- *Corresponding Author:
- P. K. Patel
Department of Pharmacology, Maliba Pharmacy College, UkaTarsadia University, Bardoli-Mahuva Road, Bardoli, Surat-394 350
E-mail: paras.pharm@gmail.com
| Date of Submission | 12 Dec 2014 |
| Date of Revision | 29 Feb 2016 |
| Date of Acceptance | 13 Apr 2016 |
| Indian J Pharm Sci, 2016;78(2):231-239 |
Abstract
The aim of present investigation was to evaluate the effect of Pedalium murex fruit extract in ethylene glycol-induced urolithiasis in male rats. Nephrolithiasis was induced in male Wistar rats by adding ethylene glycol (0.75%) in drinking water for 28 days. Animals divided in six groups each containing six animals. Vehicle control, model control, P. murex methanol extract in different doses 100, 200 and 400 mg/kg p.o. cystone (750 mg/kg, p.o.) used as standard. Hyperoxaluria as well as the increase in the excretion of calcium, phosphate, uric acid and decrease in citrate and magnesium in urine, impairment of renal function and oxidative imbalance in kidney observed in calculi induce group. Treatment with P. murex decreases hyperoxaluria, calcium, uric acid, improves renal function and also produces antioxidant effect. Crystalluria was characterized by excretion of large calcium oxalate crystals in lithogenic group but smaller in drug treated group. The histology showed depositions of large number of calcium oxalate crystals in kidney in calculi induced group while in the treated group small and fewer deposits. The result indicates antiurolithiatic activity of P. murex mediated possibly by calcium oxalate crystal inhibition, diuretic, antioxidant and maintaining balance between stone promoters and inhibitors constituents and this study rationalized its medicinal use in urolithiasis.
Keywords
Pedalium murex, fruit, ethylene glycol, urolithiasis, calcium oxalate
Urolithiasis has been a perplexing problem due to its high incidence and rate of recurrence. Calcium oxalate (CaOX) represents up to 80% of analysed stones [1]. Formation of kidney stone is a complex process that results from a succession of several physicochemical events, including supersaturation, nucleation, growth aggregation, and retention within the renal tubules [2]. Various therapies like thiazide diuretics and alkali citrate are being used in management of urolithiasis but scientific evidence for their efficacy is less convincing [3].
Present day medical management of urolithiasis mainly involves endoscopic removal of stones and techniques such as extracorporeal shock wave lithotripsy (ESWL) has revolutionized the treatment of urolithiasis but do not prevent the likelihood of new stone formation [4]. They cause side effects such as hemorrhage, hypertension, tubular necrosis, subsequent fibrosis of the kidney and also increase in stone recurrence. Moreover, they are very costly [5]. Therefore, it is worthwhile to look for an alternative to these means by using medicinal plants or phytotherapy.
A large number of plants have been used in India since ancient times, which claim the efficient cure of urinary stone [6]. Pedalium murex Linn. Belonging to family of Pedaliaceae commonly known as ‘Bada-gokhru’ found in the sandy coast of Gujarat and in peninsular India [7,8]. As per indigenous system of medicine fruits of Pedalium murex are reported to be useful in the treatment of wide range of an ailment, including urolithiasis [9]. Some of the therapeutic uses of P. murex include diuretic [10], antihypertensive effects [11], and abortifacient [12] activity. Antihyperlipidemic [13], Insecticidal [14] activity has recently been reported. However, so far no scientific study has been reported regarding antiurolithiatic property of fruits of P. murex . Therefore, present study has been planned to establish the scientific validity of the antiurolithiatic activity of Pedalium murex fruit methanol extract (PMF) using ethylene glycol induced urolithiasis.
Materials and Methods
Pedalium murex fruits were collected from the coastal region of Surat, Gujarat during the month of August-September 2014; it was identified and authenticated. A voucher specimen of the plant was deposited in the herbarium (PKP/10102008/02). The fruits were the shade dried and ground to coarse powder. The powder obtained packed in Soxhlet column and extracted with methanol at 70-75º for 24 h. The extraction procedure repeated, pooled extract was evaporated at 45º, under vacuum and stored in the airtight container. The yield of the extract 7.6% w/w was sticky brownish-black. The powder was qualitatively analysed for the presence of various phytochemical constituents [8].
Determination of methanol in extract
Extraction was made using methanol, which is class 2 residual solvent according to United States Pharmacopoeia (USP). Therefore, methanol content was determined according to the USP using gas chromatography (Shimadzu Gas Chromatograph GC-2014 with headspace autosampler HT) and minimum permeable limit of methanol content is <3000 ppm [15].
Experimental animals
Adult albino Wistar rats (male for antiurolithiatic study) and female (for acute oral toxicity study) weighing between 200-250 g housed in standard conditions of temperature (22±2°), relative humidity (55±5%) and light (12 h light-dark cycles) were used. They have been fed with standard pellet diet and water ad libitum. The study protocol was approved by the Institutional Animal Ethics Committee according to the regulation of Committee for the Purpose of Control and Supervision of Experiments on Animals. (Protocol No.: 902 dated: 26/09/2009). The experiment was conducted in accordance with internationally accepted standard guidelines for the use of animals.
