- *Corresponding Author:
-
S. Nagata
Laboratory of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Hiroshima International University, 5-1-1 Hiro-koshingai, Kure, Hiroshima 7370112,Japan
E-mail: s-nagata@ps.hirokoku-u.ac.jp
| Date of Submission | 24 July 2013 |
| Date of Revision | 03 December 2013 |
| Date of Acceptance | 10 December 2013 |
| Indian J Pharm Sci,2014; 76(1):54-61 |
Abstract
In order to create Fe2O3 and Fe2O3·H2O nanoparticles, various polymers were used as dispersing agents, and the resulting effects on the dispersibility and nanoparticulation of the iron oxides were evaluated. It was revealed that not only the solution viscosity but also the molecular length of the polymers and the surface tension of the particles affected the dispersibility of Fe 2 O 3 and Fe 2 O 3·H 2 O particles. Using the dispersing agents 7.5% hydroxypropylcellulose-SSL, 6.0% Pharmacoat 603, 5.0% and 6.5% Pharmacoat 904 and 7.0% Metolose SM-4, Fe2O3 nanoparticles were successfully fabricated by wet milling using Ultra Apex Mill. Fe2O3·H2O nanoparticles could also be produced using 5.0% hydroxypropylcellulose-SSL and 4.0 and 7.0% Pharmacoat 904. The index for dispersibility developed in this study appears to be an effective indicator of success in fabricating nanoparticles of iron oxides by wet milling using Ultra Apex Mill.
Keywords
Dispersibility, iron oxide, nanoparticle, polymer, wet-milling
The colouring agents used as pharmaceutical excipients are classified into inorganic and organic substances, and organic colouring agents are further divided into natural and synthetic substances. Because organic colouring agents are soluble in water and/or organic solvents, the colour palette of the resulting tablets or capsules is clear and the printed marks are clean-cut. However, the natural colouring agents tend to have poor stability, and although coal-tar colours are typically used as synthetic colouring agents, the authorised use of tar colours is limited to only certain countries. Among inorganic substances, titanium dioxide and iron oxide are generally used as colouring agents. Although the use of inorganic colouring agents is generally allowed worldwide, the colour palette of the resulting tablets or capsules and the printed marks are not clear because the inorganic colouring agents are not soluble in water and/or organic solvents.
Iron oxides are very important materials in various industries and are widely used as inorganic dyes, food additives and pigments for cosmetics [1,2]. There are various kinds of iron oxides such as Fe2O3, Fe2O3·H2O, FeO and Fe3O4, and these iron oxides are synthesised by various methods [3-5]. Fe2O3 and Fe2O3·H2O are used as red and yellow pigments [6], respectively, and Fe2O3 has good properties owing to its nontoxicity, chemical stability and low cost [2]. The use of iron oxides as colouring agents for medical inks is considered one of the most effective ways to avoid the problems derived from the use of tar colours and organic solvents. However, it is difficult to uniformly disperse iron oxide particles in a water solvent because of the aggregation and sedimentation of the particles; the clogging of the particles at the nozzle of an ink-jet printer also creates problems with the use of iron oxide as ink.
According to the Stokes equation, the sedimentation rate of particles decreases in proportion to the decrease in the square of particle sizes and the increase in the viscosity of the solvent [7]. In other words, the dispersibility of iron oxide particles in water can be improved by adding polymers to a solvent (thus increasing the viscosity) and milling the particles (thus decreasing the size). Clogging problems that exist with ink-jet printers could also be solved by decreasing particle size.
To enhance the capabilities of particles, many kinds of nanoparticulation techniques for various materials have been reported [2,8-10]. However, nanoparticles tend to aggregate owing to their high surface energy, thereby inducing phase separation [11]. Creating a stable nanosuspension of iron oxides is considered to be quite effective in order to utilise iron oxides for medical inks. Moreover, it enables smoother characters and logos to be printed on tablets or capsules without roughness. The usual approach for minimising particle aggregation is by adding dispersing agents such as polymers and surfactants. Various polymers are used for the stabilisation of particles, such as methylcellulose (MC), hydroxypropylcellulose (HPC) and hypermellose (HPMC). These polymers provide sufficient solution viscosity or steric barriers to inhibit contact between the coated particles [12]. However, the inhibitory effects of particle aggregation are considerably different among each substance and polymer.
In this study, in order to create a stable medical ink from iron oxides, we investigated the effects of various polymers on the dispersibility and nanoparticulation of Fe2O3 and Fe2O3·H2O using Ultra Apex Mill.
Materials and Methods
Fe2O3 and Fe2O3·H2O were provided by Kishi Kasei Ltd. Yokohama, Japan. Nisso HPC-SSL (MW: 40 000), -SL (MW: 100 000), -L (MW: 140 000), and -H (MW: 910 000) were purchased from Nippon Soda Co., Ltd., Tokyo, Japan. HPMCs such as TC-5E (Pharmacoat 603), TC-5M (Pharmacoat 645), TC-5R (Pharmacoat 606), 60SH-4000, 60SH-10000 and SB4 (Pharmacoat 904) and MCs such as Metolose SM-4 and SM-4000 were purchased from Shin-Etsu Chemical Co., Ltd., Tokyo, Japan. Pluronic® F-68 and F-108 were purchased from Adeca Alcohol Delivery Company, Japan. Plasdone® S-630 was purchased from Wako Pure Chemical Industries Ltd., Japan. All other reagents were analytical-grade commercial products.
