measuring the rate of nociception. In this study, a significant increase in the tail-flick latency was observed after 6 w of STZ induction (P<0.05). These deficits were significantly reversed after treating with ETS for 8 w (P<0.05, fig. 2).
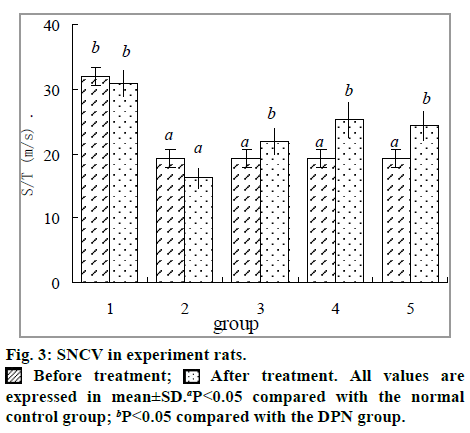
The results of effects on SNCV in DPN rats are shown in fig 3. Influenced by DM, the SNCV of DPN rats in group 2 was much slowed compared with the normal subjects in Group 1 (P<0.05). However, groups 3, 4 and 5 were notably improved than group 2 (P<0.05).
In clinical examination, the lower limbs usually reveal abnormal sensations of vibration, pressure, pain, and temperature perception[20], and DPN is manifested by deficit in nerve conduction velocity as well as thermal perception thresholds[21]. In the present study, we successfully induced DPN models by feeding them HFD for 12 w accompanied by an injection of low dose STZ. 6 w after modeling, early functional abnormalities in the peripheral nerves were observed, including thermal hyperalgesia and reduction in SNCV and by the end of the experiment, these abnormalities became more noticeable and accompanied with disordered growth factor and oxidative stress injury. The alleviation of these parameters by ETS showed the protective effect on DPN.
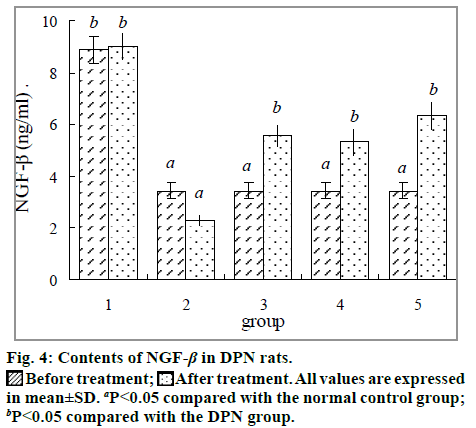
Fig. 4 shows the effect of ETS on contents of serum NGF-β before and after treatment. The concentrations of NGF-β were significantly decreased (P<0.05) before treatment compared with normal controls, which can help to manifest the neurologic damage. The NGF-β contents were apparently increased in groups treated with ETS (P<0.05).
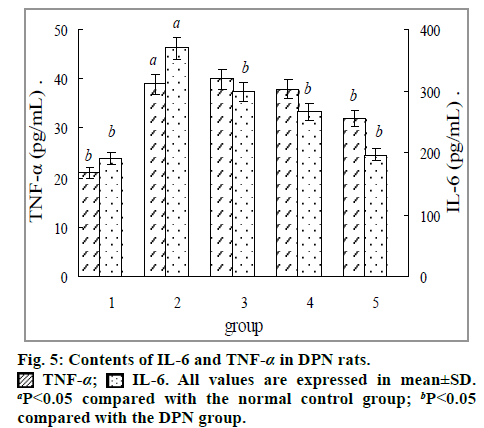
The results in fig. 5 showed that serum levels of pro-inflammatory cytokines TNF-α and IL-6 were significantly (P<0.05) increased in DPN rats compared with the normal controls. Treated with ETS significantly decreased the contents of IL-6 (P<0.05) and the effects on TNF-α showed significantly decreased only in the group treated with high dose ETS.