Acute toxicity testing
The acute oral toxicity study was carried out in female rats as per the guidelines set by the Organization for Economic Cooperation and Development (OECD) no. 425. One tenth of the median lethal dose (LD50) was taken as an effective dose [16].
Antiurolithiatic activity
Ethylene glycol model was used to induce urolithiasis [17]. Thirty six animals were randomly divided into six groups as Group I, II, III, IV, V and VI containing six animals in each. Group I served as a vehicle treated control and maintained on regular rat food and drinking water ad libitum. All remaining groups received calculi inducing treatment for 28 days, comprised of 0.75% v/v ethylene glycol in drinking water ad libitum. Group II received only ethylene glycol served as model control for 28 days. Group III received cystone (The Himalaya Drug Company, Bangalore, India, 750 mg/kg body weight) (Composition of cystone: Didymocarpus pedicellata, Saxifraga ligulata, Rubia cordifolia, Cyperus scariosus, Achyranthes aspera, Onosma bracteatum, Vernonia cinerea, Shilajeet (Purified), Hajrul yahood bhasma) from 1st day to 28th day of calculi induction. Groups IV, V and VI served as treatment groups, received extract at doses of 100, 200 and 400 mg/kg body weight, p.o. respectively from 1st day to 28th day of calculi induction. Extract and standard drugs were suspended in distilled water and given once daily by oral route using a gastric tube.
All animals were kept in individual metabolic cages and 24 h urine samples were collected on 28th day of calculi induction treatment. Following volume, pH and crystalluria determination urine was acidified with a drop of concentrated HCl and stored at -20° for determination of various parameters. Urine was analyzed for calcium [18], oxalate [19], magnesium [20], phosphate [21], uric acid [22], and citrate [23].
Serum analysis
After the experimental period, blood was collected from retro-orbital under anesthetic conditions and animals were sacrificed by cervical decapitation. Serum was separated by centrifugation at 10000×G for 10 min and analysed for creatinine, uric acid, calcium and blood urea nitrogen (BUN). The creatinine, uric acid, and BUN diagnostic kits (Span Diagnostics Ltd., India) were used and calcium was analysed according to Lorentz, 1982 [18].
Kidney histopathology and homogenate analysis
The abdomen was incised and opened, and both kidneys were removed from each animal. Isolated kidneys were cleaned off extraneous tissue, weighed and rinsed with ice-cold normal saline. The left kidney was fixed with 10% v/v neutral formalin and after harvesting, sliced horizontally and sent to histology services (Samarth Pathology Laboratory, Surat) for Hematoxylene and Eosin staining. Calcium oxalate crystal depositions were counted by light microscope using polarized filter and given a score as follows; <1=0, <10=1, <30=2, <50=3, <75=4 and >75=5. Same histology slides were subjected to microscopic examination for the presence of glomerular congestion, tubular casts, peritubular congestion, epithelial adhesion, blood vessel congestion, interstitial edema and inflammatory cells, and given the scores accordingly.
The right kidney was finely chopped and 20 % homogenate prepared in Tris-HCl buffer (pH 7.4). Total kidney homogenate was used for assaying tissue calcium and oxalate [18,19], malondialdehyde (MDA), superoxide dismutase (SOD), catalase and reduced glutathione (GSH) measured using commercially available kits.
Statistical analysis
The results were presented as mean±SEM. Difference among data was statistically analysed using ANOVA followed by Tukey’s test to find out the level of significance using SigmaPlot software (Systat Software Inc., CA, USA., Version: 10.0.1, Release year: 2007). Differences between the data were considered significant at P<0.05.
Results
Qualitative phytochemical analysis of PMF powder showed the presence of saponin, glycosides, flavanoids, and polyphenols. Methanol content in the extract was found to be 2267.56 ppm, which is less than the permeable limit of USP. From the acute oral toxicity study, the LD50 cut-off dose was found to be 2000 mg/kg body weight for the extract. Hence, therapeutic doses were taken as 100, 200 and 400 mg/kg of body weight for the evaluation.