Measurement of viscosity
Various polymers were dissolved in distilled water at various concentrations (w/w%). The viscosities of these solutions were measured using a viscometer with an S61 spindle (LVDV-I Prime, Brookfield Engineering Laboratories, Inc., Middleboro, Massachusetts, USA) at 60 rpm (11/s) and 20°.
Evaluation of dispersibility of Fe2O3 and Fe2O3·H2O particles
Fe2O3 and Fe2O3·H2O were suspended at 10 w/w% into the various polymer solutions by a homogeniser (3000 rpm (50/s), 30-45 s; HM-300, Hsiangtai Machinery Industry Co., Ltd., New Taipei, Taiwan). The suspensions were then centrifuged for 5 min using a centrifuge at 5590×g of relative centrifugal force (RCF). Each supernatant was diluted to onetenth with distilled water, and each transmittance (%) was determined at 500 nm by a spectrophotometer (UVmin-1240, Shimadzu Corporation, Kyoto, Japan). The dispersibility of Fe2O3 and Fe2O3·H2O particles was evaluated in terms of the index for dispersibility (IFD), as defined in the following equation: IFD=100 − transmittance. The RCF was calculated by the following equation: RCF (× g)=1118×R×N2×10-8, where R is the radius of the centrifuge (cm) and N is the rotational speed (rpm).
Measurement of penetration length of various polymer solutions into Fe2O3 and Fe2O3·H2O particles in glass tube
Fe2O3 (2.2 g) and Fe2O3·H2O (0.5 g) particles were placed in a glass tube (inner diameter: 6 mm), the other end of which was sealed with a paper filter. The densities of the particles were adjusted to 0.52 and 0.59 g/cm3, respectively, by tapping. Then, 1 and 0.3 ml of the various polymer solutions was added through the top of the glass tube filled with Fe2O3 and Fe2O3·H2O particles, respectively. After 3 h for Fe2O3 and 1 h for Fe2O3·H2O, the penetration length of various polymer solutions into Fe2O3 and Fe2O3·H2O was measured as the index for surface tension.
Scanning electron microscopy analysis
A scanning electron microscope (SEM, S-4100, Hitachi Ltd., Tokyo, Japan) operating at 5 keV was used to determine the shape of the iron oxides particles. Prior to SEM analyses, the iron oxides particles were dispersed onto a carbon-tape-coated aluminium stub and then coated with gold.
Nanoparticulation of iron oxide particles using the Ultra Apex Mill
Fe2O3 and Fe2O3·H2O particles were suspended at about 15 w/w% into the various polymer solutions, and wet milling was conducted using Ultra Apex Mill, which is a novel wet-mill instrument using Centri separator instead of a screen type to isolate a wetmilled substrate from beads [13-15]. The procedure has been reported in detail previously [13-15]. The milling chamber of the Ultra Apex Mill was filled with 500 g of fine zirconia beads (diameter: 0.05 mm). The beads were stirred by a rotor pin (rotation speed: 10.0 m/s); the iron oxide suspensions with various polymers were then poured into a slurry tank. The iron oxide particles were milled in the milling chamber by impact with the zirconia beads. The milled iron oxides were separated from the zirconia beads by a Centri separator and the separated milled suspension was collected. The particle sizes of the iron oxides in the various milled suspensions were immediately measured.
Measurement of particle size
The particle size distribution in the various iron oxide suspensions was measured using a laser scattering analyser (LSA, LA-950, Horiba, Japan), and the mean particle size was calculated.
Results
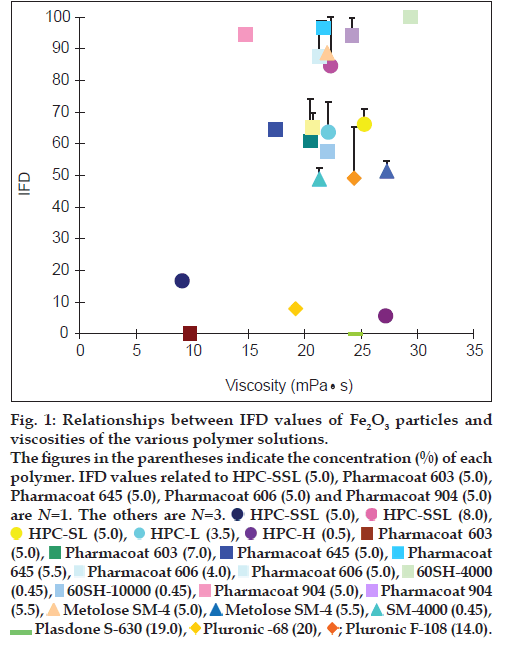
IFD values of Fe2O3 particles and viscosities of the various polymer solutions
The IFD values of the Fe2O3 particles were plotted against the viscosities of the various polymer solutions (fig. 1). The value associated with 5.0% Pharmacoat 606 was the highest (100) and the IFD value related to 19% Plasdone S-630 was the lowest (0.00) among the various IFD values. According to the Stokes equation, the IFD values of Fe2O3 particles in the polymer solutions with higher viscosity are considered to be high as compared with those with lower viscosity. Although the solutions with viscosities greater than 20 MPas seemed to provide better IFD values (greater than 50), good correlation was not obtained in this range (R2=0.0002). For example, the IFD values associated with 0.5% HPC-H and 20% Pluronic F-68 were much lower, regardless of the fact that their viscosities were greater than 20 MPas.
