- *Corresponding Author:
- Chao Ye
Department of Pharmacy, The Third Hospital of Changsha, Changsha, Hunan Province 410000, China
E-mail: chaoyelcyx@163.com
| Date of Received | 15 January 2023 |
| Date of Revision | 27 November 2023 |
| Date of Acceptance | 10 May 2024 |
| Indian J Pharm Sci 2024;86(3):1000-1007 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the role of mitoxantrone in regulating gastric cancer cell phenotypes and the underlying mechanism. To reveal mitoxantrone-induced effects on gastric cancer cell malignancy, AGS cells were divided into different groups according to various study purposes, including negative control, 5 μmol/l mitoxantrone, 10 μmol/l mitoxantrone, 20 μmol/l mitoxantrone, anti-microRNA-negative control, anti-microRNA-211-5p, 20 μmol/l mitoxantrone+microRNA-negative control, and 20 μmol/l mitoxantrone+microRNA-211-5p groups. AGS cell proliferation was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide, while apoptotic rate was quantified via flow cytometry. Bcl-2 associated X-protein, activated caspase-3, phosphorylated-p65 and phosphorylated-IκBα was detected by Western blot for protein expression. Malondialdehyde was detected via colorimetry, nicotinamide adenine dinucleotide phosphate oxidase activity was detected by chemiluminescence, superoxide dismutase activity was measured via colorimetry, and microRNA-211-5p was quantified by reverse transcription-quantitative polymerase chain reaction. AGS cell proliferation, superoxide dismutase activity, as well as microRNA-211-5p expression in mitoxantrone groups (5, 10 and 20 μmol/l) were decreased compared with the negative control group, while the apoptotic rate, Bcl-2-associated X-protein and activated caspase-3 protein expression, malondialdehyde content, and nicotinamide adenine dinucleotide phosphate oxidase activity were increased. Protein expression of phosphorylated-p65 and phosphorylated-IκBα (inhibitor of nuclear factor kappa B) was decreased in the 20 μmol/l mitoxantrone group. The microRNA-211-5p expression, cell proliferation, superoxide dismutase activity, and phosphorylated-p65 and phosphorylated-IκBα protein expression in anti-microRNA-211-5p group were significantly decreased in relative to anti-microRNA-negative control group, while cell apoptosis, Bcl-2-associated X-protein and activated caspase-3 protein expression, malondialdehyde content, and nicotinamide adenine dinucleotide phosphate oxidase activity were increased. The microRNA-211-5p expression, cell proliferation, superoxide dismutase activity, and phosphorylated-p65 and phosphorylated-IκBα protein expression in the 20 μmol/l mitoxantrone+microRNA-211-5p group were higher than in 20 μmol/l mitoxantrone+microRNA-negative control group, while apoptotic, Bcl-2-associated X-protein and activated caspase-3 protein expression, malondialdehyde content, and nicotinamide adenine dinucleotide phosphate oxidase activity were lower than those in the 20 μmol/l mitoxantrone+microRNA-negative control group. Mitoxantrone repressed gastric cancer cell tumor property by downregulating the microRNA-211-5p/nuclear factor kappa B pathway.
Keywords
Gastric cancer, mitoxantrone, apoptosis, oxidative stress, microRNA-211-5p, nuclear factor kappa B
Gastric cancer, which usually originates from gastric epithelial cells, is a deadliest cancer worldwide[1,2]. It is often asymptomatic in its early stages, leading to low rates of early diagnosis and confusion with gastritis[3]. Current standard treatment protocols for gastric cancer patients include tumor resection, chemotherapy, radiotherapy, or a combination of these approaches[4]. However, due to late-stage diagnosis, tumor recurrence, limited efficacy and side effects of chemotherapy, and the occurrence of drug resistance, there remains a need to explore alternative strategies to improve overall outcomes for gastric cancer patients[5]. The precise mechanisms underlying gastric cancer development are still poorly understood. Proposed as an important factor produced by the body in response to various harmful stimulation, oxidative stress participates in tumor initiation and progression[6]. Over time, this damage can lead to mutations and other changes that promote the growth of cancer cells. Oxidative stress participates in cancer development through several pathways, including damage to the cell membrane, gene mutation, regulation of the immune system. Free radicals can bind to lipid molecules in the biological membrane, causing membrane dysfunction and promoting tumor initiation. Free radicals can attack Deoxyribonucleic Acid (DNA) molecules, leading to DNA mutations, including genomic instability and DNA repair defects, which can increase the number of mutations. Oxidative stress can affect the development and function of immune cells, potentially participating in the complex interactions between the immune system and tumors. Therefore, understanding the role of oxidative stress in gastric cancer development may provide valuable insights into developing new therapeutic strategies.