The urinary output was significantly increased in group II (EG treated) compared to control group I (P<0.05). While in the drug treated groups, urine volume was significantly higher than that of control and EG treated groups. Similarly urinary pH was increased in calculi induce group compared to control. Drug treatments maintain pH near to normal level (Table 1).
| Urinary Parameters | Group I | Group II | Group III | Group IV | Group V | Group VI |
|---|---|---|---|---|---|---|
| Urine volume (ml/24h) | 10.76±0.44 | 15.56±1.36a* | 24.18±0.45b* | 18.51±0.38b | 20.15±0.31b* | 22.6±0.52b* |
| pH | 6.41±0.24 | 7.55±0.23a* | 6.65±0.29b* | 6.63±0.23b* | 6.9±0.17b* | 6.88±0.32b |
| Calcium (mg/24 h) | 4.74±0.23 | 7.91±0.22a* | 4.72±0.18b* | 6.28±0.23b* | 5.82±0.29b* | 5.08±0.18b* |
| Oxalate (mg/24 h) | 3.25±0.11 | 7.84±0.17a* | 3.87±0.25b* | 6.02±0.19b* | 4.85±0.22b* | 4.34±0.26b* |
| Magnesium (mg/24 h) | 2.66±0.15 | 1.36±0.13a* | 2.59±0.13b* | 1.76±0.11b* | 1.86±0.11b* | 2.3±0.16b* |
| Phosphate (mg/24 h) | 4.83±0.15 | 7.39±0.23a* | 5.58±0.22b* | 6.35±0.22b* | 6.02±0.17b* | 5.96±0.11b* |
| Citrate (mg/24 h) | 48.53±2.23 | 30.77±0.73a* | 49.82±1.07b* | 40.35±1.23b* | 43.09±1.33b* | 45.34±1.65b* |
| Uric acid (mg/24 h) | 1.43±0.12 | 3.58±0.15a* | 1.81±0.18b* | 2.14±0.13b* | 1.72±0.16b* | 1.89±0.13b* |
Values are expressed as mean ± SEM, 6 animals in each group. Comparisons are made: awith Group I, bwith Group II. Symbols represent statistical significance: *P<0.05.
Table 1: Effect Of Extract Of Pedalium Murex On Urinary Parameters In Urolithiasis Induced Rats
In the present study renal stone induced by EG administration in rats lead to development of Hyperoxaluria. There was a significant increase in calcium, oxalate, uric acid and phosphate (P<0.05) in group II compared to group I. Treatment with PMF significantly prevented these changes in the urinary calcium, oxalate, uric acid and phosphate excretion dose dependently in the group IV-VI (Table 1).
On another hand magnesium and citrate were significantly decrease in calculi induce group compared to vehicle control group (P<0.05). Supplementation with PMF significantly prevented these changes (Table 1). Number of CaOX crystals in urine was more with larger in size significantly presence in group II compared to group I. Treatment with PMF significantly decreased the number as well as size of the crystals in the group IV-VI in the dose dependent manner (fig. 1).
The markers of renal functions creatinine, uric acid, urea and BUN in serum were found to be significantly increased in calculi induce group compared to vehicle control group (P<0.05). Supplementation of the PMF improves the renal function thereby decreasing their level in the group IV-VI dose dependently (Table 2).
| Serum Parameters | Group I | Group II | Group III | Group IV | Group V | Group VI |
|---|---|---|---|---|---|---|
| Creatinine(mg/dl) | 0.72±0.03 | 2.27±0.20a* | 0.735±0.06b* | 1.32±0.12b* | 0.89±0.08b* | 0.80±0.04b* |
| Uric acid (mg/dl) | 3.14±0.18 | 5.05±0.33a* | 3.37±0.25b* | 4.30±0.16b | 3.78±0.29b* | 3.79±0.24b* |
| Urea (mg/dl) | 16.22±0.71 | 27.02±1.40a* | 17.50±1.39b* | 19.68±1.24b* | 18.35±1.44b* | 17.88±1.47b* |
| BUN (mg/dl) | 35.22±3.50 | 62.87±3.19a* | 39.05±2.17b* | 53.00±2.28b* | 48.85±2.52b* | 46.25±1.57b* |
Values are expressed as mean±SEM, 6 animals in each group. Comparisons are made: awith Group I, bwith Group II. Symbols represent statistical significance: *P<0.05.
Table 2: Effect Of Extract Of Pedalium Murex On Serum Parameters In Urolithiasis Induced Rats
The weight of the kidney was significantly (P<0.05) increased in the lithogenic group (II) compared to vehicle control. Treatment with PMF significantly decreased the kidney weight in a dose dependent manner compared to calculi induced group (Table 3).
| Serum Parameters | Group I | Group II | Group III | Group IV | Group V | Group VI |
|---|---|---|---|---|---|---|
| Kidney weight (gm) | 1.39±0.15 | 2.31±0.13a* | 1.38±0.11b* | 1.85±0.13b* | 1.80±0.10b* | 1.61±0.15b* |
| Calcium (mg/g of kidney) | 3.14±0.14 | 5.72±0.18a* | 3.63±0.23b* | 4.48±0.19b* | 4.25±0.22b* | 4.05±0.26b* |
| Oxalate (mg/g of kidney) | 1.03±0.11 | 3.85±0.18a* | 1.96±0.19b* | 2.77±0.23b* | 2.09±0.20b* | 1.99±0.19b* |
| MDA (nmol/mg of protein) | 0.58±0.05 | 4.61±0.14a* | 1.45±0.17b* | 2.93±0.22b* | 2.37±0.18b* | 1.85±0.20b* |
| SOD (U/min/mg of protein) | 6.05±0.24 | 2.70±0.13a* | 5.72±0.12b* | 3.35±0.31b | 4.62±0.18b* | 4.78±0.21b* |
| Catalase (µM/H2O2/min) | 46.41±2.17 | 20.83±1.40a* | 40.69±2.19b* | 26.48±1.28b* | 32.98±1.25b* | 35.93±2.08b* |
| GSH (µM/H2O2/min) | 9.58±0.57 | 5.59±0.17a* | 7.38±0.36b* | 6.07±0.20b | 6.70±0.18b* | 6.99±0.22b* |
MDA: Malondialdehyde, SOD: superoxide dismutase, GSH: reduced glutathione. Values are expressed as mean ± SEM, 6 animals in each group. Comparisons are made: awith Group I, bwith Group II. Symbols represent statistical significance: *P<0.05.