A series of studies have shown that, NGF-β and proinflammatory cytokines IL-6 and TNF-α are all important biomarkers of neuropathy, they might have vital implications clinically. The role of TNF-α is still controversial and Magrinelli’s clinical research showed it was not found to be associated with DPN. Data from animal models documented that TNF-α was down regulated in the dorsal root ganglion in early stages of experimental diabetes, while the opposite occurred in later stage[22,23]. In our research mean value of serum TNF-α level was found significantly increased in DPN rats and decreased in high dose ETS group compared with DPN model rats. It might thus be hypothesized that the model rats were in later stage of DPN after the whole experiment. The proinflammatory cytokines IL-6 has been linked to various diabetic complications[24]. In our research mean value of serum IL-6 level was found increased in DPN rats and decreased in three ETS groups significantly. NGF performs a vital neuroprotective role with the ability to potentiate axonal growth. A low serum NGF-β level is correlated with neural damage in type 2 DM. Evidence showed that patients with more severe symptoms had lower NGF levels[25]. Our findings suggested that ETS could significantly decrease TNF-α and increase NGF-β level, which might be important protective mechanisms against DPN.
In summary, alleviation of DPN in rats by early intervention with ETS is a result of the suppression of multiple pathogenic mechanisms, including alleviating hyperglycemia, inhibiting oxidative stress and regulating growth factor, etcetera. Nevertheless, we still do not know the underlying molecular mechanisms that ETS used to regulate these changes. In our further studies, we will separate the active components for investigating the further molecular mechanisms and contribute to adjuvant treatment for DPN.
Acknowledgments
The authors thank Prof. Fengbin Wang of Weifang Medical University for measuring the sciatic nerve conduction velocity and to Dr. Chongmei Xu of the Department of Pharmacognosy of Weifang Medical University for plant authentication.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (81274049), the Scientific and Technological Innovation Foundation of Weifang Medical University (K1302024) and the Traditional Chinese Medicine Science and Technology development projects of Shandong province (2015-231).
Conflicts of interest
There are no conflicts of interest.
References
- Boulton AJ, Vinik AI, Arezzo JC, Arezzo JC, Bril V, Feldman EL,etal. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956-62.
- Wang Y, Chen Z, Ye R, He Y, Li Y, Qiu X. Protective effect of Jiaweibugandecoction against diabetic peripheral neuropathy. Neural Regen Res 2013;8:1113-21.
- El Boghdady NA, Badr GA. Evaluation of oxidative stress markers and vascular risk factors in patients with diabetic peripheral neuropathy. Cell BiochemFunct 2012;30:328-34.
- Kim ES, Lee SW, Mo EY, Moon SD, Han JH. Inverse association between serum total bilirubin levels and diabetic peripheral neuropathy in patients with type 2 diabetes. Endocrine 2015;50:405-12.
- Wu YB, Shi LL, Wu YJ, Xu WH, Wang L, Ren MS. Protective effect of gliclazide on diabetic peripheral neuropathy through Drp-1 mediated-oxidative stress and apoptosis. NeurosciLett 2012;523:45-9.
- Fatani AJ, Al-Rejaie SS, Abuohashish HM, Al-Assaf A, Parmar MY, Ola MS,etal.Neuroprotective effects of Gymnemasylvestreon streptozotocin-induced diabetic neuropathy in rats. ExpTher Med 2015;9:1670-8,.
- Magrinelli F, Briani C, Romano M, Ruggero S, Toffanin E, Triolo G,etal. The association between serum cytokines and damage to large and small nerve fibers in diabetic peripheral neuropathy. J Diabetes Res 2015; 2015.
- Hussain G, Rizvi SA, Singhal S, Zubair M, Ahmad J. Serum levels of TNF-α in peripheral neuropathy patients and its correlation with nerve conduction velocity in type 2 diabetes mellitus. Diabetes MetabSyndr 2013;7:238-42.
- Zheng LR, Zhang YY, Han J, Sun ZW, Zhou SX, Zhao WT etal. Nerve growth factor rescues diabetic mice heart after ischemia/reperfusion injury via up-regulation of the TRPV1 receptor. J Diabetes Complications 2015;29:323-8.