As reported, Mitoxantrone (MTN) inhibits the growth of ovarian cancer cells (COC1) and induces apoptosis by increasing reactive oxygen species levels in RH35 liver cancer cells[7,8]. However, its role in gastric cancer cell tumor property is still unclear. microRNA (miRNA), a small non-coding Ribonucleic Acid (RNA), has attracted widespread attention due to its diverse and important roles in regulating gene expression. miRNA acts as either oncogenes or tumor suppressor in gastric cancer development. Recent evidence has shown the association of miR-211-5p with poor prognosis of gastric cancer[9]. miR-211-5p is capable of inhibiting gastric cancer cell proliferation and metastasis, and it can be regulated by MTN[10]. However, whether miR-211-5p is regulated via MTN remains unknown.
This work mainly investigated the effects of MTN on AGS cell biological behaviors and explored its potential mechanisms involving miR-211-5p and the Nuclear Factor-Kappa B (NF-κB) pathway. The goal is to provide new insights into the development of gastric cancer.
Materials and Methods
Materials:
Gastric cancer cells AGS (Shanghai Yaji); MTN (National Institutes for Food and Drug Control); anti-miR-NC, miR-211-5p inhibitor and mimic (Shanghai GenePharm); 3-(4,5-Dimethylthiazol- 2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) (American Sigma); Bicinchoninic Acid (BCA) protein assay kit and Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase activity quantitative detection kit (Shanghai Chengong); Malondialdehyde (MDA) and Superoxide Dismutase (SOD) analysis kits (Shanghai Beyotime), phosphorylated (p)-p65 antibody, β-actin antibody, Bcl-2-Associated X-Protein (BAX) antibody, cleaved caspase-3 antibody, (American Abcam); p-IκBα antibody (American Cell Signaling Technology); cell apoptosis detection kit (American BioVision); complementary DNA (cDNA) synthesis kit (Beijing TianGen); and SYBR Green Mix kit (Takara, Japan).
Cell culture and passaging:
Gastric cancer cells AGS were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10 % fetal bovine serum at 37° with 5 % Carbon dioxide (CO2), with 1:2 ratio for passaging.
Experimental groups and treatment:
This study contained 8 groups, including NC, 5 μmol/l MTN, 10 μmol/l MTN, 20 μmol/l MTN, anti-miR-NC, anti-miR-211-5p, 20 μmol/l MTN+miR-NC, and 20 μmol/l MTN+miR- 211-5p groups. MTN was diluted in the culture medium and subjected to 48 h incubation. During transfection, 6-well plates were seeded with AGS cells, which were transfected with oligonucleotides according to the Lipofectamine 2000 protocol when reaching approximately 70 % confluence. Cells were collected and then exposed to 20 μmol/l MTN for 48 h after cell transfection. Subsequent experiments were performed according to the above grouping.
MTT assay:
AGS cells were collected from each group and seeded into 6-well plates. Then, the culture plates were placed in incubators. After 48 h, MTT was added to the 6-well plates according to the guidebook, and the reaction was allowed to proceed for 4 h. The produced crystals were dissolved in 150 μl of Dimethyl Sulfoxide (DMSO). An enzyme-labeled microplate was applied to analyze samples.
Flow cytometry:
AGS cells from each group (2×105) were seeded in 24-well plates, which were subsequently transferred to an incubator. The cells were stained with cell apoptosis detection kit in the dark for 15 min following incubation of 48 h. Apoptosis rate was analyzed using a FACScan flow cytometer.