Table 3: Effect Of Extract Of Pedalium Murex On Kidney Parameters In Urolithiasis Induced Rats
Stone forming treatment significantly (P<0.05) increased level of MDA and decreased the activity of SOD, catalase, and GSH level compared to vehicle control. Treatment of PMF improved all of above an oxidative markers level in the dose dependently groups IV-VI (Table 3).
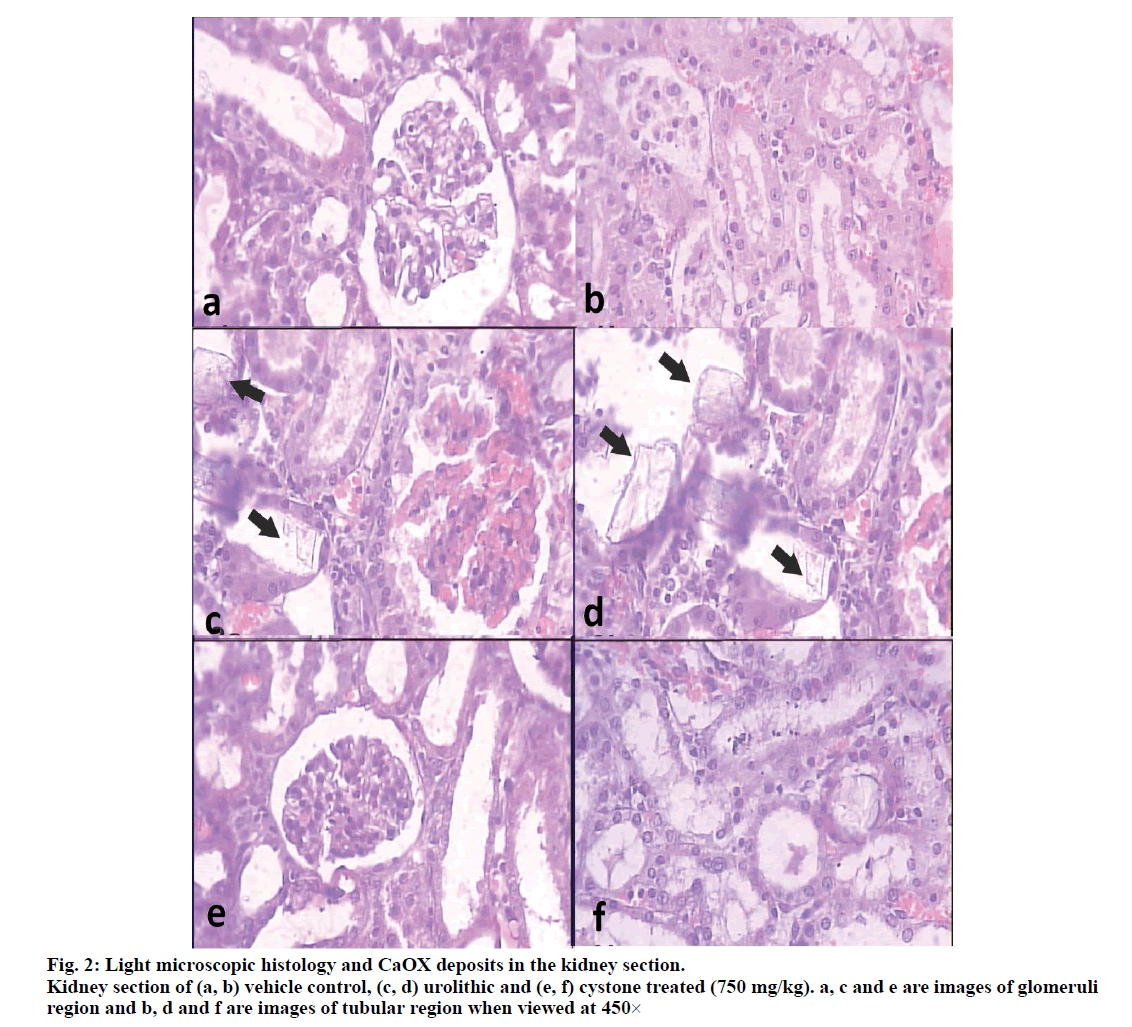
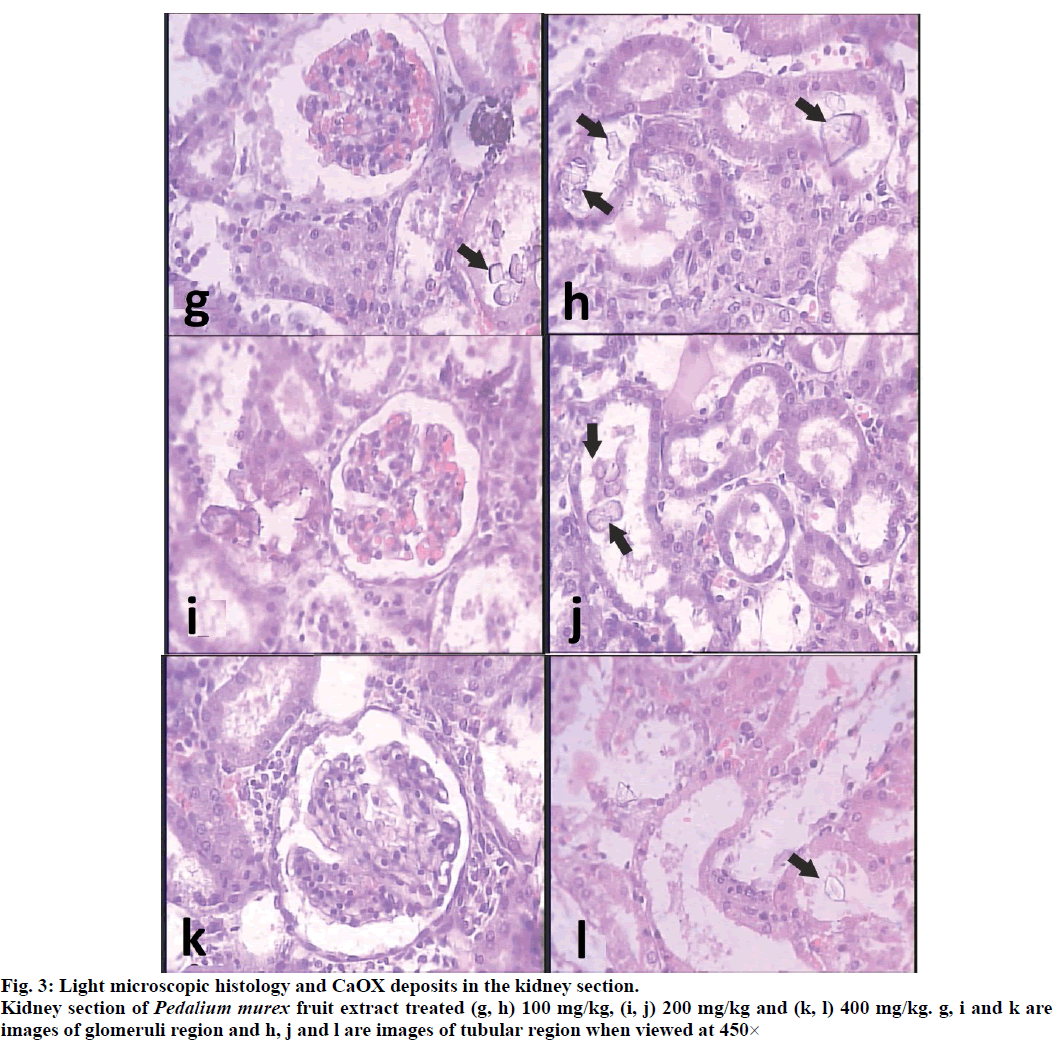
Histopathological examination revealed a normal glomeruli and tubular region with the absence of CaOX crystals (figs. 2a and 2b) while in stone forming group II severe glomeruli damage, RBCs which leads to hematuria, more numbers and large size CaOX crystals deposition in renal tubule and dilation of the proximal tubules with interstitial inflammation ware observed in the renal tissue of urolithiasis rat (figs. 2c and 2d). Treatment with PMF gradually decrease glomeruli damage, less RBCs accumulation, less no. and small size CaOX crystals deposition with fragmentation in the dose dependent manner in the group IV-VI Comparison to calculi induced group (fig. 3).
When observed under polarized light microscope, many birefringent crystalline deposits (score=05) in the histological preparations were seen in tubules of all regions of kidneys of all the animals in the untreated group compared to normal control group (score=00). In PMF treated groups, such deposits were found in rats receiving 100 mg/kg (Score=05), 200 mg/kg (Score=04) and 400 mg/kg (Score=02) while cystone treated group showed few crystal deposits (score=01) compared to model control animals. Deposits were also visibly small and less abundant compared to those in the untreated kidneys (fig. 4).
