- *Corresponding Author:
- Yukun Luo
Department of Ultrasound, The First Medical Center, Chinese PLA General Hospital, Beijing 100853, China
E-mail: lykun1123@163.com
| This article was originally published in a special issue,“Drug Discovery and Repositioning Studies in Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(4) Spl Issue “411-416” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the application value of contrast-enhanced ultrasound to evaluate the changes in blood microcirculation in the renal cortex during renal ischemia reperfusion injury in rats. 18 Sprague-Dawley rats were randomly divided into control group (sham group), renal ischemia reperfusion group (ischemia-reperfusion injury group) and quercetin intervention group (ischemia-reperfusion injury+quercetin group), with 6 rats in each group. Ischemia-reperfusion injury+quercetin group was given 100 mg/kg quercetin by intragastric administration 7 d before surgery, and all three groups underwent contra-enhanced ultrasound examination 24 h after surgery. Blood was collected from the inferior vena cava, and creatinine and urea nitrogen levels were detected by automatic biochemical analyzer. The levels of interleukin-6, tumor necrosis factor-alpha and interleukin-10 were detected by enzyme-linked immunosorbent assay kit. The left kidney tissue of rats was collected and periodic acid-Schiff staining was performed for pathological detection. In terms of inflammatory factors, compared with sham group, interleukin-6, tumor necrosis factor-alpha and interleukin-10 in ischemia-reperfusion injury group were significantly increased (p<0.05), while interleukin-6 and tumor necrosis factor-alpha were significantly decreased and IL-10 was significantly increased in ischemia-reperfusion injury+quercetin group compared with ischemia-reperfusion injury group (p<0.05). Pathologically, a large number of tubular shapes were observed in the renal interstitium of rats in the ischemia-reperfusion injury group, during which inflammatory cell infiltration was observed. The injury degree of ischemia-reperfusion injury+quercetin group was lower than that of the ischemia-reperfusion injury group and the tubular shapes were not obvious. Contrast-enhanced ultrasound can evaluate the changes of blood flow microcirculation in the renal cortex during the intervention of quercetin in renal ischemia-reperfusion injury in rats. Quercetin can improve the renal cortical microcirculation and reduce the expression of inflammatory factors during renal ischemia reperfusion injury in rats.
Keywords
Reperfusion injury, acute kidney injury, quercetin, ultrasonography
Renal Ischemia-Reperfusion Injury (IRI) is the most common complication in urology, it often occurs after trauma, renal transplantation, renal tumor resection and so on[1]. In clinical practice, IRI can lead to severe Acute Kidney Injury (AKI), which greatly increases the disability and mortality of patients[2]. Because of the complexity of the pathological mechanism of IRI, it brings great challenges to the diagnosis and treatment of clinicians. Therefore, how to effectively detect and interfere with the occurrence and development of IRI in the early stage has become the focus of scholars all over the world.
Quercetin (QU), as a flavonoid compound with a variety of biological activities, has been proved to have a certain protective effect in renal IRI, but the related therapeutic mechanism has not been fully elucidated[3,4]. In this study, contrast-enhanced ultrasound was used to monitor the changes of renal microcirculation and the expression of related inflammatory factors during the intervention of QU in rats with IRI, so as to provide reliable experimental basis for the treatment of IRI in the future.
Materials and Methods
Experimental animal grouping:
Eighteen healthy adult male Sprague-Dawley (SD) rats (weighing 200-250 g) were purchased from Spyford (Beijing) Biotechnology Co., Ltd. This research project was approved by Animal Ethics Committee of General Hospital of Chinese People’s Liberation Army (No: 2023-X19-45). They were randomly divided into control group (sham group), model group (IRI group) and QU intervention group (IRI+QU group) with 6 rats in each group. IRI+QU group was given QU 100 mg/kg (0.5 mg Sodium- Carboxymethyl Cellulose (Na-CMC) solution) intragastrically 7 d before operation, sham group and IRI group were also given the same volume of solvent (0.5 mg Na-CMC).