Figure 1: Relationships between IFD values of Fe2O3 particles and
viscosities of the various polymer solutions.
The figures in the parentheses indicate the concentration (%) of each
polymer. IFD values related to HPC-SSL (5.0), Pharmacoat 603 (5.0),
Pharmacoat 645 (5.0), Pharmacoat 606 (5.0) and Pharmacoat 904 (5.0)
are N=1. The others are N=3. HPC-SSL (5.0),
HPC-SSL (5.0), HPC-SSL (8.0),
HPC-SSL (8.0), HPC-SL (5.0),
HPC-SL (5.0), HPC-L (3.5),
HPC-L (3.5), HPC-H (0.5),
HPC-H (0.5),  Pharmacoat 603
(5.0),
Pharmacoat 603
(5.0), Pharmacoat 603 (7.0),
Pharmacoat 603 (7.0), Pharmacoat 645 (5.0),
Pharmacoat 645 (5.0), Pharmacoat
645 (5.5), Pharmacoat 606 (4.0),
Pharmacoat
645 (5.5), Pharmacoat 606 (4.0), Pharmacoat 606 (5.0),
Pharmacoat 606 (5.0), 60SH-4000
(0.45),
60SH-4000
(0.45), 60SH-10000 (0.45),
60SH-10000 (0.45),  Pharmacoat 904 (5.0),
Pharmacoat 904 (5.0), Pharmacoat 904
(5.5),
Pharmacoat 904
(5.5),  Metolose SM-4 (5.0),
Metolose SM-4 (5.0),  Metolose SM-4 (5.5),
Metolose SM-4 (5.5), SM-4000 (0.45),
SM-4000 (0.45),
 Plasdone S-630 (19.0),
Plasdone S-630 (19.0),  Pluronic -68 (20),
Pluronic -68 (20), Pluronic F-108 (14.0).
Pluronic F-108 (14.0).
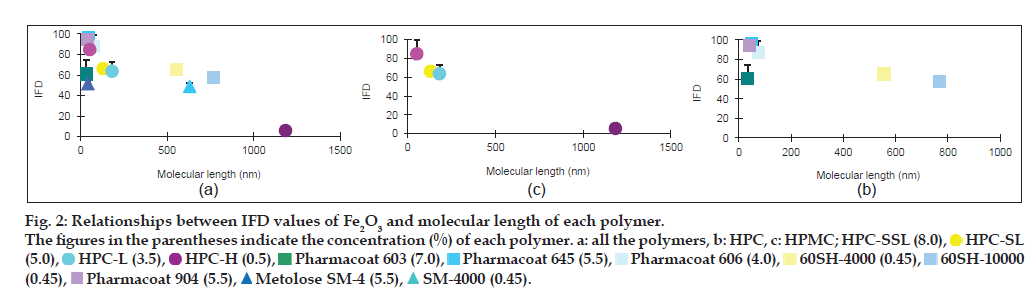
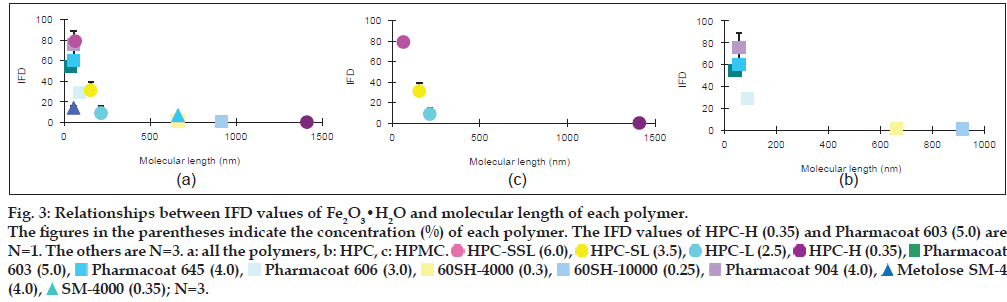
Relationships between IFD values of the iron oxides and molecular length of each polymer
In order to evaluate the effect of the molecular length of each polymer on the IFD values, the molecular lengths of HPC, HPMC and MC were calculated from each mean degree of polymerisation (DPw), using data provided by Nippon Soda Co., Ltd., and Shin-Etsu Chemical Co., Ltd., and the monomer molecular size of glucose by assuming that these polymers are straight chains (Table 1). The minimum energy of glucose was calculated by the molecular mechanics (MM2) program in Chem 3D® Pro ver. 8.0, and the glucose size from oxygen of 1C to 4C was estimated to be 0.42 nm. The relationships between the IFD values and the lengths are shown in figs. 2 and 3. The IFD values of Fe2O3 and Fe2O3·H2O evaluated from each polymer solution with almost the same viscosity (20.5-27.3 and 10.5-15.4 MPas for Fe2O3 and Fe2O3·H2O, respectively) were used to avoid the influence of viscosity on the relationships. In Fe2O3 (fig. 2a-c), the IFD in solution of HPC-SSL, Pharmacoat 645, Pharmacoat 606 and Pharmacoat 904, all of which have shorter lengths, showed high values ranging from 84.6 to 96.5. As the lengths increased, the IFD values tended to decrease, and the IFD value associated with 0.5% HPC-H with the longest length had the lowest IFD value (5.6). In case of Fe2O3·H2O (fig. 3a-c), the IFD values also decreased as the polymer lengths increased. The IFD values in solution of HPC-SSL and Pharmacoat 904 showed relatively high values of 78.9 and 75.5, respectively, whereas those of HPC-H, 60SH-4000, and 60SH-10000 were less than 1.