Western blot:
AGS cells from each group were lysed in protein extraction lysis buffer. After detecting sample concentration through a BCA protein assay. Protein samples were separated and then blocked. After taking them from incubator boxes, the membranes were incubated with primary antibodies against BAX (1:1000), cleaved caspase-3 (1:1000), p-p65 (1:2000), p-IκBα (1:1000), and β-actin (1:1000). Subsequently, protein bands were visualized using an ECL detection reagent. Band intensities were quantified using Image Lab software.
Colorimetric assay:
AGS cells from each group (1×106) were collected. As per guidebook provided with the MDA detection kit and SOD detection kit, MDA content and SOD activity in cell culture supernatant were analyzed, respectively.
Chemiluminescence assay:
AGS cells from each group (1×106) were collected after digestion using trypsin solution. NADPH oxidase activity in AGS cells was quantified using the Chemiluminescence assay with NADPH oxidase activity detection kit.
Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR):
Cell samples were digested using trypsin solution and then exposed to Trizol reagent. cDNA was synthesized utilizing the cDNA synthesis kit. After measuring their concentration, cDNA samples were diluted using double distilled Water (ddH2O) for quantitative analysis using the SYBR Green Mix kit on an Applied Biosystems ABI 7500 instrument with specific primers for miR-211-5p, (forward 5'-CGCTTCCCTTTGTCATCCT-3' and reverse 5'-TATGGTTTTTGACTGTGTGAT-3') and U6 snRNA, (forward 5'-CTCGCTTCGGCAGCACA-3' and reverse 5'-ACGCTCTCACGAATTTGCGT-3'). miR-211-5p expression was calculated using the 2-ΔΔCt method.
Statistical analysis:
Data are presented as mean±standard deviation. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 22.0 software. One-way analysis of variance, t-test and Student-Newman-Keuls (SNK) q-test was used for comparisons. p<0.05 indicated statistically significant.
Results and Discussion
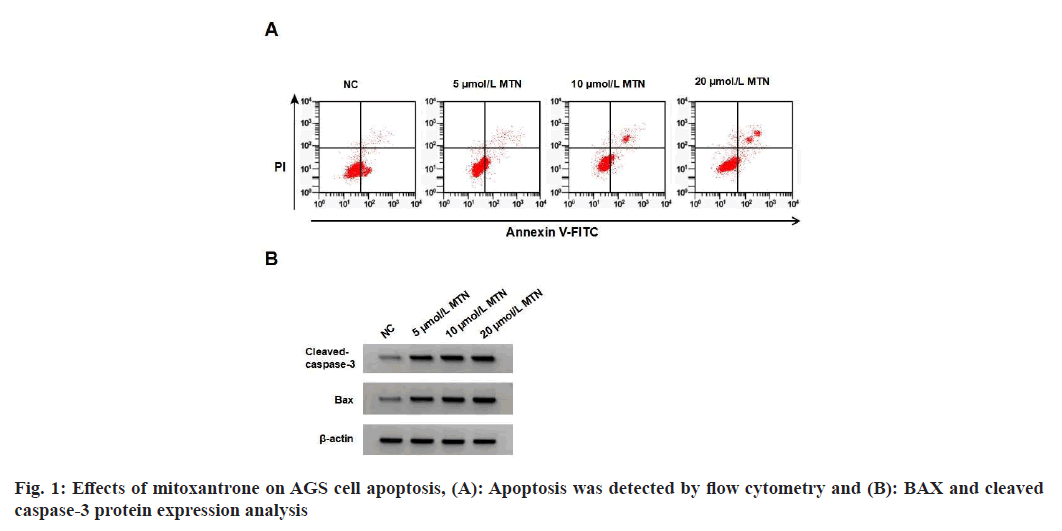
TAGS cell proliferation was decreased, apoptosis rate was increased, and BAX and cleaved caspase-3 protein levels were elevated in the MTN treatment groups when compared to the Negative Control (NC) group (Table 1 and fig. 1). MTN treatment increased MDA content and NADPH oxidase activity and decreased SOD activity (Table 2).
