- *Corresponding Author:
- A. A. Hajare
Department of Pharmaceutical Chemistry, Bharati Vidyapeeth College of Pharmacy, Kolhapur, Maharashtra 416013, India
E-mail: ashok.hajare@bharatividyapeeth.edu
| Date of Received | 13 February 2023 |
| Date of Revision | 31 July 2023 |
| Date of Acceptance | 15 March 2024 |
| Indian J Pharm Sci 2024;86(2):660-666 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The study was performed with a dual approach to develop and validate analytical method for poorly water soluble candesartan cilexetil by enhancing its solubility employing cosolvency technique. In order to enhance drug’s solubility based on its solubility characteristics, phosphate buffer (pH 6.8):ethanol (95 % v/v) mixture at 90:10 proportion was used along with Tween 80 (1 % v/v). Solubility of candesartan cilexetil in phosphate buffer (pH 6.8) enhanced due to the addition of co-solvent ethanol and Tween 80. Developed method obeyed Beer-Lambert’s law in the concentration range between 3-21 μg/ml. The regression coefficient at the wavelength 232 nm was 0.999. The analysis of tablets by the proposed method indicated a good correlation between estimated and label claims. Recovery studies showed that any small difference in drug concentration could be accurately determined. Low values of limit of detection and limit of quantitation indicated that the proposed method is sensitive, economic, accurate, precise, and robust for routine analysis of candesartan cilexetil in bulk and dosage forms.
Keywords
Method development, validation, cosolvency, cosolvent, candesartan cilexetil
Hydrophobic drugs have low solubility in the aqueous medium. Low solubility of such drugs causes them to excrete from the gastrointestinal tract prior to its complete dissolution and absorption into the systemic circulation. This results in low absorption, low bioavailability and poor dose proportionality[1]. Hydrophobic drugs of low aqueous solubility frequently present many problems in the formulation and manufacturing of oral solid dosage forms arising from poor wetting and dissolution characteristics[2]. Solubility is one of the important characteristic of a drug to achieve the desired concentration of drug in systemic circulation to exhibit pharmacological response[3]. Such drugs belong to poorly water-soluble category and categorised under Biopharmaceutics Classification System (BCS) class II and class IV[4]. In the process of absorption of the drug from the oral route, dissolution is the rate-limiting step for hydrophobic drugs; therefore it is necessary to enhance dissolution of such drugs to ensure its maximum therapeutic utility. In dissolution, solid substance goes into solution and extent to which it gets dissolved under a given set of conditions is referred to as the solubility of the substance in the solvent[5].
Various strategies have been developed to enhance the dissolution of hydrophobic drugs. These includes use of surfactants[2], application of solid dispersion, particle size reduction by micronization or nanonization[3], use of pro-drugs, cosolvency, use of water-soluble salts, or polymorphic forms[4]; microencapsulation formation of inclusion complexes, and inclusion of drug solutions[5] or liquid drugs into soft gelatin capsules or specially sealed hard shell capsules[6]; and formation of self-emulsifying drug delivery systems[1,6]. Cosolvency is the addition of water-miscible solvent to the solution of a poorly water-soluble drug to improve its aqueous solubility[7]. The common cosolvents used for analytical method development in the pharmaceutical industry are ethanol, polyethylene glycols, propylene glycol, glycerine and glycofural[8]. Cosolvent system works by reducing the interfacial tension between the aqueous solution and hydrophobic solute. Cosolvency is an effective method to increase aqueous solubility of poor water-soluble drugs and can be used to accurately estimate Active Pharmaceutical Ingredient (API) in regular dosage form analysis[9].
Candesartan is a tetrazole derivative with five-member heterocyclic ring with 4 nitrogen atoms. Candesartan cilexetil is chemically 2-ethoxy-3-[21-(1H-tetrazole-5-yl) biphenyl-4ylmethyl]-3H-benzoimiadazole-4-carboxylic acid 1-cyclohexyloxy carbonyloxy ethyl ester, (fig. 1) with chemical formula C33H34N6O6 and molecular weight 610.66 g/mol. It is white to off-white powder with a melting point of 157°-160°. It is practically soluble in dimethyl formamide, acetone, methanol, 0.1 N sodium hydroxide, and insoluble in water[10-15]. Clinically it is used in the form of an ester prodrug as candesartan cilexetil. It is an Angiotensin-Receptor Blocker (ARB) used in the treatment of hypertension[14,15]. Therefore, the present study was designed to develop and validate Ultraviolet (UV) spectrophotometric analytical method for poorly soluble candesartan cilexetil using cosolvency technique by increasing its aqueous solubility using ethanol (95 % v/v) as a cosolvent with Tween 80 (1 % v/v).
