- *Corresponding Author:
- Weiqian Tian
Department of Anesthesiology, Affiliated Hospital of Nanjing University of Chinese Medicine, Jiangsu Province Hospital of Chinese Medicine, Nanjing, Jiangsu 210029, China
E-mail: njucmtwq@163.com
| Date of Received | 15 December 2022 |
| Date of Revision | 06 July 2023 |
| Date of Acceptance | 18 January 2024 |
| Indian J Pharm Sci 2024;86(1):236-245 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To examine the potential benefits of Ginkgo biloba diterpene glumine injection as a treatment for cerebral small vascular disease, as well as the effects of the injection on oxidative stress and brain inflammation in cerebral small vascular disease model rats, as well as the cognitive function of these animals. 24 male Sprague-Dawley rats were split into three groups randomly; a diterpene glumine injection group, a model group, and a normal control group, eight rats in each group. The cerebral small vascular disease model was induced, and rats in the experimental group underwent water maze testing to evaluate their behavioral and cognitive functions. Biochemical methods were employed to analyze oxidase activity and malondialdehyde levels in brain tissue. Nitric oxide content and inducible nitric oxide synthase activity were performed in brain tissue. Brain water content was determined using the weightlessness method, and the percentage of brain infarct volume was calculated via 2,3,5-triphenyltetrazolium chloride staining. Pathological alterations in brain tissue and neuronal death were observed through terminal deoxyuridine triphosphate nick end labeling, haemotoxylin and eosin labeling techniques. Following the establishment of the cerebral small vascular disease model, there was a decrease in cognitive function, an increase in the neurological impairment score, an increase in the infarct area and brain water content, an observation of oxidative stress and inflammatory infiltration in the brain tissue, a decrease in synaptic function, and an increase in the rate of neuronal apoptosis. Following diterpene glumine injection administration, the cerebral small vascular disease model rats cognitive function progressively returned, the damage to their neurological system decreased, the extent of their cerebral infarction shrank, the levels of oxidative stress and inflammatory infiltration in the brain improved. More significantly, in rats with cerebral small vascular disease model disease, diterpene glumine injection reduced the pathogenic alterations in brain tissue, synaptic function, and neuronal death. Diterpene glumine injection can improve the cognitive function of cerebral small vascular disease model rats, reduce the damage of nerve function and the size of cerebral infarction, and improve the apoptosis and pathological development of brain neurons in cerebral small vascular disease model rats by reducing brain oxidative stress and inflammatory infiltration.
Keywords
Cerebral small vessel disease, cognitive impairment, inflammation, oxidative stress, Ginkgo diterpene lactone
The current aging process of society is quickening, and with the advancement and widespread use of imaging, middle-aged and older persons are more likely to be diagnosed with Cerebral Small Vascular Disease (CSVD), which accounts for 20 % to 25 % of ischemic stroke patients[1]. The term CSVD is utilized when discussing the clinical, radiological, and pathological symptoms of the illness brought on by the damage to tiny blood arteries in the brain[2]. The phrase "small blood vessels" encompasses the pathological process present in arterioles, capillaries, venules with vessels having diameter between 40 and 200 μm. On the other hand, the term "pathological structure" primarily denotes the pathological process found in small blood vessels situated beneath the cortex. The diagnosis of CSVD is mostly based on imaging findings, which are classified into four categories namely, cerebral microbleeds, perivascular space, leukoaraiosis, and lacunar infarction. This is because the disease lacks identifiable clinical signs. Magnetic resonance imaging of the head makes it simple to identify the two imaging categories of leukoaraiosis and lacunar infarction. CSVD and cognitive function are intimately associated with each other. Since it can be challenging to diagnose early cognitive impairment, up to 45 % of individuals with dementia develop the disease later on. The most notable clinical symptom of CSVDinduced cognitive impairment is a marked loss in executive and attention function. Other clinical manifestations include psychomotor retardation, attention, planning, delayed recall, and executive dysfunction syndrome. It also involves problems with the gait[3], emotion and behavior[4] and urinary system disorders[5], in addition to cognitive impairment.
As the disease advances, cognitive function deterioration intensifies, accompanied by increasingly apparent challenges that significantly impact the daily lives of middle-aged and elderly individuals. For patients with small cerebral vascular disease, it is important to detect cognitive impairment, control risk factors as early as possible, and formulate individual specific treatment plans according to its etiology and pathogenesis before without delaying the development of the disease. Studies have shown that the chronic low-level inflammatory process can lead to the injury and increase of permeability of endothelial cells in small cerebral vessels[6], which promotes the occurrence of vasculitis. In the pathological progression of CSVD, inflammation (including Tumor Necrosis Factor (TNF) and Interleukin (IL)) leads to immune cell infiltration (such as macrophages and lymphocytes), forming inflammatory foci around small blood vessels in the brain[7,8]. In addition, oxidative stress is a process of cellular damage caused by the accumulation of free radicals and oxidizing substances. In CSVD, oxidative stress can accelerate the aging and failure of endothelial cells in brain and small blood vessels can decrease the stability of blood vessel walls[9]. Oxidative stress can also affect cerebral blood flow and metabolism, exacerbating the development of small cerebral vascular diseases[10]. Generally, inflammation and oxidative stress represent crucial mechanisms underlying the onset and progression of CSVD. The interplay between these processes contributes to structural and functional abnormalities in cerebral micro vessels, ultimately impacting the normal brain function. Therefore, controlling inflammation and oxidative stress may become one of the important strategies for the prevention and treatment of CSVD.