| Polymers | Mean degree of polymerisation (DPw) | Molecular length (nm) |
|---|---|---|
| HPC‑SSL | 126 | 52.9 |
| HPC‑SL | 310 | 130.2 |
| HPC‑L | 433 | 181.9 |
| HPC‑H | 2821 | 1184.8 |
| Pharmacoat 603 | 79 | 33.2 |
| Pharmacoat 645 | 112 | 47.0 |
| Pharmacoat 606 | 175 | 73.5 |
| 60SH‑4000 | 1323 | 555.7 |
| 60SH‑10000 | 1828 | 767.8 |
| Pharmacoat 904 | 100 | 42.0 |
| Metolose SM‑4 | 100 | 42.0 |
| SM‑4000 | 1500 | 630.0 |
Each polymer was assumed straight chain
Table 1: Mean Degree Of Polymerisation And Molecular Length Of The Various Polymers
Figure 2: Relationships between IFD values of Fe2O3 and molecular length of each polymer.
The figures in the parentheses indicate the concentration (%) of each polymer. a: all the polymers, b: HPC, c: HPMC; HPC-SSL (8.0),  HPC-SL
(5.0),
HPC-SL
(5.0), HPC-L (3.5),
HPC-L (3.5), HPC-H (0.5),
HPC-H (0.5), ![]() Pharmacoat 603 (7.0),
Pharmacoat 603 (7.0), Pharmacoat 645 (5.5),
Pharmacoat 645 (5.5),  Pharmacoat 606 (4.0),
Pharmacoat 606 (4.0),  60SH-4000 (0.45),
60SH-4000 (0.45),  60SH-10000
(0.45),
60SH-10000
(0.45),  Pharmacoat 904 (5.5),
Pharmacoat 904 (5.5),  Metolose SM-4 (5.5),
Metolose SM-4 (5.5),  SM-4000 (0.45).
SM-4000 (0.45).
Figure 3: Relationships between IFD values of Fe2O3•H2O and molecular length of each polymer. The figures in the parentheses indicate the concentration (%) of each polymer. The IFD values of HPC-H (0.35) and Pharmacoat 603 (5.0) are N=1. The others are N=3. a: all the polymers, b: HPC, c: HPMC. 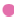 HPC-SSL (6.0),
HPC-SSL (6.0),  HPC-SL (3.5),
HPC-SL (3.5),  HPC-L (2.5),
HPC-L (2.5), HPC-H (0.35),
HPC-H (0.35), ![]() Pharmacoat 603 (5.0),
Pharmacoat 603 (5.0),  Pharmacoat 645 (4.0),
Pharmacoat 645 (4.0), Pharmacoat 606 (3.0),
Pharmacoat 606 (3.0),  60SH-4000 (0.3),
60SH-4000 (0.3),  60SH-10000 (0.25),
60SH-10000 (0.25),  Pharmacoat 904 (4.0),
Pharmacoat 904 (4.0),  Metolose SM-4 (4.0),
Metolose SM-4 (4.0),  SM-4000 (0.35); N=3.
SM-4000 (0.35); N=3.