Materials and Methods
Materials:
Candesartan cilexetil was a kind gift by Smilax Laboratories Ltd. Hyderabad; ethanol and Tween 80 were obtained from Research Lab, Mumbai. All other chemicals, reagents, and solutions used were of analytical grade. The spectrophotometric determinations were performed on UV-visible double beam spectrophotometer (JASCO V-550 UV and UV-1800, Shimadzu, Japan) and digital analytical balance (Shimadzu Aux 220, Japan).
Methods:
Solubility study: The solubility of candesartan cilexetil was estimated by saturation solubility study in Phosphate Buffer Solution (PBS) pH 6.8. An excess amount of drug was added to 10 ml vials containing PBS (pH 6.8) and shaken for 72 h on a rotary shaker at 28±1° to obtain saturated solution. In order to estimate solubility, candesartan cilexetil saturated solution was diluted with PBS (pH 6.8):ethanol (95 %) mixture (9:1) along with 1 % Tween 80 to dissolve the drug completely. Ethanol was employed to enhance the drug solubility in PBS (pH 6.8) through its polarizability whereas tween 80 was used as solubilizer due to its high Hydrophilic–Lipophilic Balance (HLB) (>10). Prepared solutions were filtered using Whatman filter paper #41, and the resulting filtrates were suitably diluted and analyzed spectrophotometrically against solvent blank[15].
Preparation of working standard stock solution:
A solution of 1000 μg/ml strength was prepared by dissolving 100 mg of drug in 90 ml of 9:1 mixture of PBS (pH 6.8):ethanol (95%) in 100 ml volumetric flask. The contents were dissolved with aid of drop-wise addition of 1 % Tween 80 solutions with shaking and sonication for 15 min and final volume was made up to 100 ml with solvent mixture. Accurately 10 ml of this solution was transferred to 100 ml of volumetric flask and the final volume was made up with PBS (pH 6.8) to obtain resultant solution with concentration of 100 μg/ml and used as stock solution.
Preparation of sample solution:
Serial dilutions of stock solution (100 μg/ml) were made to obtain test solutions of concentration 3, 6, 9, 12, 15, 18 and 21 μg/ml.
Determination of absorption maxima:
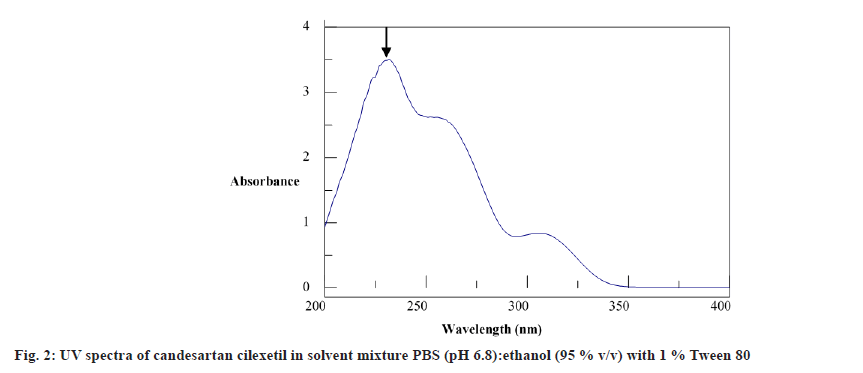
The stock solution of the drug was scanned in the spectrum mode within the wavelength range of 200-400 nm. The λmax of candesartan cilexetil was found to be 232 nm.
Preparation of standard calibration curve:
Absorbance of serially diluted solutions was recorded at 232 nm and the calibration curve was constructed to acquire the linearity and regression equation.