According to the Compendium of Materia Medica, Ginkgo has the effect of "dispersing toxins". Now, the ancient Chinese term "dispersing toxins" means removing inflammation and oxidation states from the body. The phrase "dispersive toxin" led to the original idea of today's anti-inflammatory and antioxidant studies of Ginkgo biloba in Acute Cerebral Infarction (ACI). At present, Ginkgo biloba extract has shown various pharmacological effects, especially in the treatment of ACI[11,12]. The main components of Ginkgo Diterpene Lactone Meglumine Injection (GDLMI) are extracted from Ginkgo biloba leaves, including Ginkgo biloba A, B, C, J, K, L and M. Among them, A, B and K are the main active components of GDLMI[13]. Ginkgolides A, B, and K exhibit widespread distribution in different organs and tissues, with the kidney, liver, and intestine displaying the highest concentrations, while the brain shows comparatively lower levels[14]. The main components of GDLMI have been pretested in a wide range of diseases and have shown effective therapeutic effects. Studies have found that GDLMI can improve depression-like behavior in patients. Clinical study has shown that GDLMI can improve migraine symptoms in patients, while ginkgolide B can prevent aura migraine[15]. Some studies have also found that GDLMI can improve cognitive function, reduce memory impairment thereby enhancing the learning ability, and is a potential drug for the treatment of vascular cognitive impairment and Alzheimers Disease (AD)[16,17]. In addition, ginkgolide can promote neuronal differentiation through related pathways, and can also reduce nerve cell apoptosis in alphasynuclein aggregates, raising the prospect of new therapies for future neurodegenerative diseases.
Based on the role of Ginkgo diterpene in alleviating inflammation and participating in immune regulation, this study studied the effects of Diterpene Glumine Injection (DGMI) on cognitive function, brain inflammation and oxidative stress in CSVD model rats, and investigated the effects of DGMI on brain pathological changes and neuronal apoptosis in CSVD model rats. It is expected to provide new experimental data support for the treatment of CSVD.
Materials and Methods
Animal origin:
The experimental Sprague-Dawley (SD) rats were purchased, weighing 230~250 g. Experimental animals were fed adaptively for 1 w to 2 w. Implement the Research Animal Ethics Committee (IAEC) animal testing guidelines in accordance with the system.
Animal grouping and modeling:
The animals were divided into three groups (n=8); control, model, and DGMI. In the control group, only the neck was incised and sutured. The model group adopts Bilateral Carotid Artery Occlusion (BCAO) to establish the CSVD model of low perfusion injury[18]. The specific methods are as follows: The rats were anesthetized and fixed in the supine position, and the bilateral common carotid arteries were permanently overlapped with surgical sutures. Based on the model group, 6 mg/ (kg) DGMI (Jiangsu Kangyuan Pharmaceutical Co., Ltd., specification: 5 mg: 1 ml, lot number: 20171124) was injected intraperitoneally daily in DGMI group while the model group was injected with normal saline of equal proportions for 7 d.
Detection index:
Neural functional deficit score: 10-point scale was performed[19,20] and the score according to the symptoms were given. 1 point for upper limb holding wrist flexure, 2 points for elbow flexure, 3 points for shoulder flexure and 1 point for circle crawling, 1~3 points were given to withdrawal resistance and muscle tone decline.
Cognitive ability test: In this experiment, the water temperature of the Morris water maze was maintained at (25°±2°), and a transparent circular platform with a diameter of 12 cm was placed underwater at 2 cm. The positioning voyage began at 10 am every day for 5 consecutive d; after 1 w of rest, the positioning sailing experiment was repeated once. During the experiment, the virtual 4 quadrants of the pool were studied. The rats were put into the water 1 h in advance from the fixed drop point facing the pool wall, and the time to find the platform within 60 y was recorded as the escape incubation period. The rats that did not find the platform within 60 s were guided to the platform with a small stick and allowed to stop on the platform for 15 s. The escape latency was recorded with the memory spatial marker for 60 s. The rats that successfully reached the platform within 60 s were also allowed to rest on the platform for 15 s. At the end of the experiment, each rat’s route and escape latency were recorded each time they swam. After 24 h of completion of all the positioning navigation experiments, the space exploration experiment was carried out, the platform was removed, and the rats were allowed to enter the water in the opposite quadrant of the original platform. The swimming route of the rats was recorded in the pool for 60 s, and the number of times they passed the original platform and the percentage of time they spent in the original platform quadrant were calculated.