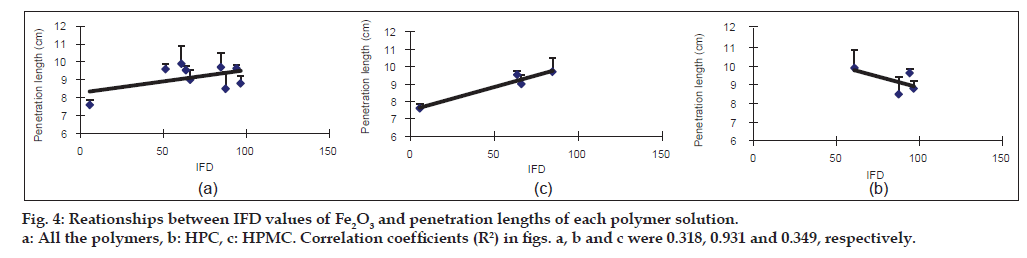
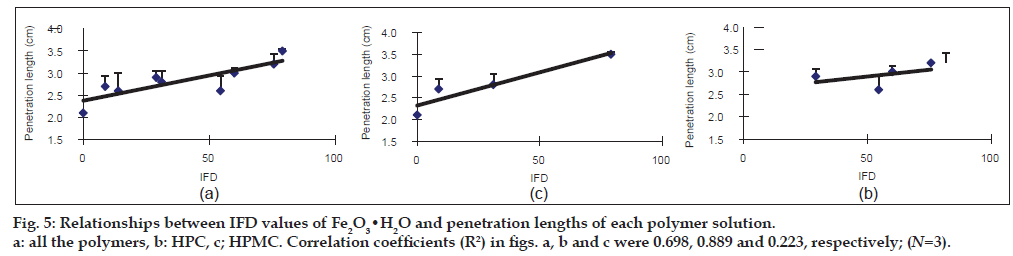
Relationships between IFD values of the iron oxides and the penetration lengths of each polymer solution into Fe2O3 and Fe2O3·H2O particles filled in glass tube
The penetration length was measured as an index for surface tension. To avoid the influence of the viscosity of the polymer solution on the penetration length, the viscosity was constantly adjusted from 18.1 to 20.5 MPas (Table 2). The penetration length of the 6.5% Pharmacoat 603 solution into Fe2O3 showed the highest value (9.9 cm), followed by that of 7.5% HPC-SSL (9.7 cm) and Pharmacoat 904 (9.6 cm). The IFD of Fe2O3 in these polymer solutions showed a moderate value in 6.5% Pharmacoat 603 (60.8) and high values in both 7.5% HPC-SSL and Pharmacoat 904 (84.6 and 94.1, respectively). In the case of Fe2O3·H2O, the penetration lengths of 7.5% HPC-SSL, 5.0% Pharmacoat 904 and 5.0% Pharmacoat 645 were greater than 3.0 cm and the IFD values related to these polymers were also high (59.9-78.9). Then, the regression lines between the IFD values of the iron oxides and the penetration lengths were calculated (figs. 4 and 5). In Fe2O3, the correlation coefficients were 0.931 and 0.349 for HPC and HPMC, respectively (fig. 4b and c, respectively). Although a good correlation was observed in HPC, a negative slope was obtained in HPMC. Overall, the correlation coefficient (R2=0.318) was low (fig. 4a). In Fe2O3·H2O, a relatively good correlation (R2=0.698) between IFD values and the penetration lengths of all the polymer solutions was observed (fig. 5a). The correlation coefficient was high in HPC (R2 = 0.889) and low in HPMC (R2 = 0.223).
| Polymers (%) | Viscosity(MPas) | IFD | Penetration length(cm±SD) | ||
|---|---|---|---|---|---|
| Fe2O3 | Fe2O3•H2O | Fe2O3 | Fe2O3•H2O | ||
| HPC‑SSL (7.5) | 18.7 | 84.6 | 78.9 | 9.7±0.79 | 3.5±0.05 |
| HPC‑SL (5) | 20.4 | 66.1 | 31.1 | 9.0±0.52 | 2.8±0.25 |
| HPC‑L (3.5) | 20.4 | 63.6 | 8.8 | 9.5±0.21 | 2.7±0.24 |
| HPC‑H (0.45) | 20.5 | 5.6 | 0.1 | 7.6±0.28 | 2.1±0.00 |
| Pharmacoat | 20.3 | 60.8 | 54.4 | 9.9±0.99 | 2.6±0.33 |
| 603 (6.5) | 19.1 | 96.5 | 59.9 | 8.8±0.39 | 3.0±0.12 |
| Pharmacoat | |||||
| 645 (5.0) | 18.1 | 87.5 | 29.0 | 8.5±0.92 | 2.9±0.16 |
| Pharmacoat | |||||
| 606 (4.0) | 18.5 | 94.1 | 75.5 | 9.6±0.21 | 3.2±0.22 |
| Pharmacoat | |||||
| 904 (5.0) | |||||
Table 2: Ifd Values Of Fe2O3 And Fe2O3 • H2O (N=1) And Penetration Length Of Each Polymer Solution (N=3)
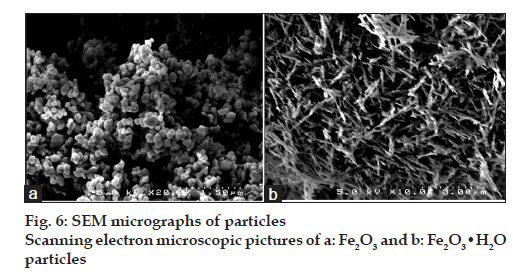
Particle morphology
The morphological characteristics of the Fe2O3 and Fe2O3·H2O particles were analysed using SEM (fig. 6). The SEM micrographs of the Fe2O3 particles revealed that nanosized particles (about 100-300 nm) were aggregated (fig. 6a). In the case of the Fe2O3·H2O particles, the morphology included porous aggregates composed of many micro-sized crystals having needle-like structures (fig. 6b).