Assay of marketed formulation:
The amount of candesartan cilexetil present in marketed formulation was determined by the method described in the assay[11]. Commercially available candesartan cilexetil tablets were purchased from a local market. Twenty tablets containing a label claim of 8 mg of the drug were weighed and powdered in mortar for analysis. The tablet powder equivalent to 8 mg of candesartan cilexetil was weighed and dissolved in a sufficient quantity of PBS (pH 6.8):ethanol (95 %) mixture 9:1 with 1 % Tween 80 by shaking and sonicated for 15 min. The solution was filtered through Whatman filter paper #41, suitably diluted with PBS (pH 6.8) to make final volume 100 ml, and assayed at 232 nm, using a UV-visible double beam spectrophotometer. None of the excipients employed in the formulation of the drug interfered in the analysis by the proposed method[15].
Method validation:
The proposed method was validated for the following parameters as per International Council for Harmonisation (ICH) guidelines[16-20].
Accuracy: Accuracy of the method was estimated by standard addition recovery method. A standard stock solution of candesartan cilexetil of known concentration was added at three different concentrations to the previously examined sample solutions, namely 80 %, 100 % and 120 %. These alternatives were re-examined and at each level, solutions were analyzed thrice and the accuracy was calculated and reported as a percentage of the recovery. To ensure the accuracy and reproducibility of the results, tablet powder equivalent to 8 mg of candesartan cilexetil was transferred to a 100 ml of volumetric flask and dissolved in a sufficient quantity of PBS (pH 6.8):ethanol (95 %) (9:1) with 1 % Tween 80. Accurately, 2 mg candesartan cilexetil in bulk form was added to the same volumetric flask. The content of the flask is dissolved by shaking and sonication for 15 min. The solution was filtered through Whatman filter paper #41, suitably diluted with PBS (pH 6.8) to make up volume 100 ml and absorbance was measured at 232 nm against corresponding solvent blank. Drug content was calculated and percentage recovery was calculated. A similar procedure was adopted using 4 mg and 6 mg of candesartan cilexetil bulk drug. The drug concentration was determined, and percent recoveries were estimated.
Inter- and intra-day precision: The inter-day concentration of the drug was calculated by measuring the absorbance of the working standard solutions on the same day at an interval of 1 h, whereas the intra-day concentration of the drug was calculated on next 3 d at the same laboratory conditions.
Detection limit and quantification limit: Limit of Detection (LOD) and Limit of Quantitation (LOQ) of candesartan cilexetil by the proposed method were determined using the calibration curve. LOD and LOQ were calculated as 3.3 σ/S and 10 σ/S, respectively, where S is the slope of the calibration curve and σ is the Standard Deviation (SD) of response.
Short-term stability: In performing stability studies, prepared solutions were kept for 24 h at ambient temperature, and the absorbance of this solution was recorded on a subsequent day (Table 1).
| Concentration (µg/ml) | Absorbance (nm)* |
|---|---|
| 0 | 0 |
| 3 | 0.155 |
| 6 | 0.2829 |
| 9 | 0.4402 |
| 12 | 0.606 |
| 15 | 0.7399 |
| 18 | 0.8697 |
| 21 | 1.0151 |
Note: *n=3
Table 1: Linearity table of candesartan cilexetil in working standard solution.
Ruggedness: Ruggedness of the method was determined by performing analysis employing two different analysts and determining absorbance of solution having concentration 21 μg/ml. The results were calculated as percentage Relative Standard Deviation (RSD) and are presented in Table 2.
| Concentration (μg/ml) | Absorbance (λmax=203nm) | Calculated amount (μg/ml) | Statistical analysis | |
|---|---|---|---|---|
| Analyst-1 | 21 | 1.0151 | 21 | Mean=21.08 SD=0.0816 % RSD=0.0809 |
| 21 | 1.0191 | 21.1 | ||
| 21 | 1.0221 | 21.2 | ||
| 21 | 1.0181 | 21 | ||
| 21 | 1.0191 | 21.1 | ||
| Analyst-2 | 21 | 1.0162 | 21.2 | Mean=21.13 SD=0.0471 % RSD=0.0458 |
| 21 | 1.0185 | 21.1 | ||
| 21 | 1.0188 | 21.1 | ||
| 21 | 1.019 | 21.1 | ||
| 21 | 1.0159 | 21 |
Table 2: Statistical analysis for ruggedness of the proposed method.