Discussion
In the present study, male rats were selected for induction of urolithiasis because the urinary system of male rats resembles that of humans and earlier studies shown that the amount of stone depositions was significantly less [24]. Moreover, estrogen inhibits the formation of calcium oxalate crystals in female rat [25].
Urinary supersaturation with respect to stone-forming constituents is generally considered to be one of the causative factors in calculogenesis. Previous studies indicated that, 28-days administrations of ethylene glycol to the male albino rats resulted into the formation of renal calculi composed mainly of calcium oxalate. The biochemical mechanism for this process is related to an increase in the urinary concentration of oxalate. Stone formation in ethylene glycol fed is caused by hyperoxaluria, which causes increased renal retention and excretion of oxalate [26]. Renal calcium oxalate deposition by ethylene glycol 0.75% v/v for 28 days in rats is frequently used to mimic the urinary stone formation in humans [17,27].
Urinary volume is markedly increased in experimental groups. Treatment with PMF causes diuresis by increasing urinary volume which deceases supersaturation process which one of the favourable requisition factor for stone formation by crystallization. Similar results are also observed when lupeol is used as an antilithic agent [28]. Acidic urine is usually found in humans with idiopathic renal calcium oxalate stone formation, whereas chronic hyperoxaluric rats had alkaline urine [29]. The mechanism of alkaline urine production after ethylene glycol treatment and its possible correlation with nephrolithiasis in this rat model remains unclear and needs further study.
The analysis of crystalluria showed that rats of lithogenic group (II) excreted more and large size CaOX crystals than drug treated groups. This may be important as large particles have a greater chance of being trapped within the renal tubules, whereas small particles can be flushed easily from the kidney. Crystalluria could occur similarly in both healthy and stone-forming subjects, but the latter might tend to excrete large and aggregated particles [30]. Treatment with PMF gradually decreased in the number as well as smaller in size crystals in the group IV-VI.
In the present study, calcium and oxalate excretion are progressively increased in calculi induced animals (group II). It has been reported that oxalate plays an important role in stone formation and have about 15- fold greater effect than urinary calcium. The changes in the urinary oxalate level are relatively much more important than those of calcium [24]. Elevated urinary calcium is a favouring factor for nucleation of calcium oxalate from urine and further crystal growth [31]. Though P. murex fruit extract lower the levels of calcium and oxalate dose dependently.
Urinary phosphate is increased in calculi induced group (II). Increased urinary phosphate excretion along with oxalate stress seems to provide an environment appropriate for stone formation by forming calcium phosphate crystals, which epitaxial induces calcium oxalate deposition [32]. Administration of P. murex fruit extract reinstates the level of phosphate and reduced risk of stone formation.
A declined level of urinary citrate was observed in ethylene glycol induced urolithic rats. Hypocitraturia is the main metabolic abnormality in patients with renal stones. Investigations of citrate metabolism in stone formers have shown that tubular citrate reabsorption is the main mechanism regulating urinary citrate excretion [24]. The PMF treatment brought the urinary citrate excretion near to normal in Groups IV-VI.
Normal urine contains many inorganic and organic inhibitors of crystallization. Magnesium is one such well-known inhibitor. Low levels of magnesium are also encountered in stone formers as well as in stoneforming rats. The magnesium levels return to normal on drug treatment [17]. Promising results in preventing recurrence have been shown in patients treated with potassium magnesium citrate. Magnesium can reduce the supersaturation of calcium oxalate by reducing the saturation of calcium oxalate. Magnesium has been found to decrease the growth and nucleation rates of calcium oxalate crystals [33]. Urinary magnesium was significantly diminished in ethylene glycol induced urolithic rats. The PMF treatment restored the magnesium excretion and thus reduces the growth of calcium oxalate crystals in Groups IV–VI.
The increase in uric acid excretion is observed in calculi induce group (II). Increased excretion of uric acid has been reported in stone formers and hyperoxaluric rats. Uric acid interferes with CaOX solubility, and it binds and reduces the inhibitory activity of glycosaminoglycans [17]. The predominance of uric acid crystals in CaOX stones and the observation that uric acid binding proteins is capable of binding to CaOX and modulate its crystallization also suggests its primary role in stone formation [34]. Drug treatment reduces uric acid levels near to normal thus reducing the risk of stone formation.
In urolithiasis, the glomerular filtration rate decreases due to the obstruction to the flow of urine by stones in the urinary system. Due to this, the waste products, particularly nitrogenous substances such as urea, creatinine, BUN and uric acid get accumulated in blood [24]. In calculi-induced rats, marked renal damage was seen as indicated by the elevated serum levels of creatinine, urea, BUN and uric acid, which are markers of glomerular and tubular damage. Treatment of PMF showed to prevent the elevation of serum levels of these markers by improving glomerular filtration as it causes diuresis.