Establishment of ischemia-reperfusion model:
The rats were anesthetized by intraperitoneal injection of 2 % pentobarbital sodium, and the bilateral lumbar and back median incision was performed to expose the bilateral renal area, and the bilateral renal pedicle was clipped by artery clip, when the color of the kidney changed from red to dark purple, it was suggested that the ischemia was successful. After 40 min, the artery clamp was loosened, and the color of the kidney changed from dark purple to red, suggesting that the reperfusion was successful, and the incision was sutured layer by layer. After the operation, the rats were anesthetized and awake, and given water and feed for routine feeding.
Contrast-enhanced ultrasound examination:
After 24 h of reperfusion, the rats were anesthetized with 2 % pentobarbital sodium 50 mg/kg and fixed in lateral position. Ultrasound examination using Mindray M9 color ultrasound instrument, L12-4 s linear array probe, Mechanical Index (MI=0.098) and frequency (7~13 Mhz). It was fixed on the largest coronal section of the left kidney area of the rat, and 5 ml normal saline was injected into the contrast agent SonoVue® according to the instructions, contrast agent was injected into the tail vein of rats at a dose of 0.2 ml/kg, and then 0.5 ml normal saline was injected into the tube, immediately began to store angiography images, continuous storage of dynamic images for 3 min. In the same approximate depth and location area of the renal cortex, three regions of interest were selected to automatically generate signal Time Intensity Curves (TIC) to generate and record relevant parameters; Area Under Curve (AUC), Arrival Time (AT), Peak Intensity (PI) and Ascending Slope (AS).
Serum index detection:
After the radiography, blood was collected from the inferior vena cava, centrifuged at 3000 rpm for 15 min, and the serum was taken for biochemical examination. The creatinine value and urea nitrogen level were detected by automatic biochemical analyzer (Hitachi 7600, Japan).
Detection of inflammatory factors:
The levels of Interleukin (IL)-6, IL-10 and Tumor Necrosis Factor-Alpha (TNF-α) in rat serum were detected by Enzyme-Linked Immunosorbent Assay (ELISA) (CZKEWEI, China) kit, and the experimental procedures were carried out according to the instructions provided by the kit.
Pathological observations:
After the blood was taken from the inferior vena cava, the rats were sacrificed. The left kidney of rats was fixed with 4 % formaldehyde, embedded, sliced and other processed tissue sections for Periodic Acid-Schiff (PAS) staining, and the renal pathology was observed under a microscope.
Statistical method:
Statistical Package for the Social Sciences (SPSS) 27.0 statistical analysis software and GraphPad Prism 9 software were used for plotting, and one-way analysis of variance was performed between groups. Measurement data conforming to normal distribution were represented by x? ±s, and one-way analysis of variance was performed between groups; for nonnormally distributed data, Kruskal-Wallis test was used. p<0.05 was considered statistically significant.
Results and Discussion
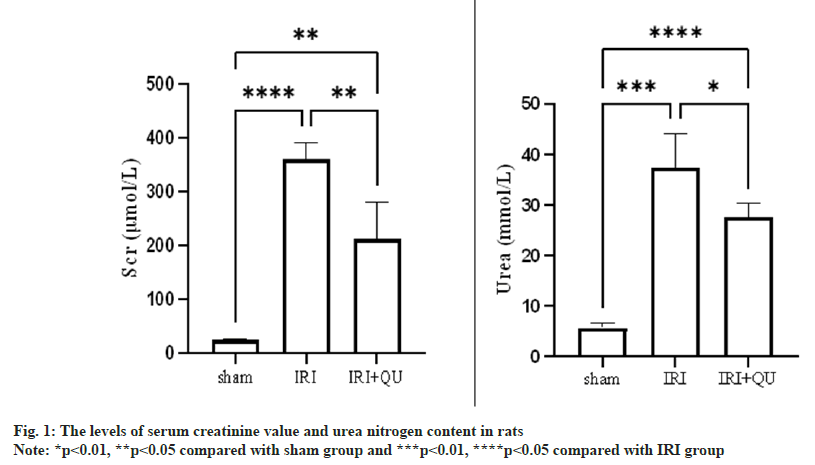
Compared with the Sham group, the creatinine value and urea nitrogen level in the IRI group were significantly increased, and the difference was statistically significant (p<0.05); compared with the IRI group, the creatinine value and urea nitrogen level in the IRI+QU group were decreased, and the difference was statistically significant (p<0.05, fig. 1).