Particle size measurement
The mean sizes of the Fe2O3 and Fe2O3·H2O particles with and without polymers were measured using a LSA (Table 3). The sizes of the Fe2O3 and Fe2O3·H2O particles were 32.8 and 41.8 mm, respectively. After adding the 7.5% HPC-SSL, 6.0% Pharmacoat 603, 5.0% and 6.5% Pharmacoat 904 and 7.0% Metolose SM4 to Fe2O3 followed by wet milling using the Ultra Apex Mill, the particles became nanosized particles with sizes ranging from 0.45 to 0.76 mm. However, the other Fe2O3 particle sizes (milled with 0.75 or 0.90% HPC-H or 4.0% Pharmacoat 603) did not reach the nanometer range (1.12-1.59 mm).
| Iron oxide Polymers (%) | Mean particle size (◦m) | |
|---|---|---|
| Fe2O3 | HPC‑SSL (7.5) | 32.8 |
| 0.45 | ||
| HPC‑H (0.75) | 1.59 | |
| HPC‑H (0.90) | 1.37 | |
| Pharmacoat 603 (4.0) | 1.12 | |
| Pharmacoat 603 (6.0) | 0.75 | |
| Pharmacoat 904 (5.0) | 0.76 | |
| Pharmacoat 904 (6.5) | 0.59 | |
| Metolose SM‑4 (7.0) | 0.70 | |
| Fe2O3•H2O | HPC‑SSL (3.5) | 41.8 |
| 2.21 | ||
| HPC‑SSL (5.0) | 0.91 | |
| Pharmacoat 904 (4.0) | 0.51 | |
| Pharmacoat 904 (7.0) | 0.26 | |
Table 3: Mean particle sizes of Fe2O3 and Fe2O3•H2O in various suspensions after or before wet‑milling
The Fe2O3·H2O particle size in the suspensions was 0.26-0.91 mm after milling with 5.0% HPC-SSL and with 4.0 and 7.0% Pharmacoat 904, but the particles were much larger when milled with 3.5% HPC-SSL (2.21 mm).
Discussion
In order to develop a medical ink made from Fe2O3 and Fe2O3·H2O nanoparticles, the effects of various polymers on the dispersibility and nanoparticulation were investigated. According to the Stokes equation, the sedimentation rate of particles decreases inversely with the viscosity of the solvent [7]. We therefore first compared the viscosities of the various polymer solutions to the IFD values of the Fe2O3 particles (fig. 1). The Fe2O3 particles showed a tendency toward relatively high dispersibilities in solutions with viscosities of more than 20 MPas, but the correlation was not good among them (R2=0.0002). These results indicate that not only the solution viscosity but also other factors such as the molecular length of the polymers and the surface tension on the particles have an impact on the dispersibility of Fe2O3 particles.
The relationship between the IFD values and the molecular lengths of HPMC, HPC and MC was evaluated under the condition that the viscosities of the polymer solutions were almost constant. Although these polymers do not generally form a straight chain and they are partially self-associated by nonspecific bindings such as hydrogen bonding, it is considered that the rough molecular lengths of these polymers could be estimated from the monomer glucose size. The IFD values increased with decreasing length of the polymers in both Fe2O3 and Fe2O3·H2O (figs. 2 and 3). It is generally known that adding polymers inhibits aggregation of particles by coating substrates and providing steric barriers to hinder contacts between polymer-coated particles [16-19]. Under the constant viscosity of the polymer solutions, the polymer concentration (w/w%) decreased with increasing molecular length of each polymer (figs. 2 and 3), indicating that the mole number is much smaller in longer-length polymers. The reason for this may be due to insufficient coating of longer-length polymers onto the Fe2O3 and Fe2O3·H2O particles because of the low mole number.
Next, the penetration length of various polymers for Fe2O3 and Fe2O3·H2O was measured as an index for particle surface tension (Table 2, figs. 4 and 5). Wettability on the particle surface is also an important determinant for inhibiting particle aggregation [11,20,21].
Polymers are known to improve the wettability of hydrophobic substrates following their adsorption onto the particle surface by reducing the solid/liquid interfacial tension [22-25]. However, the IFD values of Fe2O3 did not correlate with the penetration length in the case of the HPMC solution. This may be due to the relatively lesser interaction of the Fe2O3 surface with HPMC molecules. Further investigation is needed for this. The penetration length of 6.5% Pharmacoat 603 and 5.0% Pharmacoat 904 to Fe2O3 was relatively high (9.9 and 9.6 cm, respectively) and the molecular lengths were short (33.2 and 47.0 nm, respectively). However, the IFD value in 6.5% Pharmacoat 603 was not very high (60.8) as compared with that in 5.0% Pharmacoat 904 (94.1), regardless of their similar properties. It might be considered that other factors such as the substituent content of methoxy and hydroxypropoxy groups are involved in the dispersibility of Fe2O3 particles [26].