| Group | A value | Apoptotic rate (%) | BAX | Cleaved caspase-3 |
|---|---|---|---|---|
| NC | 1.086±0.10 | 7.16±0.61 | 0.32±0.03 | 0.43±0.03 |
| 5 μmol/l MTN | 0.821±0.07* | 13.46±1.02* | 0.56±0.04* | 0.610.05* |
| 10 μmol/l MTN | 0.601±0.05* | 19.76±1.61* | 0.71±0.06* | 0.78±0.06* |
| 20 μmol/l MTN | 0.517±0.05* | 25.01±2.14* | 0.83±0.07* | 0.91±0.08* |
| F | 117.148 | 250.831 | 158.073 | 116.664 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with NC, *p<0.05
Table 1: Effects of mitoxantrone on AGS aell proliferation and apoptosis (X?±S, N=9)
| Group | MDA (μmol/g) | SOD (U/mg) | NADPH oxidase activity |
|---|---|---|---|
| NC | 19.86±1.53 | 16.13±1.22 | 0.94±0.09 |
| 5 μmol/l MTN | 25.93±1.72* | 13.01±1.05* | 1.68±0.12* |
| 10 μmol/l MTN | 31.25±2.73* | 8.35±0.73* | 2.28±0.18* |
| 20 μmol/l MTN | 39.16±3.24* | 5.86±0.43* | 3.17±0.25* |
| F | 103.868 | 231.004 | 273.125 |
| p | 0.000 | 0.000 | 0.000 |
Note: Compared with NC, *p<0.05
Table 2: Effects Of mitoxantrone on MDA content, SOD activity and NADPH Oxidase activity in AGS cells (X?±S, N=9)
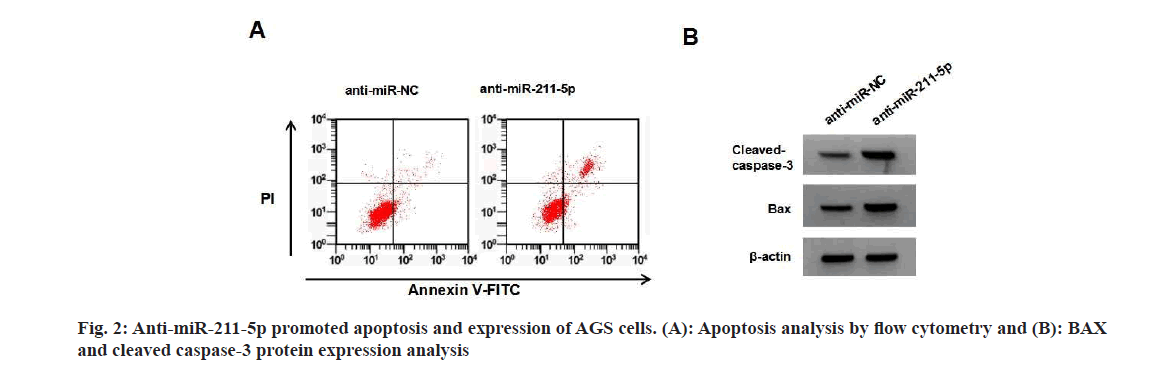
miR-211-5p expression in AGS cells was reduced by 0.17, 0.39, and 0.57 after MTN treatment (5, 10, and 20 μmol/l) (Table 3). miR-211-5p expression, cell proliferation and SOD activity were lower in the anti-miR-211-5p group, while apoptosis rate, BAX, cleaved caspase-3 protein levels, MDA content and NADPH oxidase activity were higher compared to the anti-miR-NC group (Table 4 and fig. 2).