Robustness: Robustness is the reliability of an analysis with respect to deliberate variations in method parameters. Hence, robustness of the method was determined by evaluating linearity of method using two different instruments namely; Jasco UV V-550 and Shimadzu UV-1800, Japan. The results are presented in as % RSD (Table 3).
| Concentration (μg/ml) | Instrument absorbance (λmax=232 nm) | Mean | ||
|---|---|---|---|---|
| Instrument 1 | ||||
| 0 | 0 | 0 | 0 | 0 |
| 3 | 0.155 | 0.1495 | 0.1634 | 0.1559 |
| 6 | 0.2829 | 0.2905 | 0.3144 | 0.2959 |
| 9 | 0.4402 | 0.4202 | 0.4702 | 0.4435 |
| 12 | 0.606 | 0.5821 | 0.6001 | 0.596 |
| 15 | 0.7399 | 0.7212 | 0.7199 | 0.727 |
| 18 | 0.8697 | 0.8934 | 0.8897 | 0.8897 |
| 21 | 1.0151 | 1.0011 | 1.0022 | 1.0061 |
| Instrument 2 | ||||
| 0 | 0 | 0 | 0 | 0 |
| 3 | 0.1459 | 0.1478 | 0.1537 | 0.1491 |
| 6 | 0.2766 | 0.2845 | 0.3054 | 0.2888 |
| 9 | 0.4488 | 0.419 | 0.4646 | 0.4441 |
| 12 | 0.6102 | 0.5959 | 0.6023 | 0.6028 |
| 15 | 0.7222 | 0.7275 | 0.7278 | 0.7258 |
| 18 | 0.8597 | 0.8923 | 0.8891 | 0.8803 |
| 21 | 0.9999 | 1.0345 | 1.0234 | 1.0192 |
Table 3: Statistical analysis for robustness of the proposed method.
Results and Discussion
The results of saturation solubility study of candesartan cilexetil indicated an increase in its solubility in PBS (pH 6.8) due to the addition of co-solvent ethanol and 1 % Tween 80. Solubility of candesartan cilexetil in PBS was 0.012 mg/ml, which was significantly improved to 0.6 mg/ml in presence of 95 % v/v ethanol and 1 % Tween 80. Therefore, this solvent mixture was optimized to determine its proportionate solvent in the analysis of the tablet formulation. Accuracy of the developed method with recovery studies indicated that any small change in the drug concentration in its solution could be accurately determined by the proposed method. Accuracy, reproducibility and precision of the proposed method were further confirmed from percent recovery values, which were close to 100, with a low value of SD and Standard Error (SE) (Table 4).
| Concentration level | Amount API in tablet powder (mg) | Amount of bulk drug added (mg) | % Recovery mean±SD |
|---|---|---|---|
| 0 % | 4 | 2 | 99.9557±0.2020 |
| 6 | 4 | 99.66±0.4667 | |
| 8 | 6 | 101.15±0.2350 | |
| 100 % | 4 | 4 | 99.48±0.4758 |
| 6 | 6 | 99.5763±0.5281 | |
| 8 | 8 | 99.67±0.5140 | |
| 120 % | 4 | 6 | 99.243±0.6772 |
| 6 | 8 | 100.0917±0.0672 | |
| 8 | 10 | 101.07±0.6050 |
Table 4: Statistical analysis of accuracy and recovery studies.
Beer-Lambert’s range for candesartan cilexetil in solution employing 95 % v/v ethanol and 1 % v/v Tween 80 as cosolvent was obtained between 3-21 μg/ml (Table 1). Repetition of result signified precision of method under the same operating conditions over a short interval time and inter-assay precision. In both, inter-day and intra-day precision studies, co-efficient variation was not more than 1 % indicating good intermediate precision (Table 5).
| Concentration found (μg/ml) | Intra-day precision | Inter-day precision | % RSD | |
|---|---|---|---|---|
| Concentration found (μg/ml) mean±SD | % RSD | Concentration found (μg/ml) mean±SD | ||
| 12 | 12.4833±0.3447 | 0.4221 | 11.96±0.1395 | 0.1708 |
| 15 | 14.9022±0.5343 | 0.6544 | 15.28±0.2808 | 0.3439 |
| 18 | 18.2318±0.3234 | 0.3961 | 18.3433±0.4235 | 0.5186 |
Note: *Values are mean±SD, n=3
Table 5: Results of statistical analysis of intra-day assay and inter-day assay.