Finally, the effects of adding polymers on the nanoparticulation of iron oxides were investigated (Table 3). Although the particle sizes of Fe2O3 and Fe2O3·H2O were about 100-300 nm and 1-3 mm, respectively, according to the SEM micrographs (fig. 6a and b, respectively), the particles formed aggregates with sizes of 32.8 mm in Fe2O3 and 41.8 mm in Fe2O3·H2O in distilled water.
After wet milling to disperse the iron oxides by Ultra Apex Mill with various polymers, the Fe2O3 particles milled with 0.75% or 0.90% HPC-H or 4.0% Pharmacoat 603 and Fe2O3·H2O milled with 3.5% HPC-SSL did not reach nanometre size. Although the IFD value of Fe2O3 in the 0.45% and 0.5% HPC-H solutions was low (figs. 1 and 2 and Table 2), the particles were not dispersed to nanometre size even at the higher concentrations of HPC-H (0.75% and 0.90%). As mentioned earlier, adding polymers provides steric barriers to hinder reaggregation [16-19]. Therefore, it might be difficult for particles to decrease to a size that is less than the molecular length of the polymer added in a suspension, that is, the final particle sizes might depend on the size of each polymer because polymers with longer lengths (on the order of more than a micrometer) cannot make a sufficient coating layer on the nanoparticle surface owing to the larger polymer size. Yamamoto et al. reported on the stability of probucol nanoparticles using sodium dodecyl sulphate (SDS) and polyvinylpyrrolidone (PVP) K12 or K17 as dispersing agents, and they reported the importance of a sufficient coating layer of polymers for improved stability [27-30].
As it stands now, there are no effective ways to select an appropriate type and concentration of polymer to fabricate nanoparticles by wet milling, and it is a fact that various preexperimentations using various types and concentrations of polymers and surfactant are needed for nanoparticulation [13,15,31]. However, the IFD might provide a good way to indicate this. In Fe2O3, the IFD showed a relatively high value in the 6.5% Pharmacoat 603 solution (about 61, Table 2). Although the particle size did not reach into the nanometre range by wet milling with a lower concentration of Pharmacoat 603 (4.0%), the size was 0.75 mm in the 6.0% concentration (near the 6.5% concentration). In addition, the particles were in the nanometre size range with 5.0% and 6.5% Pharmacoat 904. The concentrations were around 5.5%, which provided a high IFD value (about 95, figs. 1 and 2). In Fe2O3·H2O, the IFD value was high in the 6.0% HPC-SSL solution (about 80, fig. 3). Use of 5.0% HPC-SSL (near the 6.0% concentration) achieved nanoparticulation (0.91 mm), but the use of 3.5% did not. When the IFD value of iron oxides is more than 60, nanomilling seems possible. By using the IFD value as a measure of nanoparticulation, cost, time and the wastage of active pharmaceutical ingredients could be avoided.
To develop stable medical inks of iron oxides, the dispersibility and nanoparticulation using various types and concentrations of polymers were investigated. The polymer concentration, surface tension and molecular length were found to affect the dispersibility of iron oxides and the solution viscosity. The optimal concentration of HPC-SSL, Pharmacoat 603, Pharmacoat 904 and Metolose SM-4, all of which have short molecular lengths, enabled successful fabrication of iron oxide nanoparticles by wet milling. In addition, we created an effective indicator for nanoparticulation, namely, IFD, for wet milling using Ultra Apex Mill. We believe that these methods and the information they provide would be useful in the pharmaceutical industry
References
- Montes-Hernandez G., Pironon J, Villieras F. Synthesis of a red iron oxide/montmorillonite pigment in a CO2-rich brine solution. J Colloid Interface Sci 2006;303:472-6.
- Dengxin L, Guolong G., Fanling M, Chong J. Preparation of nano-iron oxide red pigment powders by use of cyanided tailings. J Hazard Mater 2008;155:369-77.
- Cai CQ. Discussion on real seed during synthesis of ammonium-based iron oxide red. Paint Coat Ind.2006;36:472-6.
- Itoh H, Sugimoto T. Systematic control of size, shape, structure, and magnetic properties of uniform magnetite and maghemite particles. J Colloid Interface Sci 2003;265:283-95.
- Legodi MA, de Waal D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigm 2007;74:161-8.
- Rowe RC, Sheskey PJ, Weller PJ. editors. Handbook of Pharmaceutical Excipients. 4th ed. Atlanta, GA: APhA Publications; 2003. p. 171.
- Martin A, Swarbrick J, Cammarata A. Physical Pharmacy. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 1983. p. 478-9.
- Gilbert B, Lu G., Kim CS. Stable cluster formation in aqueous suspensions of iron oxyhydroxide nanoparticles. J Colloid Interface Sci 2007;313:152-9.