| Group | miR-211-5p |
|---|---|
| NC | 1.00±0.10 |
| 5 μmol/l MTN | 0.83±0.08* |
| 10 μmol/l MTN | 0.61±0.05* |
| 20 μmol/l MTN | 0.43±0.02* |
| F | 116.067 |
| p | 0.000 |
Note: Compared with NC, *p<0.05
Table 3: Effects of mitoxantrone on miR-211-5p expression (X?±S, N=9)
| Group | miR-211-5p | A value | Apoptotic rate (%) | BAX | Cleaved caspase-3 | MDA (μmol/g) | SOD (U/mg) | NADPH oxidase activity |
|---|---|---|---|---|---|---|---|---|
| Anti-miR-NC | 1.00±0.09 | 1.091±0.09 | 7.20±0.58 | 0.33±0.03 | 0.45±0.04 | 19.73±1.61 | 16.03±1.31 | 0.96±0.10 |
| nti-miR-211-5p | 0.47±0.03* | 0.534±0.05* | 21.04±1.73* | 0.76±0.07* | 0.91±0.08* | 37.15±2.43* | 7.02±0.61* | 2.38±0.17* |
| F | 16.76 | 16.23 | 22.755 | 16.939 | 15.429 | 17.928 | 18.705 | 21.599 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with anti-miR-NC, *p<0.05
Table 4: anti-miR-211-5p inhibited the proliferation and promoted oxidative stress and apoptosis Of AGS Cells (X?±S, N=9)
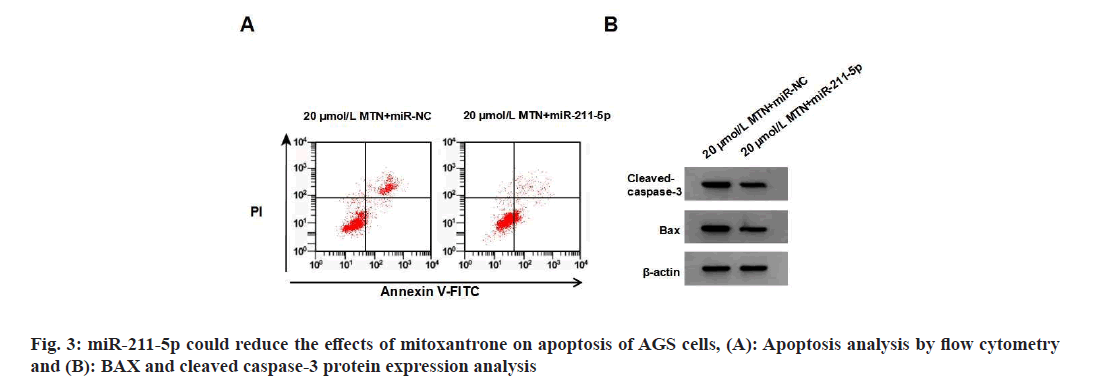
miR-211-5p expression, cell proliferation and SOD activity were higher in the 20 μmol/l MTN+miR- 211-5p group than in the 20 μmol/l MTN+miRNC group, while apoptosis rate, BAX, cleaved caspase-3 protein expression levels, MDA content, and NADPH oxidase activity were lower compared to the 20 μmol/l MTN+miR-NC group (Table 5 and fig. 3).
| Group | miR-211-5p | A values | Apoptotic rate (%) | BAX | Cleaved caspase-3 | MDA (μmol/g) | SOD (U/mg) | NADPH oxidase activity |
|---|---|---|---|---|---|---|---|---|
| 20 μmol/l MTN+miR-NC | 1.00±0.10 | 0.520±0.05 | 25.10±2.07 | 0.85±0.07 | 0.93±0.07 | 39.25±3.17 | 5.81±0.41 | 3.14±0.24 |
| 20 μmol/l MTN+miR-211-5p | 1.92±0.15* | 0.943±0.08* | 9.13±0.73* | 0.41±0.03* | 0.45±0.04* | 15.73±1.25* | 15.34±1.19* | 1.17±0.10* |
| t | 15.310 | 13.451 | 21.827 | 17.332 | 17.861 | 20.707 | 22.715 | 22.731 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with 20 μmol/l MTN+miR-NC, *p<0.05
Table 5: Mir-211-5p reduced the effects of mitoxantrone on proliferation, apoptosis and oxidative stress Of AGS cells (X?±S, N=9)
p-p65 and p-IκBα protein levels were decreased by 0.42 and 0.41, respectively, in the 20 μmol/l MTN group compared to the NC group. Compared to the 20 μmol/l MTN+miR-NC group, p-p65 and p-IκBα protein levels were increased by 0.44 and 0.41, respectively, in the 20 μmol/l MTN+miR-211-5p group (Table 6 and fig. 4).