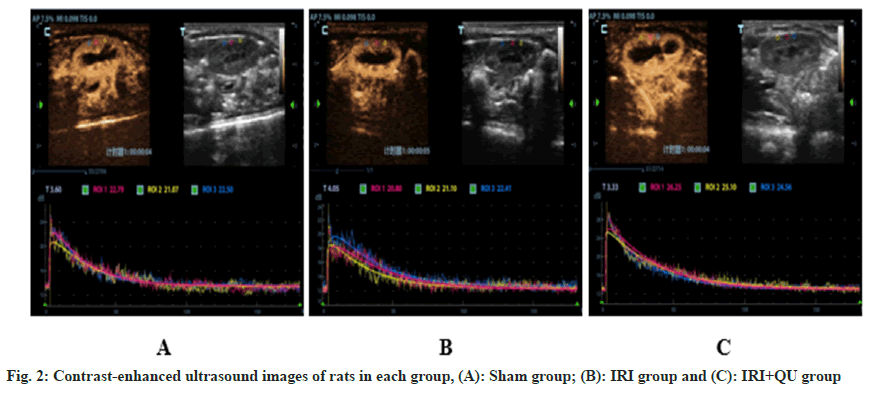
Compared with sham group, the AUC and PI of renal cortex increased in IRI group, the difference was statistically significant (p<0.05), AT prolonged, AS decreased, and the difference was not statistically significant. Compared with the IRI group, the AUC of the renal cortex in the IRI+QU group decreased, the AT decreased, and the AS increased, the difference was statistically significant (p<0.05), and the difference in PI was not statistically significant (fig. 2 and Table 1).
| Group | AUC (dB/s) | AT (s) | PI (dB) | AS (dB/s) |
|---|---|---|---|---|
| Sham | 2631.11±188.57 | 2.98±0.97 | 20.61±1.73 | 0.53±0.15 |
| IRI | 3300.90±264.30?? | 3.97±0.83 | 23.62±1.86? | 0.38±0.09 |
| IRI+QU | 2661.39±92.95** | 2.30±0.59** | 22.60±1.79 | 0.72±0.16** |
| F | 22.58 | 6.32 | 4.36 | 9.35 |
| p | <0.001 | 0.012 | 0.032 | 0.003 |
Note: ?p<0.05, ??p<0.01 compared with sham group and *p<0.05, **p<0.01 compared with IRI group (n=6)
Table 1: The Level of Renal Cortical Parameters of Rats in Each Group by Contrast-Enhanced Ultrasound (X?±S)
Compared with the sham group, the levels of IL-6, TNF-α and IL-10 in the IRI group were significantly increased (p<0.05). Compared with the IRI group, the pro-inflammatory factors IL-6 and TNF-α in the IRI+QU group were decreased, and IL-10 was increased. The difference was statistically significant (p<0.05, Table 2).