- Wang CC, Tsai YJ, Hsieh YC, Lin RJ, Lin CL. The aqueous extract fromToonasinensisleaves inhibits microglia-mediated neuroinflammation. Kaohsiung J Med Sci 2014;30:73-81.
- Liu H, Tsai Y, Chang S. Toonasinensisleaf extract inhibits lipid accumulation through up-regulation of genes involved in lipolysis and fatty acid oxidation in adipocytes. J Agric. Food Chem 2014;62:5887-96.
- Yu WJ, Chang CC, Kuo TF, Tsai TC, Chang SJ.ToonasinensisRoem leaf extracts improve antioxidant activity in the liver of rats under oxidative stress. Food ChemToxicol 2012;50:1860-5.
- Kakumu A, Ninomiya M, Efdi M, Adfa M, Hayashi M, Tanaka K,etal. Phytochemical analysis and antileukemic activity of polyphenolic constituents of Toonasinensis. Bioorg Med ChemLett 2014;24:4286-90.
- Yang HL, Chen SC, Lin KY, Wang MT, Chen YC, Huang HC,etal. Antioxidant activities of aqueous leaf extracts of Toonasinensison free radical-induced endothelial cell damage. J Ethnopharmacol 2011;37:669-80.
- Fu R, Zhang YT, Guo YR, Huang QL, Peng T, Xu Y,etal. Antioxidant and anti-inflammatory activities of the phenolic extracts of Sapiumsebiferum(L.) Roxb leaves. J Ethnopharmacol 2013;147:517-24.
- Tembhurne SV, Sakarkar DM. Effect of Fluoxetine on an Experimental Model of Diabetes- induced Neuropathic Pain Perception in the Rat. Indian J Pharm Sci 2011;73:621-5.
- Cui XP, Li BY, Gao HQ, Wei N, Wang WL, Lu M. Effects of grape seed proanthocyanidin extracts on peripheral nerves in streptozocin-induced diabetic rats. J NutrSciVitaminol (Tokyo) 2008;54:321-8.
- Stavniichuk R, Drel VR, Shevalye H, Maksimchyk Y, Kuchmerovska TM, Nadler JL,etal. Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative-nitrosative stress and p38 MAPK activation. ExpNeurol 2011;230:106-13.
- Koneri RB, Samaddar S, Simi SM, Rao ST. Neuroprotective effect of a triterpenoidsaponin isolated fromMomordicacymbalariaFenzl in diabetic peripheral neuropathy. Indian J Pharmacol 2014;46:76-81.
- Ding Y, Dai X, Jiang Y, Zhang Z, Li Y. Functional and morphological effects of grape seed proanthocyanidins on peripheral neuropathy in rats with type 2 diabetes mellitus. Phytother Res 2014;28:1082-7.
- Kambiz S, van Neck JW, Cosgun SG, van Velzen MH, Janssen JA, Avazverdi N, etal. An early diagnostic tool for diabetic peripheral neuropathy in rats. PLoS One 2015;10(5):e0126892.
- Cavusoglu T, Karadeniz T, Cagiltay E, Karadeniz M, Yigitturk G, Acikgoz E,etal. The protective effect of losartan on diabetic neuropathy in a diabetic rat model. ExpClinEndocrinol Diabetes. 2015; 123:479-84.
- Saleh A, Smith DR, Balakrishnan S, Dunn L, Martens C, Tweed CW etal. Tumor necrosis factor-α elevatesneurite outgrowth through an NF-κB-dependent pathway in cultured adult sensory neurons: Diminished expression in diabetes may contribute to sensory neuropathy. Brain Res 2011;1423:87-95.
- Dhamodharan U, Viswanathan V, Krishnamoorthy E, Rajaram R, Aravindhan V. Genetic association of IL-6, TNF-α and SDF-1 polymorphisms with serum cytokine levels in diabetic foot ulcer. Gene Jul 2015;565:62-7.
- Kim HC, Cho YJ, Ahn CW, Park KS, Kim JC, Nam JS,etal. Nerve growth factor and expression of its receptors in patients with diabetic neuropathy. Diabet Med 2009;26:1228-34.