Cerebral water content and infarct volume percentage: Three rats were randomly selected from each group according to the random number table method, and the brain tissues were taken after anesthesia, and immediately after weighing (W1), they were roasted at 110° for 48 h to constant weight, and weighed again (W2). The weight difference between the two rats was weight loss (ΔW=W1-W2), and the brain water content (%)=(ΔW/W1)×100 %. Brain tissues of 3 rats were taken from each group, frozen at -20° for 15 min, and were sliced with a coronal thickness of 2 mm, and then incubated with 2 % 2,3,5-Triphenyltetrazolium Chloride (TTC) solution in the dark for 30 min (the slices were turned over for every 5 min). Finally, the percentage of infarct volume was calculated.
Biochemical examination of brain tissue: The rat brain tissue was taken, and the tissue homogenate was prepared with a concentration of 10 % after proper amount of cold lysate was added. After centrifuging the brain homogenate after 15 min at 4° (3000 r/min) to get the supernatant, colorimetry was used to measure the activity of Superoxide Dismutase (SOD), Catalase (CAT), and Malondialdehyde (MDA). The enzyme-linked immunosorbent assay was utilized to ascertain the concentrations of TNF-Alpha (α), IL-6, and IL-1 Beta (β) in brain tissue.
Pathological examination of brain tissue: Slices of rat brain tissues were paraffin embedded, 2 μm thick, dewaxed, hydrated, and stained with HE after being fixed for 72 h in a 10 % formaldehyde solution. The histological morphological and structural changes of the ischemic brain were identified using an optical microscope.
Observation of neuronal apoptosis: Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) staining was performed according to the kit instructions and the apoptosis of neurons in ischemic cerebral tissue was observed by optical microscope. The total number of neurons in the visual field and the number of apoptotic neurons were counted
Apoptotic Index (AI, %)=(number of apoptotic neurons/total number of neurons)×100 %
Western blotting: Radioimmunoprecipitation Assay (RIPA) (Beyotime, China) was utilized to lyse the cells. Using a Bicinchoninic Acid (BCA) assay kit, the total protein was measured. It was then separated using a 10 % Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel sample and electrophoretically transferred to a Polyvinylidene Difluoride (PVDF) membrane. Subsequent to blocking, the membranes were treated overnight at 4° with primary antibodies against synaptophysin, PSD95, Bcl-2-Associated X Protein (BAX) and B-Cell Lymphoma 2 (Bcl2). Tubulin is used as the internal parameter. The membranes were subsequently treated the next day with a Horseradish Peroxidase (HRP)-conjugated secondary antibody. The protein bands were imaged and analyzed after exposure.
Statistical analysis:
For analysis, Statistical Package for the Social Sciences (SPSS) 15.0 software was utilized. For the purpose of comparing means across several groups, one-way Analysis of Variance (ANOVA) was employed, whereas the Least Significant Difference (LSD)-t test was utilized to compare pairings. The test standard was set at α=0.05 and the measurement data were reported as mean±standard deviation p<0.05 was regarded as statistically significant.
Results and Discussion
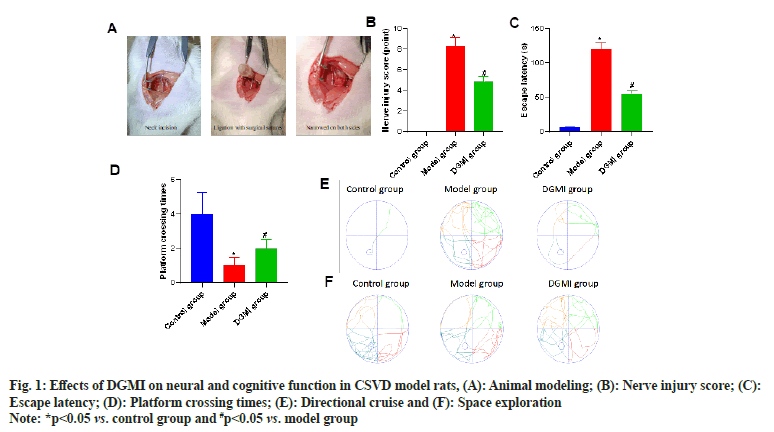
Fig. 1 displays the animal modeling used for CSVD. The model group’s directional cruise and space exploration route was disrupted (p<0.05), the number of platform crossings was less (p<0.05), the escape latency time was considerably longer (p<0.05), and the model group’s nerve damage score was higher (p<0.05) than that of the control group. Rat’s brain function, learning, and memory function all significantly improved after DGMI intervention as compared to the model group (p<0.05) as shown in fig. 1.
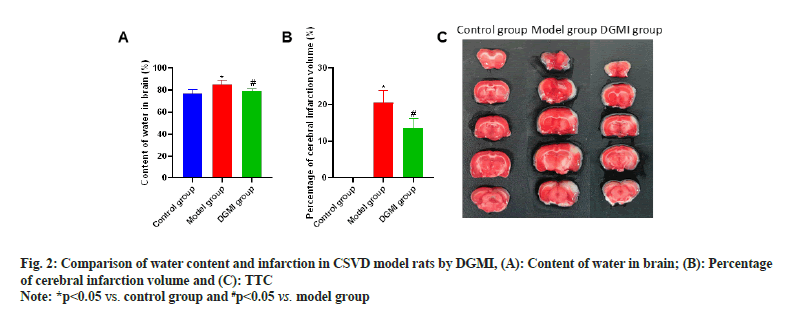
The water content and infarct volume of the brain tissue were compared after the neurological and cognitive function tests were finished. It was discovered that the CSVD model rat’s brain tissue had higher water content and an infarct volume percentage than that of the control group (p<0.05). Following DGMI intervention, there was a substantial reduction (p<0.05) in both the water content and infarct volume percentage of the brain tissue as shown in fig. 2.