| Group | TNF-α (pg/ml) | IL-6 (pg/ml) | IL-10 (pg/ml) |
|---|---|---|---|
| Sham | 74.81±5.91 | 60.04±9.16 | 39.78±6.33 |
| IRI | 184.77±9.43?? | 183.23±6.70?? | 116.17±3.37? |
| IRI+QU | 134.81±6.65** | 122.89±5.80** | 158.79±5.69** |
| F | 324.48 | 420.29 | 780.66 |
| p | <0.001 | <0.001 | <0.001 |
Note: ?p<0.05, ??p<0.01 compared with sham group and *p<0.05, **p<0.01 compared with IRI group (n=6)
Table 2: The Expression Levels of Inflammatory Factors in Rats of Each Group (X?±S)
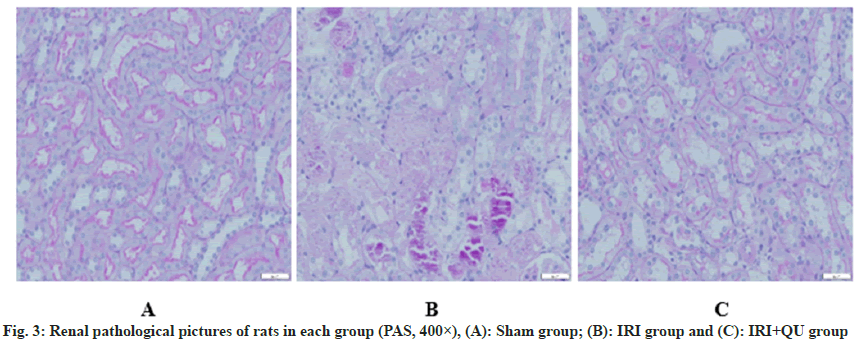
PAS staining showed that renal tubules and glomeruli were clearly displayed in sham group, and no obvious abnormality was found in renal tubulointerstitium; the number of renal interstitial lymphocytes in the IRI group was significantly increased, and obvious inflammatory cell infiltration was observed, and the renal tubular lumen was narrowed. The renal tubular epithelial cells were necrotic and exfoliated, and nuclear condensation and nuclear fragmentation could be seen in the renal tubular epithelial cells. The degree of damage to the renal tissues of rats in the IRI+QU group was significantly lower than that of the IRI group, and the morphology of the renal tubules was still acceptable, scattered irregular necrosis and tubular formation of renal tubules were seen (fig. 3).
AKI is defined as a clinical complication of a sharp decline in renal function due to various reasons. The degree of renal injury and prognosis are usually assessed by measuring creatinine values[5]. However, due to the strong compensatory ability of the kidney, the creatinine value is not sensitive to early or mild AKI, the creatinine value increases significantly only when the Glomerular Filtration Rate (GFR) is reduced to 50 % of the normal value, therefore, there are obvious limitations in using abnormal creatinine value as a diagnostic criterion for early AKI[6]. As a common cause of AKI, the high morbidity and mortality of IRI have been perplexing clinicians, and the perfusion of renal cortex is often related to the degree of IRI renal injury[7,8]. Therefore, monitoring the blood perfusion of renal cortex can indirectly reflect disease progression and efficacy evaluation. As a non-invasive, repeatable and economical imaging method, contrast-enhanced ultrasound is widely used in the diagnosis of kidney diseases[9-12]. Numerous studies have shown that ultrasonography can dynamically monitor changes in renal perfusion as well as changes in blood flow after pharmacologic interventions[13-16]. Therefore, in this study, contrastenhanced ultrasound was used to monitor the changes of renal cortex blood perfusion of rats treated with QU in IRI, and the experimental basis of IRI protection of QU was put forward from the level of microcirculation, which provided a preliminary theoretical basis for follow-up research.
Endothelial cells and smooth muscle cells of the renal vasculature play an important role in the pathologic process of AKI during renal IRI[5]. Damage to endothelial cells leads to more intense constriction of small renal blood vessels due to an increase in vasoconstrictor factors such as endothelin-1, angiotensin II, thromboxane A2, and prostaglandin H in the tissues, as well as a decrease in other vasodilator substances, such as nitric oxide and L-arginine, which ultimately results in the socalled phenomenon of no reflux. It is characterized by a lack of effective and sufficient perfusion at the microcirculation level, although the kidneys are reperfused[17,18]. With the injury of renal tubular endothelial cells, it will lead to the continuous release of inflammatory cells and pro-inflammatory factors. A large number of inflammatory cells and pro-inflammatory factors gather and adhere to the vascular endothelium, causing extensive interstitial edema and oppressing the microcirculation of renal small vessels. Eventually cause damage to renal function[19,20].