The development of tissue injury probably depends on the balance between the generation of reactive oxygen species (ROS) and the tissue antioxidant defence mechanism. Decreased tissue antioxidant enzymes may follow elevated free radical production in the early stage in the later stages of nephrolithiasis, which may put the renal tissue under oxidative stress. This hypothesis is strengthened by the report that patients with kidney stones have less activity of antioxidant enzyme with increased lipid peroxidation [35]. Oxidative damage as reflected from increased level of marker of oxidative injury by higher MDA and decreased antioxidant enzymes activity like SOD, catalase and GSH level in the kidney as well as deteriorate since kidney functions as observed in calculi induced rats. Treatment with PMF decrease MDA level and increase the activity of antioxidant enzymes and level of GSH indicate that it protected against oxidative stress induce tissue damage.
Microscopic examination of kidney sections derived from ethylene glycol induced urolithic rats showed polymorphic irregular crystal deposits inside the tubules, which cause dilation of the proximal tubules along with interstitial inflammation and severe glomeruli damage with hematuria indicated by RBCs deposition that might be attributed to oxalate. Co-treatment with the PMF decreased the number and size of calcium oxalate deposits in different parts of the renal tubules and also prevented damages to the tubules.
The result showed the nephroprotective effect of fruit of P. murex Linn. in ethylene glycol induced urolithiasis model. It is suggested that the effect could be by maintaining balance between stone promoters and inhibitors, reducing deposition and excretion of small particles of CaOX from the kidney, maintaining the antioxidant environment and reducing the chance of them being retained in the urinary tract. A number of herbal extracts and their isolated constituents have also shown a protective effect against stone renal formation [30].
The result showed the nephro protective effect of fruit of P. murex in the ethylene glycol induced urolithiasis model. It is suggested that the effect could be by maintaining balance between stone promoters and inhibitors, reducing deposition and excretion of small particles of CaOX from the kidney, maintaining the antioxidant environment and reducing the chance of them being retained in the urinary tract. A number of herbal extracts and their isolated constituents have also shown a protective effect against renal stone formation [30,40].
Saponin, a flavonoid is the major phytoconsituent present in PMF [36-38]. Saponin derivatives appears as a component of the great number of medicinal herbs with claimed antiurolithiasis properties [39]. Flavonoid present in the fenugreek extract responsible for its antiurolithiatic activity [41].
The result indicates that administration of methanolic extract of P. murex fruit to rat with ethylene glycol induced lithiasis, reduced and prevented growth of urinary stones, diuresis, antioxidant activity and maintaining balance between stone promoters and inhibitors constituents. This supports the information regarding antiurolithiatic activity of plant.
Acknowledgements
The authors are thankful to Dr. S. A. Shah, Principal, Maliba Pharmacy College, Uka Tarsadia University, India for providing the necessary facilities to carry out the research work and also thankful to Dr. Samir Patel, MD (Patho), Samarth Pathology Laboratory, Surat, India for providing a facility of biochemical and histopathological analysis. Authors would like to thank Prof. Minoo Parabia, Head of the Department of Bioscience, Veer Narmad South Gujarat University, Surat, for authentication of plant material.
Financial Assistance
None.
Conflict of Interests
None declared.
References
- Daudon M, Bader CA, Jungers P. Urinary calculi: review of classification methods and correlations with etiology. Scanning Microsc 1993;7:1082-106.
- Laroubi A, Touhami M, Farouk L, Zrara IRA, Benharref A, Chait A. Prophylaxis effect of Trigonella foenum graecum L. seeds on renal stone formation in rats. Phytother Res 2007;21:921-5.
- Hess B. Pathophysiology, diagnosis and conservative therapy in clinical calculi. Revue Therapeutique 2003;60:79-87.
- Bashir S, Gilani AH. Antiurolithic effect of Begenia ligulata rahizomes: an explanation of underlying mechanisms. J Ethnopharmacol 2009;122:106-16.
- Begun FP, Knoll CE, Gottlieb M, Lawson RK. Chronic effects of focused electrohydraulic shock-waves on renal function and hypertention. J Urol 1991;145:635-39.
- Mukharjee T, Bhalla N, Aulakh GS and Jain HC. Herbal drugs for urinary stones – literature appraisal. Indian Drugs 1984;21:224-8.
- Anonymous. The Wealth of India, Raw Materials. Vol. 7. New Delhi: Publications and Information Directorate, CSIR; 1998, p. 284.
- Anonymous. Quality standard of Indian medicinal plants. Vol. 4. New Delhi: Indian Council of Medical Research; 2006, p. 178-83.
- Chunekar KC, Pandey GS. Bhāvprakāsa Nighantu (Indian Materia Medica) of Śrī Bhāvamiśra. Varanasi: Chaukhambha Bharati Academy; 1999, p. 293-4.
- Haravey SK. A preliminary experimental study of diuretic activity of some indigenous drugs. Indian J Med Res 1966;54:774-8.
- Agrawal SS, Bhardwaj S, Gupta L. Some pharmacological studies on Pedalium murex Linn. J Res Indian Med Yoga Homoeop 1976;11:107-8.