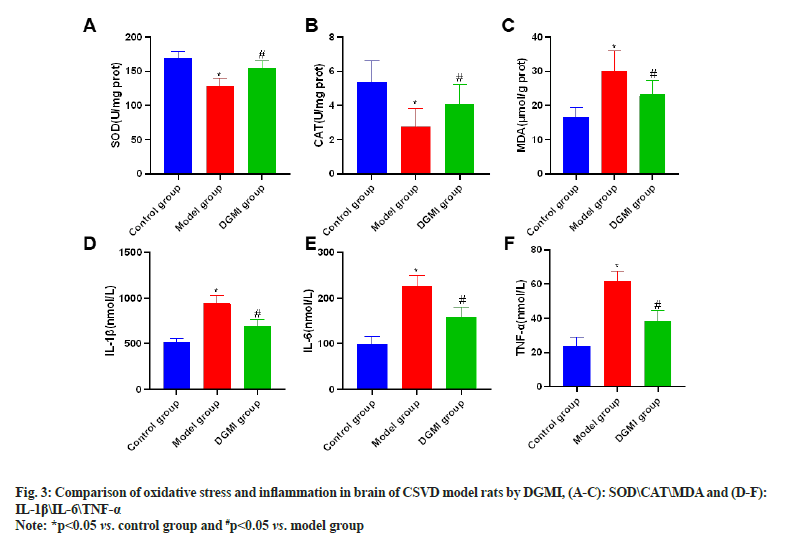
Brain oxidative stress and inflammatory detection revealed that following CSVD modeling, the contents of MDA, TNF-α, IL-1β, and IL-6 were dramatically elevated (p<0.05), while the activities of SOD and CAT were significantly lowered (p<0.05). Following the DGMI intervention, there was a substantial (p<0.05) drop in the contents of MDA, TNF-α, IL-1β, and IL-6, and an increase in the activities of SOD and CAT (p<0.05) as shown in fig. 3.
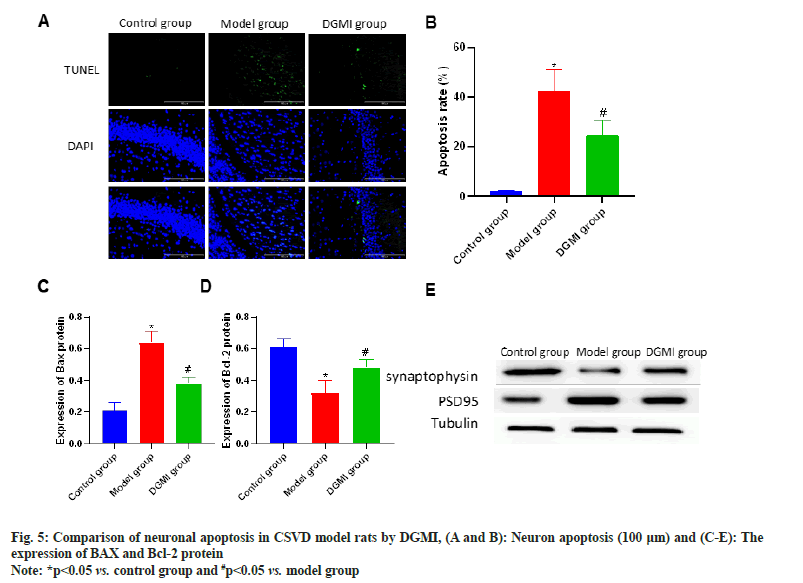
Sections were taken at the end of the experimental study, and no abnormalities were found in the control group. In the model group, the brain tissue neurons showed obvious morphological and structural pathological changes, including spongy degeneration of the brain tissue, reduction of the number of neurons, cytoplasmic contraction, vacuolar degeneration, uneven cytoplasmic coloring, and deep staining of the nucleus. The model group’s brain lesions can be considerably improved by DGMI treatments as compared to the model group. Synapsin and PSD95 protein expression were considerably (p<0.05) lower in the model group. Following DGMI stimulation, there was a substantial (p<0.05) increase in the expressions of Postsynaptic Density protein-95 (PSD-95) and synaptophysin as shown in fig. 4.
Neuronal apoptosis detection revealed that whereas a significant number of apoptotic neurons emerged in the model group (p<0.05), there were none in the sham surgery group. Following DGMI treatments, there was a substantial (p<0.05) decrease in the number of neuronal apoptosis. Apoptosis-related proteins were detected, and it was shown that following DGMI intervention, BAX protein rose and Bcl-2 protein decreased in the model group as shown in fig. 5.