Spectrum analysis was done and λmax was found to be 232 nm, fig. 2. The regression value at this wavelength was found 0.999 which was close to 1 indicating good correlation between the amount of drug estimated and the label claim. The sensitivity of the proposed method was estimated by Sandell’s sensitivity17 (μg/cm2/0.001 Abs unit) was found at 0.1764 μg cm-2 (Table 6). The low values of LOD, 0.2697 μg/ml and LOQ, 0.5874 μg/ml for drug in PBS (pH 6.8):ethanol (95 %) with 1 % Tween 80 indicated good sensitivity of the proposed method (Table 6).
| Parameter | Observed result |
|---|---|
| Wavelength of maximum absorption (λmax) | 232 nm |
| Beers law limit | 3-21 μg/ml |
| Molar absorptivity | 3.5986×104 |
| Regression equation (y=mx±c) | y=0.048x+0.006 |
| Correlation coefficient* | 0.999 |
| Slope* | 0.048 |
| Intercept* | 0.006 |
| LOD* | 0.2697 μg/ml |
| LOQ* | 0.5874 μg/ml |
| Sandell’s Sensitivity* | 0.1764 μg cm-2 |
Note: *n=3
Table 6: Spectral characteristics of candesartan cilexetil.
To estimate drug precipitation and its stability in co-solvent, a part of the solution was kept at room temperature for 48 h (Table 7). The result claims that estimation of candesartan cilexetil can be done without substantial effect on drug stability as no precipitation was observed. The findings indicated absence of ethanol and tween 80 interference in the determination of drug at the wavelength of 232 nm.
| Concentration (μg/ml) | The concentration found at 24 hrs mean±SD, (μg/ml) | % drug content* mean±SD | % RSD |
|---|---|---|---|
| 15 | 14.9646±0.0033 | 99.78±0.0244 | 0.0244 |
Note: *Values are mean±SD, n=3
Table 7: Short term stability study.
The ruggedness of the method was confirmed by the analysis of formulation by two different analysts for that % RSD was found to be 0.08095 and 0.04589. Robustness was determined by evaluating linearity of method and there were no noticeable changes in linearity (Table 3). The estimated percent label claim was found to be 99.506±0.225 with a SE=0.565 for brand I and 99.123±0.344 with a SE of 0.467 for brand II, respectively (Table 8). The lower values of ruggedness and robustness indicates that use of 95 % v/v ethanol and 1 % v/v Tween 80 as co-solvents would be an effective approach to determine the candesartan cilexetil content in the tablet formulation by UV analysis without any interference. It can thus be concluded that solubilization of candesartan cilexetil through cosolvency approach using 95 % v/v ethanol and 1 % v/v Tween 80 as cosolvent is a good approach to develop simple, accurate, cost effective and precise analytical method over the use of costlier, toxic organic solvents. This method can be successfully employed for the estimation of poor water soluble drugs in routine analysis of dosage forms.
| Tablet formulation | Label claim (mg) | % Label claim estimated* | Standard error |
|---|---|---|---|
| Brand I | 8 mg | 99.506±0.225 | 0.565 |
| Brand II | 8 mg | 99.123±0.344 | 0.467 |
Note: *Values are mean±SD, n=3
Table 8: Analysis of candesartan cilexetil in marketed tablet formulation.
The cosolvency approach applied to develop and validate an analytical method for poorly soluble candesartan cilexetil successfully produced a selective, accurate, precise, sensitive and robust UV spectroscopic method for concurrent quantitative analysis of candesartan cilexetil in bulk and tablet formulations. The developed method showed good recovery of the analyte without interference of excipients in the formulation. As determined by the validation study, the developed UV spectrophotometric method can be exploited for routine analysis of the candesartan cilexetil in bulk and pharmaceutical formulations.