- Aswal BS, Goel AK, Kulshreshtha Dk, Mehrotra BN, Patnaik GK. Screening of Indian plants for biological activity. Part XV. Indian J Exp Biol 1996;34:444-67.
- Balasubramanium MN, Murlidharan P, Balamurugan G. Antihyperlipidemic activity of Pedalium murex (Linn.) fruits on high fat diet fad rat. Int J Pharmacol 2008;4:310-3.
- Sahayaraj K, Venkateshwari M, Balasubramanium R. Insecticidal and antifeedant effect of Pedalium murex Linn. Root and on Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). J Agri Technol 2008;4:73-80.
- Anonymous. Chapter 467: Residual solvents. United States Pharmacopoeia 30. Rockville, MD: U.S. Pharmacopeial Convention; 2007.
- Handa SS, Anupama S. Hepatoprotective activity of andrographolide from Andrographis paniculate against CCl4. Indian J Med Res 1990;92:276.
- Shukkur MF, Abdul SE, Devarajan A, Ramasamy S, Sethumadhvan S, Nachiappa GR, et al. Credential of Spiruina diet on stability and flux related properties on the biomineralization process during oxalate mediated renal calcification in rats. Clin Nutr 2005;24:932-42.
- Lorentz K. Improve determination of calcium with ortho-cresolphthalein complexone. Clin Chim Acta 1982;126:327-33.
- Hodgkinson A. Determination of oxalic acid in biological material. Clin Chem 1970;16:547-57.
- Heaton FW. Determination of magnesium by the titan yellow and ammonium phosphate method. J Clin Pathol 1960;13:358-60.
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem 1925;66:375-81.
- Verley H. Practical Clinical Biochemistry. New Delhi: CBS Publishers; 2003, p. 356-61.
- Rajagopal G. A simple colorimetric procedure for estimation of citric acid in urine. Indian J Exp Biol 1984;22:391-2.
- Karadi RV, Palkar MB, Gaviraj EN, Gadge NB, Mannur VS, Alagawadi KR. Antiurolithiatic property of Moringa oleifera root bark. Pharm Biol 2008;46:861-5.
- Lee YH, Huang WC, Huang JK, Chang LS. Testosterone enhances whereas estrogen inhibits calcium oxalate stone formation in ethylene glycol treated rats. J Urol 1996;156:502-5.
- Divakar K, Pawar AT, Chandrashekhar SB, Dighe SB, Divakar G. Protective effect of the hydroalcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rat. Food Chem Toxicol 2010;48:1013-8.
- Karadi RV, Gadge N, Alagawadi KR, Savadi RV. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 2006;105:306-11.
- Anand R, Patanaik GK, Roy K, Bhaduri AP. Antioxaluric and anticalciuric activity of lupeol derivatives. Indian J Pharmacol 1995;27:265-9.
- Huang HS, Chen J, Chen CF. Circulating adhesion molecules and neutral endopeptidase enzymuria in patients with urolithiasis and hydronephrosis. Urology 2000;55:961.
- Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsute on experimentally induced nephrolithiasis in rats. Brit J Urol Int 2003;92:137-40.
- Lemann J, Jr, Worcestor EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis 1991;27:386-91
- Roger K, Low MD, Stoller ML. Uric acid nephrolithiasis. Urol Clinics North Am 1997;24:135-48.
- Grases F, Genestar C, Conte A, March P, Costa-Bauza A. Inhibitory effect of pyrophosphate, citrate, magnesium and chondroitin sulfate in calcium oxalate urolithiasis. Brit J Urol 1989;64:235-7.
- Kalaiselvi P, Udayapriya KL, Selvam R. Uric acid binding proteins in calcium oxalate crystallization. Brit J Urol 1999;83:919-23.
- Huang HS, Ma MC, Chen J, Chen CF. Changes in the oxidant–antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Urol 2002;167,2584-93.
- Das VSR, Rao KN, Rao JVS. Phenolic acid in some members of pedaliaceae. Curr sci 1966; 35:160.
- Zafar R, Gupta M. Flavone from the stem and fruits of Pedalium murex. Indian Drugs 1989;27:202.
- Bhakuni RS, Shukla YN, Thakur R. Flavonoids and other constituents from Pedalium murex. Phytochemistry 1992;31:2917-8.
- Lakhsminarasimhan V, Mahimainathan L, Palaninathan V. Evaluation of the effect of triterpenes on urinary risk factors of stone formation in pyridoxine deficient hyperoxiluric rats. Phytother Res 2002;16:514-8.
- Amine L, Mohammed T, Loubna F, Ibtissam Z, Rachida A, Ahmmed B, et al. Prophylaxis effect of Trigonella foenum graecum seeds on renal stone formation in rats. Phytother Res 2007;21:921-5.
- Selvam R, Kalaiselvi P, Govindaraj A, Murugan VB, Kumar ASS. Effect of A. lanatai leaf extract and Vediuppu Chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental Hyperoxaluria. Pharmacol Res 2001;43:89-93.