As a common clinical cerebrovascular disease, CSVD has been paid more and more attention with the development of imaging. However, the onset of small cerebral vascular disease is hidden, and the early asymptomatic clinical manifestations are often ignored, thus delaying the early diagnosis and treatment of the disease. CSVD is a highly age-related disease, and the clinical and imaging manifestations of CSVD in people over 65 y old reach about 80 %[21]. It is difficult to observe the pathological characteristics of CSVD, which is usually asymptomatic, and gradually develops various neurological function deficits, mainly including cognitive affective disorders, gait abnormalities, voiding disorders, etc. Among various clinical manifestations, cognitive function impairment has received much attention[22-26]. The cognitive function impairment of CSVD patients shows a gradual development pattern. In the early stage, the patients may only have impairments in executive ability, attention and orientation, but in the course of disease development, the impairments in language ability and computing ability will gradually appear, and in the late stage, vascular dementia will develop[27]. Therefore, the early diagnosis of CSVD patients and the discovery of risk factors are particularly important. In our study, we established a rat model of CSVD using BCAO, and detected the neural and cognitive functions of the model rats. It was found that cognitive function declined and neurological function injury score increased after CSVD modeling. Further testing also found that along with the increase of brain tissue water content and infarct size, this was very similar to the clinical manifestations of CSVD. It is suggested that BCAO has successfully established a rat model of CSVD, which can be applied to the discovery of risk factors in subsequent experiments.
Oxidative stress plays a major role in the changes of cerebral vascular structure[28-30]. Cerebral blood flow changes with changes in the structure of cerebral vessels, which is affected in a complex way. Internal and external remodeling can also lead to the remodeling of small blood vessel walls, which leads to the reduction of the diameter of cerebral vessels[31]. The occurrence of vascular cognitive dysfunction is also directly and closely related to oxidative stress and chronic inflammation of central neuropathy[32]. For example, small cerebral vascular disease causes the formation of microglia and astroglia cells, thus increasing the oxidative stress in the brain, leading to the discharge of inflammatory mediators, and then secondary damage to small blood vessels, leading to neurological and vascular dysfunction[29]. Studies have found that when the brain is completely hypoxic[33], under the stimulation of ischemic and hypoxic environment, microglia nerve cells can activate and proliferate through stimulating protein kinase channels, thus forming cytotoxic proteins such as serine protein kinase and collagenase, and producing inflammatory mediators such as TNF-α and leukocyte mediators β. Nerve cells are destroyed by these intervening inflammatory factors. In view of the mechanism of oxidative stress and inflammation in the brain, they can be used as targets for new drug research. DGMI mainly contains ginkgolides A, B and K, and in the current study, ginkgolides A, B and K have been found to have extensive pharmacological effects[34]. A large amount of evidence has shown that ginkgolide A, B and K have important effects on central nervous system function[35,36], from enhancing cognitive function in dementia patients to promoting neuroprotection and recovery of acute hypoxic/ ischemic injury. However, the study of ginkgolide in CSVD is still lacking at home and abroad. In our study, we found that after DGMI intervention, the cognitive function of CSVD model rats was gradually restored, the neurological function damage was reduced, and the cerebral infarction area was less. This might be because ginkgolide A enhances rat memory and inhibits amyloidinduced depolarization of cortical neurons. In the study of oxidative stress and inflammation, we also found that the phenomenon of oxidative stress and inflammatory infiltration appeared in the brain tissue of rats in the model group, and after DGMI intervention, the oxidative stress and inflammatory infiltration in the brain were also improved. It is suggested that DGMI can effectively relieve oxidative stress and inflammatory infiltration in brain. Studies have reported that ginkgolide A can improve non-alcoholic fatty liver disease induced by high-fat diet in mice by inducing apoptosis of cellular lipids and inhibiting cellular inflammatory response[37]. Ginkgolide B can reduce the damage of rat hippocampal neurons induced by hypoxia by inhibiting oxidative stress and apoptosis[38-41]. Ginkgolide K also has antioxidant stress and neuroprotective effects and has been used for hundreds of years to treat cerebrovascular and cardiovascular diseases[42,43]. Our study is consistent with previous research.
The role of DGMI is not only that, neuron synapse is the biological material basis of learning and memory, and its dysfunction will lead to cognitive decline. Synaptophysin is located in the synaptic vesicular membrane, and its protein expression affects the density and distribution of synapses[44]. PSD95 can regulate the development and maturation of synapses, and is a critical component involved in dendrite morphogenesis, synaptic plasticity and glutamate transmission in the process of neural maturation[45-49]. In the study of brain histology in CSVD model rats, we found that after DGMI intervention, the brain histology was improved, the expression of synaptophysin and PSD95 protein was significantly increased, and neuronal apoptosis was also significantly decreased, suggesting that CSVD can effectively alleviate brain tissue lesions, synaptic function changes and neuronal apoptosis in CSVD model rats. The discovery of neuronal apoptosis is also related to inflammation and oxidative stress, CSVD can improve oxidative stress and inflammatory infiltration, and then affect neuronal apoptosis.
In conclusion, DGMI can improve the cognitive function of CSVD model rats, reduce the damage of nerve function and the size of cerebral infarction, and improve the apoptosis and pathological development of brain neurons in CSVD model rats by reducing brain oxidative stress and inflammatory infiltration.