| Group | p-p65 | p-IκBα |
|---|---|---|
| NC | 0.82±0.07 | 0.73±0.06 |
| 20 μmol/l MTN | 0.40±0.03* | 0.32±0.03* |
| 20 μmol/l MTN+miR-NC | 0.41±0.04 | 0.34±0.03 |
| 20 μmol/l MTN+miR-211-5p | 0.85±0.08# | 0.75±0.06# |
| F | 161.217 | 224.667 |
| p | 0.000 | 0.000 |
Note: Compared with NC, *p<0.05 and compared with 20 μmol/l MTN, #p<0.05
Table 6: Effects of MTN and miR-211-5p on p-p65 and p-IκBα protein expression (X?±S, N=9)
Research has shown that MTN can weaken the proliferative activity of cancer cells[7-11] and induce COC1 and RH35 cell apoptosis via enhancing activated caspase-3 and BAX protein expression. These studies confirm the inhibitory effect of MTN on tumor cell malignancy as well as its anticancer potential. The present work investigated the therapeutic effect of MTN on gastric cancer and found that MTN inhibited AGS cell proliferation and induced cell apoptosis. Oxidative stress refers to a state where oxidation reactions outnumber antioxidant reactions, leading to an imbalance between oxidation and antioxidation that can cause increased secretion of inflammatory cytokines, promote cell senescence, and lead to diseases. This is a characteristic of tumor cells that can be throughout the entire process of tumor development[12]. SOD can catalyze the disproportionation of hydrogen peroxide and oxygen. Therefore, the expression and activation of SOD have a significant impact on cellular oxidative reactions[13]. MDA is a common oxidative indicator that can reflect the degree of peroxidation[14]. NADPH oxidase is a multi-component complex that catalyzes the reaction from molecular oxygen to superoxide and hydrogen peroxide[15]. We discovered MTN could promote oxidative stress in AGS cells, manifested as a decrease in SOD activity, an increase in MDA content, and an increase in NADPH oxidase activity. Therefore, MTN could inhibit AGS cell proliferation, promote oxidative stress, and induce cell apoptosis.
Some studies have shown anti-tumor property of miR-211-5p, such as in bladder cancer where it is downregulated, inhibits bladder cancer cell proliferation and motility, and increased apoptotic rates[16-19]. This miRNA binds to proline-rich protein 11 to promote osteosarcoma cell apoptosis and inhibit cell migration[20]. However, several studies reported miR-211-5p levels were elevated in gastric cancer tissues[9] as well as lung cancer cells[21], and miR-211 can target and negatively regulate Phosphatase and Tensin homolog (PTEN) to accelerate human lung squamous cell carcinoma cell proliferation[22]. In addition, in an in vitro model of Alzheimer's disease, the overexpression of circ_0001588 through upregulating pathways such as miR-211-5p reduces the production of reactive oxygen species and MDA levels while increasing SOD and glutathione levels[23]. The detailed function of this miRNA in gastric cancer remains to be clarified. This study confirmed anti-miR-211-5p could inhibit gastric cancer cell proliferation, SOD activity, and promote apoptosis, MDA content, and NADPH oxidase activity in AGS cells, which were similar to latter studies[9,21,22]. In addition, MTN could downregulate miR-211-5p expression, as revealed by cell assays. miR-211- 5p could weaken the effect of MTN on AGS cell malignancy. This indicates that MTN’s inhibitory effect on gastric cancer cells may be achieved through downregulating miR-211-5p.