In this experiment, it was found that after 24 h of IRI, AUC increased, AT prolonged and AS decreased. It was consistent with previous literature[19]. AUC represents the cumulative effect of selecting contrast media in Region of Interest (ROI) in a certain period of time. In the process of IRI reperfusion, a large number of inflammatory cells infiltrated, resulting in a large number of phagocytosis of inflammatory cells to contrast microbubbles, resulting in an increase in AUC. Studies have shown that QU has a potential therapeutic effect on neurodegenerative diseases by targeting the Nuclear Factor Kappa B (NF-κB) pathway and NOD-Like Receptor Protein 3 (NLRP3) inflammasome[20,21]. Probably due to the anti-inflammatory and inhibitory effect of QU, the phagocytosis of contrast microbubbles by inflammatory cells was improved, and the AUC of the IRI+QU group was reduced, with a statistically significant difference compared to the IRI group (p<0.05). AT reflects the time when the contrast medium reaches the renal vessels and AS indicates the average blood flow velocity and local tissue perfusion rate reached by the contrast medium in the ROI[19]. The main reason for the prolongation of AT and the decrease of AS in IRI group may be the contraction of renal blood flow, which leads to the increase of renal vascular resistance and the decrease of intrarenal blood perfusion velocity. It may be due to the anti-inflammatory effect of QU, which reduces the degree of contraction of small and medium sized arteries in the kidney and relaxes small and medium-sized blood vessels, thereby improving renal microcirculation perfusion. In this experiment, it was also found that there was no significant difference in PI between IRI group and IRI+QU group, and there was no statistical significance, probably because it only reflected the instantaneous maximum signal intensity in ROI, but did not have cumulative effect. Therefore, the contrast-enhanced ultrasound parameters cannot fully represent the vascular perfusion of renal cortex. The AT and AS in the IRI+QU group were significantly improved compared with the IRI group, which was consistent with the results of inflammatory factor detection.
In the detection of inflammatory factors, it was found that the levels of pro-inflammatory cytokines IL-6 and TNF-α in IRI+QU group were lower in the IRI+QU group than in the IRI group, and the level of anti-inflammatory factor IL-10 was increased, and the difference was statistically significant. Studies have shown that IL-10 has certain anti-inflammatory and anti-apoptotic effects in IRI[22], and the increase of IL-10 content indicates that the anti-inflammatory mechanism is activated. The increase of IL-10 content suggests that the anti-inflammatory mechanism of the body is activated. In this study, it was found that QU could reduce the expression of IL-6 and TNF-α proinflammatory factors after 24 h of renal IRI in rats to a certain extent, and did not reduce the expression of anti-inflammatory factor IL-10, thus demonstrating that QU did not destroy the anti-inflammatory effect mediated by IL-10 in the pathological process of rat renal IRI. Combined with the related parameters of contrast-enhanced ultrasound, it is inferred that QU may improve the microcirculation perfusion of renal cortex by reducing the expression of inflammatory factors in renal IRI.
In this experiment, the sample size of animals is small, there are individual differences among rats, and the pathophysiological mechanisms of rats and humans are not the same and are affected by various clinical factors. For these reasons, more comprehensive preclinical studies are needed to elucidate the pathological mechanism of IRI and provide reliable experimental basis for the treatment of IRI.
In summary, contrast-enhanced ultrasound can quantitatively evaluate the effect of QU on the changes of cortical microcirculation blood flow in rats with renal IRI, and QU can improve the renal microcirculation of rats with renal IRI and play a renal protective role.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wang L, Chen H, Liu XH, Chen ZY, Weng XD, Qiu T, et al. Ozone oxidative preconditioning inhibits renal fibrosis induced by ischemia and reperfusion injury in rats. Exp Ther Med 2014;8(6):1764-8.
[Google Scholar] [PubMed]
- Zhao H, Alam A, Soo AP, George AJ, Ma D. Ischemia-reperfusion injury reduces long term renal graft survival: Mechanism and beyond. EBioMedicine 2018;28:31-42.
[Crossref] [Google Scholar] [PubMed]
- Chen BL, Wang LT, Huang KH, Wang CC, Chiang CK, Liu SH. Quercetin attenuates renal ischemia/reperfusion injury via an activation of AMP-activated protein kinase-regulated autophagy pathway. J Nutr Biochem 2014;25(11):1226-34.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res 2021;28:231-43.
[Crossref] [Google Scholar] [PubMed]
- Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 2011;121(11):4210-21.
[Crossref] [Google Scholar] [PubMed]
- Sprenkle P, Russo P. Molecular markers for ischemia, do we have something better then creatinine and glomerular filtration rate? Arch Esp Urol 2013;66(1):99-114.
[Google Scholar] [PubMed]
- Harrois A, Grillot N, Figueiredo S, Duranteau J. Acute kidney injury is associated with a decrease in cortical renal perfusion during septic shock. Crit Care 2018;22(1):161.