Conflict of interests:
The authors declared no conflict of interests.
References
- Markus HS, de Leeuw FE. Cerebral small vessel disease: Recent advances and future directions. Int J Stroke 2023;18(1):4-14.
[Crossref] [Google Scholar] [PubMed]
- Chojdak-Łukasiewicz J, Dziadkowiak E, Zimny A, Paradowski B. Cerebral small vessel disease: A review. Adv Clin Exp Med 2021;30(3):349-56.
[Crossref] [Google Scholar] [PubMed]
- Zotin MC, Sveikata L, Viswanathan A, Yilmaz P. Cerebral small vessel disease and vascular cognitive impairment: From diagnosis to management. Curr Opin Neurol 2021;34(2):246.
[Crossref] [Google Scholar] [PubMed]
- Benveniste H, Nedergaard M. Cerebral small vessel disease: A glymphopathy? Curr Opin Neurobiol 2022;72:15-21.
[Crossref] [Google Scholar] [PubMed]
- Jiang L, Cai X, Yao D, Jing J, Mei L, Yang Y, et al. Association of inflammatory markers with cerebral small vessel disease in community-based population. J Neuroinflammation 2022;19(1):106.
[Crossref] [Google Scholar] [PubMed]
- Muller A, Krause B, Kerstein-Stahle A, Comduhr S, Klapa S, Ullrich S, et al. Granulomatous inflammation in ANCA-associated vasculitis. Int J Mol Sci 2021;22(12):6474.
[Crossref] [Google Scholar] [PubMed]
- Sacoto G, Boukhlal S, Specks U, Flores-Suárez LF, Cornec D. Lung involvement in ANCA-associated vasculitis. Presse Med 2020;49(3):104039.
[Crossref] [Google Scholar] [PubMed]
- Baumal CR, Bodaghi B, Singer M, Tanzer DJ, Seres A, Joshi MR, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina 2021;5(6):519-27.
[Crossref] [Google Scholar] [PubMed]
- Del Cuore A, Pacinella G, Riolo R, Tuttolomondo A. The role of immunosenescence in cerebral small vessel disease: A review. Int J Mol Sci 2022;23(13):7136.
[Crossref] [Google Scholar] [PubMed]
- Liao FF, Lin G, Chen X, Chen L, Zheng W, Raghow R, et al. Endothelial nitric oxide synthase-deficient mice: A model of spontaneous cerebral small-vessel disease. Am J Pathol 2021;191(11):1932-45.
[Crossref] [Google Scholar] [PubMed]
- Peng H, Li YF, Sun SG. Effects of Ginkgo biloba extract on acute cerebral ischemia in rats analyzed by magnetic resonance spectroscopy. Acta Pharmacol Sin 2003;24(5):467-71.
[Google Scholar] [PubMed]
- Wu C, Zhao X, Zhang X, Liu S, Zhao H, Chen Y. Effect of Ginkgo biloba extract on apoptosis of brain tissues in rats with acute cerebral infarction and related gene expression. Genet Mol Res 2015;14(2):6387-94.
[Crossref] [Google Scholar] [PubMed]
- Chen C, Lv H, Shan L, Long X, Guo C, Huo Y, et al. Antiplatelet effect of ginkgo diterpene lactone meglumine injection in acute ischemic stroke: A randomized, double‐blind, placebo‐controlled clinical trial. Phytother Res 2023;37(5):1986-96.
[Crossref] [Google Scholar] [PubMed]
- Fan Q, Zhou J, Wang Y, Xi T, Ma H, Wang Z, et al. Chip-based serum proteomics approach to reveal the potential protein markers in the sub-acute stroke patients receiving the treatment of ginkgo diterpene lactone meglumine injection. J Ethnopharmacol 2020;260:112964.
[Crossref] [Google Scholar] [PubMed]
- Zhou L, Gao Y, Lai XX, Xu TS, Yu M, Wang Y, et al. Post-marketing study on clinical safety of ginkgo diterpene lactone meglumine injection in 6300 patients with ischemic stroke. Zhongguo Zhong Yao Za Zhi 2017;42(24):4744-9.
[Google Scholar] [PubMed]
- Tonali N, Kaffy J, Soulier JL, Gelmi ML, Erba E, Taverna M, et al. Structure-activity relationships of β-hairpin mimics as modulators of amyloid β-peptide aggregation. Eur J Med Chem 2018;154:280-93.
[Crossref] [Google Scholar] [PubMed]
- Pellegrino S, Tonali N, Erba E, Kaffy J, Taverna M, Contini A, et al. β-Hairpin mimics containing a piperidine-pyrrolidine scaffold modulate the β-amyloid aggregation process preserving the monomer species. Chem Sci 2017;8(2):1295-302.
- Wang J, Yang C, Wang H, Li D, Li T, Sun Y, et al. A new rat model of chronic cerebral hypoperfusion resulting in early-stage vascular cognitive impairment. Front Aging Neurosci 2020;12:86.
[Crossref] [Google Scholar] [PubMed]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986;17(3):472-6.