NF-κB, an important and well-studied transcription factor, regulates a lot of downstream stimuli oncogene in various tumors, including gastric cancer[24]. The pathway regulates cell proliferation, survival, angiogenesis, and cancer metastasis. Aberrant activation of NF-κB can promote cell proliferation, and suppress tumor suppressor genes such as p65[25]. The NF-κB pathway has been shown to be aberrantly activated in gastric cancer, and associated with poor prognosis[26]. As reported, aloe-emodin inhibited gastric cancer cell growth through regulation of NADPH oxidase and oxidative stress-mediated activation of NF- κB pathway[24]. Astragaloside IV exhibits antiproliferative and pro-apoptotic effects in liver cancer cells (HepG2) by modulating oxidative stress and NF-κB pathway[27]. We found p-p65 and p-IκBα protein expression were significantly inhibited by MTN, suggesting that MTN can inhibit the activation of NF-κB pathway. However, cell assays subsequently confirmed that this effect could be attenuated by miR-211-5p. The evidence indicates that inhibitory effects of MTN on AGS cell proliferation as well as promoting effects on oxidative stress, and apoptosis involved the miR- 211-5p/NF-κB pathway.
In summary, this study found that MTN could attenuate AGS cell proliferation, induce oxidative stress, and promote apoptosis by inhibiting the miR-211-5p/NF-κB pathway. However, we only discuss the effects and mechanisms of MTN in an in vitro model, which has certain limitations. Nevertheless, this research provides a foundation for gastric cancer therapy using MTN and offers new potential drug targets for personalized medicine.
Funding:
This work was supported by Hunan Provincial Natural Science Foundation Project (2021J80015).
Conflict of interests:
The authors declared no conflict of interests.
References
- Matsuoka T, Yashiro M. Novel biomarkers for early detection of gastric cancer. World J Gastroenterol 2023;29(17):2515.
- Hou W, Zhao Y, Zhu H. Predictive biomarkers for immunotherapy in gastric cancer: Current status and emerging prospects. Int J Mol Sci 2023;24(20):15321.
[Crossref] [Google Scholar] [PubMed]
- Li S, Zhang F, Li J, Hu X, Zhao W, Zhang K, et al. The role of the Epstein-Barr virus-encoded BARF1 gene expressed in human gastric epithelial cells. Turk J Gastroenterol 2020;31(11):775.
[Crossref] [Google Scholar] [PubMed]
- Yu Y, Wu Y, Zhang Y, Lu M, Su X. Oxidative stress in the tumor microenvironment in gastric cancer and its potential role in immunotherapy. FEBS Open Bio 2023;13(7):1238-52.
[Crossref] [Google Scholar] [PubMed]
- Nakonieczna S, Grabarska A, Kukula-Koch W. The potential anticancer activity of phytoconstituents against gastric cancer: A review on in vitro, in vivo, and clinical studies. Int J Mol Sci 2020;21(21):8307.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Wang L, Wen Z, Yao J. Integrated analysis identifies oxidative stress genes associated with progression and prognosis in gastric cancer. Sci Rep 2021;11(1):3292.
[Crossref] [Google Scholar] [PubMed]
- Wei J, Ma W, Li H. Proliferation and apoptosis of ovarian cancer cell COC1 induced by MXT and its in vitro mechanism. Public Med Forum Magazine 2014;18(31):4185-8.
- ZhangY, Gan Y, Qi J. Mitoxantrone induced apoptosis through ROS in rat hepatoma RH35 cell line. Chin Animal Husbandry Veterinary Med 2016;43(12):3245-50.
- Zhang Y, Guo X, Gao X. Correlation between expression of miR-221-5p and p27Kip1 in gastric cancer tissues and clinicopathogical features. Int J Digest Dis 2020;40(6):371-6.
- Wang D, Xu B, Yang T. Etomidate inhibits proliferation, migration and invasion of gastric cancer cells by regulating the expression of microRNA-211-5p/ROBO1. Chin Pharm J 2020;55(20):1686-95.
- Li C, Liu X, Liang N. Effects of mitoxantrone on proliferation of MCF-7 cell lines. J Mod Oncol 2010;18(2):236-8.
- Sun X, Xue D, Zhang K, Jiang F, Li D. Acrid-release and bitter-downbearing therapy and Banxia Xiexin decoction regulate Wnt/β-catenin pathway, inhibit proliferation and invasion, and induce apoptosis in gastric cancer cells. Am J Transl Res 2021;13(6):6211.