Acknowledgement:
The author(s) would like to thank Smilax laboratories Ltd., Hyderabad for providing gift samples of candesartan cilexetil.
Conflict of interests:
The authors declare no competing interests.
References
- Elkordy AA, Tan XN, Essa EA. Spironolactone release from liquisolid formulations prepared with Capryol™ 90, Solutol® HS-15 and Kollicoat® SR 30 D as non-volatile liquid vehicles. Eur J Pharm Biopharm 2013;83(2):203-23.
[Crossref] [Google Scholar] [PubMed]
- Brown S, Rowley G, Pearson JT. Surface treatment of the hydrophobic drug danazol to improve drug dissolution. Int J Pharm 1998;165(2):227-37.
- Doney AB. Mechanism of solubility of liquisolid formulation in non-volatile solvent: A review. Int J Pharm Pharm Sci 2012;4(3):710-5.
- Manogar PG, Hari BV, Devi DR. Emerging liquisolid compact technology for solubility enhancement of BCS class-II drug. J Pharm Sci Res 2011;3(12):1604.
- Sahil MG, Sharad SP, Shirish VS, Kisan RJ, Vilasrao JK. Liquisolid compact: A new technique for enhancement of drug dissolution. Int J Res Pharm Chem 2011;1(3).
- Javadzadeh Y, Siahi MR, Asnaashari S, Nokhodchi A. Liquisolid technique as a tool for the enhancement of poorly water-soluble drugs and evaluation of their physicochemical properties. Acta pharmaceutica 2007;57(1):99-109.
[Crossref] [Google Scholar] [PubMed]
- Lingam M, Venkateswarlu V. Enhancement of solubility and dissolution rate of poorly water soluble drug using cosolvency and solid dispersion techniques. Int J Pharm Sci Nanotechnol 2008;1(4):349-56.
- Jouyban A. Review of the cosolvency models for predicting solubility of drugs in water-cosolvent mixtures. J Pharm Pharm Sci 2008;11(1):32-58.
[Crossref] [Google Scholar] [PubMed]
- Vittal GV, Deveswaran R, Bharath S, Basavaraj BV, Madhavan V. Development of an analytical method for spectrophotometric estimation of ketoprofen using mixed co solvency approach. Int J Pharm Sci Res 2012;3(4):1053-6.
- O'Neil MJ, editor. The Merck index: An encyclopedia of chemicals, drugs, and biologicals. RSC Publishing; 2013. p. 977.
- Pharmacopoeia I. The Indian Pharmacopoeia commission, central Indian pharmacopoeial laboratory. Government of India, Ministry of health and family welfare, Ghaziabad, India. 2007;1:78-9.
- Remington JP. Remington: The science and practice of pharmacy. Lippincott williams and wilkins; 2006. p. 149.
- Martindale C. The complete drug reference. Sweetman SC, editor. London: Pharmaceutical press; 2009.
- Husain A, Azim MS, Mitra M, Bhasin PS. A review on candesartan: Pharmacological and pharmaceutical profile. J Appl Pharm Sci 2011:12-7.
- Yadav PS, Kondawar MS, Varne BS. Enhancement of dissolution properties of candesartan using liquisolid technique. Int J Adv Pharm Res 2013;4(2):2503-13.
- Ashok PT, Ananda HA. Development and validation of an HPLC-UV Method for the determination of melphalan from lyophilized nanosuspension. Indian J Pharm Educ Res 2019;53(2):316-24.
- Agrawal YP, Agrawal MY, Jadhav SB, Shinde RJ. Development and validation of novel UV spectrophotometric method for the determination of evogliptin tartarate in pharmaceutical dosage form. Indian J Pharm Edu Res 2020;54(4):1174-9.
- Patil KS, Pore YV, Bhise SB. Spectrophotometric estimation of zolpidem in tablets. J Pharm Sci Res 2010;2(1):1.
- Guideline IH. Validation of analytical procedures: Text and methodology. Q2 (R1). 2005;1(20):05.
- Yadav PS, Hajare AA, Patil KS. Development and validation of UV spectrophotometric method for doxazosin mesylate in bulk and tablets. Res J Pharm Technol 2022;15(6):2675-80.