[Crossref] [Google Scholar] [PubMed]
- Yang Q, Wei X, Deng B, Chang Z, Jin D, Huang Y, et al. Cerebral small vessel disease alters neurovascular unit regulation of microcirculation integrity involved in vascular cognitive impairment. Neurobiol Dis 2022;170:105750.
[Crossref] [Google Scholar] [PubMed]
- van Den Brink H, Doubal FN, Duering M. Advanced MRI in cerebral small vessel disease. Int J Stroke 2023;18(1):28-35.
[Crossref] [Google Scholar] [PubMed]
- Markus HS, van Der Flier WM, Smith EE, Bath P, Biessels GJ, Briceno E, et al. Framework for clinical trials in cerebral small vessel disease (FINESSE): A review. JAMA Neurol 2022;79(11):1187-98.
[Crossref] [Google Scholar] [PubMed]
- Wardlaw JM, Benveniste H, Williams A. Cerebral vascular dysfunctions detected in human small vessel disease and implications for preclinical studies. Ann Rev Physiol 2022;84:409-34.
[Crossref] [Google Scholar] [PubMed]
- Kumar AA, Yeo N, Whittaker M, Attra P, Barrick TR, Bridges LR, et al. Vascular collagen type-IV in hypertension and cerebral small vessel disease. Stroke 2022;53(12):3696-705.
[Crossref] [Google Scholar] [PubMed]
- Wang ZY, Li MZ, Li WJ, Ouyang JF, Gou XJ, Huang Y. Mechanism of action of Daqinjiao decoction in treating cerebral small vessel disease explored using network pharmacology and molecular docking technology. Phytomedicine 2023;108:154538.
[Crossref] [Google Scholar] [PubMed]
- Whittaker E, Thrippleton S, Chong LY, Collins VG, Ferguson AC, Henshall DE, et al. Systematic review of cerebral phenotypes associated with monogenic cerebral small‐vessel disease. J Am Heart Assoc 2022;11(12):e025629.
[Crossref] [Google Scholar] [PubMed]
- Inoue Y, Shue F, Bu G, Kanekiyo T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol Neurodegener 2023;18(1):46.
[Crossref] [Google Scholar] [PubMed]
- Chen S, Li L, Peng C, Bian C, Ocak PE, Zhang JH, et al. Targeting oxidative stress and inflammatory response for blood–brain barrier protection in intracerebral hemorrhage. Antioxid Redox Signal 2022;37(1-3):115-34.
[Crossref] [Google Scholar] [PubMed]
- Chung TD, Linville RM, Guo Z, Ye R, Jha R, Grifno GN, et al. Effects of acute and chronic oxidative stress on the blood-brain barrier in 2D and 3D in vitro models. Fluids Barriers CNS 2022;19(1):1-17.
[Crossref] [Google Scholar] [PubMed]
- Al Ahmad A, Gassmann M, Ogunshola OO. Involvement of oxidative stress in hypoxia-induced blood-brain barrier breakdown. Microvasc Res 2012;84(2):222-5.
[Crossref] [Google Scholar] [PubMed]
- Abdul-Muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 2015;51(3):966-79.
[Crossref] [Google Scholar] [PubMed]
- Yan N, Xu Z, Qu C, Zhang J. Dimethyl fumarate improves cognitive deficits in chronic cerebral hypoperfusion rats by alleviating inflammation, oxidative stress, and ferroptosis via NRF2/ARE/NF-κB signal pathway. Int Immunopharmacol 2021;98:107844.
[Crossref] [Google Scholar] [PubMed]
- Hahad O, Prochaska JH, Daiber A, Muenzel T. Environmental noise-induced effects on stress hormones, oxidative stress, and vascular dysfunction: Key factors in the relationship between cerebro cardiovascular and psychological disorders. Oxid Med Cell Longev 2019;2019:4623109.
[Crossref] [Google Scholar] [PubMed]
- Huang T, Huang W, Zhang Z, Yu L, Xie C, Zhu D, et al. Hypoxia-inducible factor-1α upregulation in microglia following hypoxia protects against ischemia-induced cerebral infarction. Neuroreport 2014;25(14):1122-8.
[Crossref] [Google Scholar] [PubMed]
- Feng Z, Sun Q, Chen W, Bai Y, Hu D, Xie X. The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: A literature review. Mol Med 2019;25(1):57.
[Crossref] [Google Scholar] [PubMed]
- Niu TT, Yuan BY, Liu GZ. Ginkgolides and bilobalide for treatment of Alzheimer’s disease and COVID-19: Potential mechanisms of action. Eur Rev Med Pharmacol Sci 2022;26(24):9502-10.
[Crossref] [Google Scholar] [PubMed]
- Eckert A. Mitochondrial effects of Ginkgo biloba extract. Int Psychogeriatr 2012;24(S1):S18-20.
[Crossref] [Google Scholar] [PubMed]
- Jeong HS, Kim KH, Lee IS, Park JY, Kim Y, Kim KS, et al. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomed Pharmacother 2017;88:625-34.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Lei Q, Zhao S, Xu W, Dong W, Ran J, et al. Ginkgolide B maintains calcium homeostasis in hypoxic hippocampal neurons by inhibiting calcium influx and intracellular calcium release. Front Cell Neurosci 2021;14:627846.