[Google Scholar] [PubMed]
- Kim SH, Lim JW, Kim H. Astaxanthin prevents decreases in superoxide dismutase 2 level and superoxide dismutase activity in Helicobacter pylori-infected gastric epithelial cells. J Cancer Prev 2019;24(1):54-8.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Chen X, Huang Z, Chen D, Yu B, Yu J, et al. Dietary ferulic acid supplementation improves antioxidant capacity and lipid metabolism in weaned piglets. Nutrients 2020;12(12):3811.
[Crossref] [Google Scholar] [PubMed]
- Liang S, Ma HY, Zhong Z, Dhar D, Liu X, Xu J, et al. NADPH oxidase 1 in liver macrophages promotes inflammation and tumor development in mice. Gastroenterology 2019;156(4):1156-72.
[Crossref] [Google Scholar] [PubMed]
- Qin X, Zhang J, Lin Y, Sun XM, Zhang JN, Cheng ZQ. Identification of miR-211-5p as a tumor suppressor by targeting ACSL4 in hepatocellular carcinoma. J Transl Med 2020;18:326.
[Crossref] [Google Scholar] [PubMed]
- Wu L, Xiao H, Hong Y, Xie M, Yu Y, Jiang L. CircRNA circ_0000118 regulates malignancy of cervical cancer cells by regulating miR-211-5p/miR-377-3p/AKT2 axis. Biochem Genet 2023;61(4):1-20.
[Crossref] [Google Scholar] [PubMed]
- Chen X, Xu Y, Zhou Z, Zhao P, Zhou Z, Wang F, et al. CircUSP10 promotes liver cancer progression by regulating miR-211-5p/TCF12/EMT signaling pathway. Heliyon 2023;9(10):e20649.
[Crossref] [Google Scholar] [PubMed]
- Wang W, Liu Z, Zhang X, Liu J, Gui J, Cui M, et al. miR-211-5p is down-regulated and a prognostic marker in bladder cancer. J Gene Med 2020;22(12):e3270.
[Crossref] [Google Scholar] [PubMed]
- Song D, Yang K, Wang W, Tian R, Wang H, Wang K. MicroRNA-211-5p promotes apoptosis and inhibits the migration of osteosarcoma cells by targeting proline-rich protein PRR11. Biochem Cell Biol 2020;98(2):258-66.
[Crossref] [Google Scholar] [PubMed]
- Kang M, Shi J, Li B, Luo M, Xu S, Liu X. LncRNA DGCR5 regulates the non-small cell lung cancer cell growth, migration, and invasion through regulating miR-211-5p/EPHB6 axis. Biofactors 2019;45(5):788-94.
[Crossref] [Google Scholar] [PubMed]
- Jia R, Wang H. miR-211 proliferates lung squamous cell carcinoma proliferation and invasion through targeting PTEN gene. J Clin Pulm Med 2018;23(11):76-80.
- Zhu R, Qi X, Liu C, Wang D, Li L, Liu X, et al. The silent information regulator 1 pathway attenuates ROS-induced oxidative stress in Alzheimer’s disease. J Integr Neurosci 2020;19(2):321-32.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Tang T, Wang S, Cai T, Tao H, Zhang Q, et al. Aloin inhibits the proliferation and migration of gastric cancer cells by regulating NOX2–ROS mediated pro-survival signal pathways. Drug Design Dev Ther 2020;14:145-55.
[Crossref] [Google Scholar] [PubMed]
- Wu Z, Li J, Zhang Y, Hu L, Peng X. CFTR regulates the proliferation, migration and invasion of cervical cancer cells by inhibiting the NF-κB signalling pathway. Cancer Manag Res 2020;12:4685-97.
[Crossref] [Google Scholar] [PubMed]
- Chen R, Yang M, Huang W, Wang B. Cascades between miRNAs, lncRNAs and the NF-κB signaling pathway in gastric cancer. Exp Ther Med 2021;22(1):769.
[Crossref] [Google Scholar] [PubMed]
- An XC, Zhu RX, Lin SM, Shi L. Mechanism of astragaloside IV promoting proliferation and apoptosis of hepatoma cells by inhibiting ROS/NF-κB signaling pathway. Mod Digestion Int 2019;12:1399-403.