- Gahoi S, Jain GK, Tripathi R, Pandey SK, Anwar M, Warsi MH, et al. Ahmad FJ. Enhanced antimalarial activity of lumefantrine nanopowder prepared by wet-milling DYNO MILL technique. Colloids Surf B Biointerfaces 2012;95:16-22.
- Kim M, Jung J, Lee J, Na K, Park S, Hyun J. Amphiphilic comblike polymers enhance the colloidal stability of Fe3O4 nanoparticles. Colloids Surf B Biointerfaces 2010;76:236-40.
- Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci 2003;18:113-20.
- Comba S, Sethi R. Stabilization of highly concentrated suspensions of iron nanoparticles using shear-thinning gels of xanthan gum. Water Res 2009;43:3717-26.
- Tanaka Y, Inkyo M, Yumoto R, Nagai J, Takano M, Nagata S. Nanoparticulation of poorly water soluble drugs using a wet -mill process and physicochemical properties of the nanopowders. Chem Pharm Bull 2009;57:1050-7.
- Tanaka Y, Inkyo M, Yumoto R, Nagai J, Takano M, Nagata S. Evaluation of in vitro dissolution and in vivo oral absorption of drug nanopowders prepared by novel wet-milling equipment. Curr Nano Sci 2010;6:571-6.
- Tanaka Y, Inkyo M, Yumoto R, Nagai J, Takano M, Nagata S. Nanoparticulation of probucol, a poorly water-soluble drug, using a novel wet-milling process to improve in vitro dissolution and in vivo oral absorption. Drug Dev Ind Pharm 2012;38:1015-23.
- Egorov SA, Binder K. Effect of solvent quality on the dispersibility of polymer-grafted spherical nanoparticles in polymer solutions. J Chem Phys 2012;137:094901.
- Lin HC, Hsieh BZ, Lin YL, Sheng YJ, Lin JJ. Effect of grafting architecture on the surfactant-like behavior of clay-poly (NiPAAm) nanohybrids. J Colloid Interface Sci 2012;387:106-14.
- Wei CC, Ge ZQ. Influence of electrolyte and poloxamer 188 on the aggregation kinetics of solid lipid nanoparticles (SLNs). Drug Dev Ind Pharm 2012;38:1084-9.
- Bhakay A, Merwade M, Bilgili E, Dave RN. Novel aspects of wet milling for the production of microsuspensions and nanosuspensions of poorly water-soluble drugs. Drug Dev Ind Pharm 2011;37:963-76.
- Cheng Y, Liu X, Guo J, Liu F, Li Z, Xu G, et al. Fabrication of uniform core-shell structural calcium and titanium precipitation particles and enhanced electrorheological activities. Nanotechnology 2009;20:055604.
- Overhoff KA, McConville JT, Yang W, Johnston KP, Peters JI, Williams RO 3rd. Effect of stabilizer on the maximum degree and extent of supersaturation and oral absorption of tacrolimus made by ultra-rapid freezing. Pharm Res 2008;25:167-75.
- Sinswat P, Gao X, Yacaman MJ, Williams RO 3rd, Johnston KP. Stabilizer choice for rapid dissolving high potency itraconazole particles formed by evaporative precipitation into aqueous solution. Int J Pharm 2005;302:113-24.
- Wong SM, Kellaway IW, Murdan S. Enhancement of the dissolution rate and oral absorption of a poorly water-soluble drug by formation of surfactant-containing microparticles. Int J Pharm 2006;317:61-8.
- Zaccone A, Wu H, Lattuada M, Morbidelli M. Correlation between colloidal stability and surfactant adsorption/association phenomena studied by light scattering. J Phys Chem B 2008;112:1976-86.
- Merisko-Liversidge EM, Liversidge GG. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol Pathol 2008;36:43-8.
- Shin-Etsu Chemical Co., Ltd., Brochure USP Hydroxypropyl methylcellulose Pharmacot. 1998. p. 16.
- Itoh K, Pongpeerapat A, Tozuka Y, Oguchi T, Yamamoto K. Nanoparticle formation of poorly water-soluble drugs from ternary ground mixtures with PVP and SDS. Chem Pharm Bull 2003;51:171-4.
- Shudo J, Pongpeerapat A, Wanawongthai C, Moribe K, Yamamoto K. In vivo assessment of oral administration of probucol nanoparticles in rats. Biol Pharm Bull 2008;31:321-5.
- Pongpeerapat A, Wanawongthai C, Tozuka Y, Moribe K, Yamamoto K. Formation mechanism of colloidal nanoparticles obtained from probucol/PVP/SDS ternary ground mixture. Int J Pharm 2008;352:309-16.
- Moribe K, Wanawongthai C, Shudo J, Higashi K, Yamamoto K. Morphology and surface States of colloidal probucol nanoparticles evaluated by atomic force microscopy. Chem Pharm Bull 2008;56:878-80.
- Niwa T, Miura S, Danjo K. Universal wet -milling technique to prepare oral nanosuspension focused on discovery and preclinical animal studies: Development of particle design method. Int J Pharm 2011;405:218-27.