- Wang SJ, Chen HH. Ginkgolide B, a constituent of Ginkgo biloba, facilitates glutamate exocytosis from rat hippocampal nerve terminals. Eur J Pharmacol 2005;514(2-3):141-9.
[Crossref] [Google Scholar] [PubMed]
- Zheng PD, Mungur R, Zhou HJ, Hassan M, Jiang SN, Zheng JS. Ginkgolide B promotes the proliferation and differentiation of neural stem cells following cerebral ischemia/reperfusion injury, both in vivo and in vitro. Neural Regen Res 2018;13(7):1204-11.
- Xiao Q, Wang C, Li J, Hou Q, Li J, Ma J, et al. Ginkgolide B protects hippocampal neurons from apoptosis induced by beta-amyloid 25–35 partly via up-regulation of brain-derived neurotrophic factor. Eur J Pharmacol 2010;647(1-3):48-54.
[Crossref] [Google Scholar] [PubMed]
- Chen M, Zou W, Chen M, Cao L, Ding J, Xiao W, et al. Ginkgolide K promotes angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 pathway. Eur J Pharmacol 2018;833:221-9.
[Crossref] [Google Scholar] [PubMed]
- Ma S, Yin H, Chen L, Liu H, Zhao M, Zhang X. Neuroprotective effect of ginkgolide K against acute ischemic stroke on middle cerebral ischemia occlusion in rats. J Nat Med 2012;66(1):25-31.
[Crossref] [Google Scholar] [PubMed]
- Zhao L, Liu JW, Kan BH, Shi HY, Yang LP, Liu XY. Acupuncture accelerates neural regeneration and synaptophysin production after neural stem cells transplantation in mice. World J Stem Cells 2020;12(12):1576-90.
[Crossref] [Google Scholar] [PubMed]
- Coley AA, Gao WJ. PSD95: A synaptic protein implicated in schizophrenia or autism? Prog Neuro Psychopharmacol Biol Psychiatry 2018;82:187-94.
[Crossref] [Google Scholar] [PubMed]
- Yeh H, Woodbury ME, Dixie KL, Ikezu T, Ikezu S. Microglial WNT5A supports dendritic spines maturation and neuronal firing. Brain Behav Immun 2023;107:403-13.
[Crossref] [Google Scholar] [PubMed]
- Chen H, Qiao D, Wang C, Zhang B, Wang Z, Tang L, et al. Fragile X mental retardation protein mediates the effects of androgen on hippocampal PSD95 expression and dendritic spines density/morphology and autism-like behaviors through miR-125a. Front Cell Neurosci 2022;16:872347.
[Crossref] [Google Scholar] [PubMed]
- Li H, McLaurin KA, Mactutus CF, Booze RM. Microglia proliferation underlies synaptic dysfunction in the prefrontal cortex: Implications for the pathogenesis of HIV-1-associated neurocognitive and affective alterations. J Neurovirol 2023;29(4):460-71.
[Crossref] [Google Scholar] [PubMed]
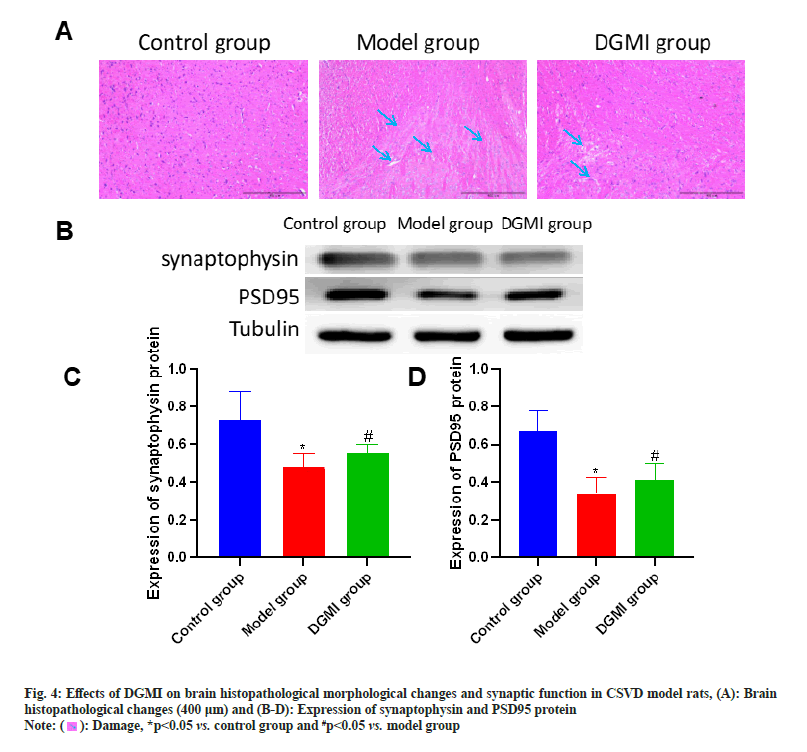
 ): Damage, *p<0.05 vs. control group and #p<0.05 vs. model group
): Damage, *p<0.05 vs. control group and #p<0.05 vs. model group