[Crossref] [Google Scholar] [PubMed]
- Kwiatkowska E, Kwiatkowski S, Dziedziejko V, Tomasiewicz I, Domanski L. Renal microcirculation injury as the main cause of ischemic acute kidney injury development. Biology 2023;12(2):327.
[Crossref] [Google Scholar] [PubMed]
- Barr RG. Use of lumason/sonovue in contrast-enhanced ultrasound of the kidney for characterization of renal masses-A meta-analysis. Abdom Radiol 2022;47(1):272-87.
[Crossref] [Google Scholar] [PubMed]
- Siracusano S, Bertolotto M, Ciciliato S, Valentino M, Liguori G, Visalli F. The current role of Contrast-Enhanced Ultrasound (CEUS) imaging in the evaluation of renal pathology. World J Urol 2011;29(5):633-8.
[Crossref] [Google Scholar] [PubMed]
- Zhu JN, Li JB, Luo YK. Application of conventional ultrasound and contrast-enhanced ultrasound in the diagnosis of adrenal tumors. Chin Res Hosp 2022;9(3):57-60.
- Huang SX, Wang D, Wang KY. Progress of ultrasonography in interventional medicine. Chin Res Hosp 2023;10(3):56-60.
- Zhao P, Li Q, Wang S, Wang Y, Zhu J, Zhu L, et al. Quantitative analysis of renal perfusion in rhabdomyolysis-induced acute kidney injury using contrast-enhanced ultrasound: An experimental study. Ultrasound Med Biol 2022;48(10):2110-8.
[Crossref] [Google Scholar] [PubMed]
- Wang S, Zhao P, Zhang Y, Zhu L, Zhu J, Luo Y, et al. The therapeutic effects of curcumin in early septic acute kidney injury: An experimental study. Drug Design Dev Ther 2021;15:4243-55.
[Crossref] [Google Scholar] [PubMed]
- Sun X, Kuang B, Dai Y, Xiong C, Li M, Luo Z. Quantitative evaluation of dexamethasone treatment effects in renal ischemia-reperfusion injury using contrast enhanced ultrasonography in rats. Clin Hemorheol Microcirc 2020;76(1):99-110.
[Crossref] [Google Scholar] [PubMed]
- Cao W, Cui S, Yang L, Wu C, Liu J, Yang F, et al. Contrast-enhanced ultrasound for assessing renal perfusion impairment and predicting acute kidney injury to chronic kidney disease progression. Antioxid Redox Signal 2017;27(17):1397-411.
[Crossref] [Google Scholar] [PubMed]
- da Silveira KD, Pompermayer Bosco KS, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, et al. ACE2–angiotensin-(1-7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci 2010;119(9):385-94.
[Crossref] [Google Scholar] [PubMed]
- Kurata H, Takaoka M, Kubo Y, Katayama T, Tsutsui H, Takayama J, et al. Protective effect of nitric oxide on ischemia/reperfusion-induced renal injury and endothelin-1 overproduction. Eur J Pharmacol 2005;517(3):232-9.
[Crossref] [Google Scholar] [PubMed]
- Luo Z, Liu Y, Tang Z, Liu J, Xu X, Li M, et al. Quantitative evaluation of renal cortex perfusion using contrast-enhanced ultrasound imaging parameters in ischemia-reperfusion injury in rabbits. Ultrasound Med Biol 2021;47(11):3253-62.
[Crossref] [Google Scholar] [PubMed]
- Bianchi ME. DAMPs, PAMPs and alarmins: All we need to know about danger. J Leucocyte Biol 2007;81(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Chiang MC, Tsai TY, Wang CJ. The potential benefits of quercetin for brain health: A review of anti-inflammatory and neuroprotective mechanisms. Int J Mol Sci 2023;24(7):6328.
[Crossref] [Google Scholar] [PubMed]
- Sakai K, Nozaki Y, Murao Y, Yano T, Ri J, Niki K, et al. Protective effect and mechanism of IL-10 on renal ischemia-reperfusion injury. Lab Invest 2019;99(5):671-83.
[Crossref] [Google Scholar] [PubMed]