- *Corresponding Author:
- Juan Liu
Department of Otolaryngology, Jinan People's Hospital Affiliated to Shandong First Medical University, Jinan 271100, China
E-mail: liujuanxcsy@163.com
| This article was originally published in a special issue, “Therapeutic Perspectives in Biomedical Research and Pharmaceutical Sciences and their Nursing Methods” |
| Indian J Pharm Sci 2021:83(4)Spl issue “228-234” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the mechanism of microRNA-125a reversing the resistance of laryngeal cancer stem cells to cisplatin from mitochondrial autophagy. The human laryngeal carcinoma cell line Hep-2 was purchased from Shanghai Bioleaf Biotechnology Co., Ltd. The expression of microRNA-125a messenger RNA in each group of cells was analyzed by reverse transcription-polymerase chain reaction. The cell migration and invasion ability of each group was evaluated by Transwell assay. Cell apoptosis was detected by flow cytometry. Western blot analysis was conducted to evaluate the protein expression. The expression of microRNA-125a messenger RNA in the Hep-2/diamminedichloroplatinum group was lower than that in the control group (p<0.05) and the expression of microRNA-125a messenger RNA in the microRNA-125a mimic group was higher than that in the Hep-2/diamminedichloroplatinum group (p<0.05). Compared with the Hep-2/diamminedichloroplatinum group, the microRNA-125a mimic group had more mitochondrial fragments and the average branch length of mitochondria decreased (p<0.05). The cell migration, invasion and viability of the Hep-2/diamminedichloroplatinum group increased (p<0.05) and the apoptosis rate decreased (p<0.05). The protein expression of phosphoinositide 3-kinase, protein kinase B and mammalian target of rapamycin in the Hep-2/diamminedichloroplatinum group was higher than that in the control group (p<0.05) and their expression in the microRNA-125a mimic group was lower than that in the Hep-2/ diamminedichloroplatinum group (p<0.05). The dysregulation of microRNA-125a was related to diamminedichloroplatinum resistance in laryngeal cancer and diamminedichloroplatinum resistance was induced by microRNA-125a through phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin pathway mediated autophagy inhibition. Overexpression of microRNA-125a inhibited the growth and metastasis of Hep-2/diamminedichloroplatinum cells through the phosphoinositide 3-kinase/ protein kinase B/mammalian target of rapamycin pathway and induced their apoptosis and autophagy.
Keywords
Mitochondria, autophagy, microRNA-125a, laryngeal carcinoma, cisplatin
Laryngeal cancer is one of the most common malignant tumors of head and neck in the world. At present, surgery, chemotherapy and radiotherapy are still the main methods to treat primary laryngeal cancer [1]. Although the treatment of cancer has been improved in the past decades, many patients died of cancer due to its metastasis to important organs after operation. For patients with advanced laryngeal cancer, chemotherapy is considered as the only strategy to treat cancer. However, the chemical resistance of laryngeal cancer has become the main obstacle to the therapeutic effect. Cancer stem cells are a group of cells with self-renewal ability [2]. They are responsible for the formation and development of tumors. Micro ribonucleic acid (miRNA) is a small non-coding RNA molecule, which can silence gene expression at post-transcriptional level by targeting messenger RNA transcripts and plays a vital role in various cellular processes [3]. MicroRNA- 125a (miR-125a) is a cancer-inhibiting miRNA, which can inhibit the proliferation, invasion and metastasis of various human cancer cells including laryngeal cancer [4]. Mitochondrial dynamics is a highly disordered process in cancer. Apoptosis and mitochondrial fission are two simultaneous events, in which increased mitochondrial fragmentation is a sign of apoptosis [5]. Recently, many studies have reported that miR-125a can regulate phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway to mediate chemotherapy drug resistance of various types of tumors [6]. Mammalian target of rapamycin (mTOR), the target of PI3K/Akt mechanism, is the key anti-apoptosis and proliferation pathway in cells and the activation of this pathway is closely related to the development of malignant tumors. Autophagy, also known as type II programmed cell death, is a highly conserved process in eukaryotes, which is used for the recovery and degradation of damaged organelles and biomacromolecules in cells [7]. Similar to the dual use of autophagy in cell death or promoting cell survival, the role of autophagy in tumor cell survival under anti-cancer drug treatment is also contradictory, because autophagy can enhance tumor inhibitory activity and weaken the anti-cancer effect of chemotherapy [8]. Studies have shown that inhibiting autophagy can promote the resistance of osteosarcoma to chemotherapy drugs. In addition, autophagy can increase the sensitivity of lung cancer to diamminedichloroplatinum (DDP) [9]. However, it is unclear whether autophagy has cytoprotective effect and how to influence the resistance of laryngeal cancer stem cells to DDP through specific molecular mechanism. In this study, we explored the mechanism of miR-125a reversing the resistance of laryngeal cancer stem cells to DDP from mitochondrial autophagy.
Materials and Methods
Experimental materials:
Cell culture and DDP drug resistance culture: Human laryngeal cancer cell line (Hep-2) (purchased from Shanghai Bioleaf Biotechnology Co., Ltd., China) was kept in Dulbecco's modified eagle medium (DMEM) containing 10 % fetal bovine serum (FBS, Gibco, Invitrogen) and placed in an incubator at 37° with 5 % CO2. TransDetect polymerase chain reaction (PCR) mycoplasma detection kit (TransGen Biotech, Beijing, China) was used to confirm that the cell line did not contain mycoplasma. The initial half maximum inhibitory concentration (IC50) of DDP of Hep-2 cells was determined by incubating cells with different DDP concentrations in 96-well plates. Cells were supplemented with DDP in the 6-well plates at a rate of 1×104 cells/well and the concentration was just below their IC50. Every week, the concentration of DDP was slowly increased by 0.25 μM. 8 mo later, a DDP-resistant cell line Hep-2/DDP was established and maintained by incubation in DDP.
Transfection of miR-125a mimics: For transfection, 50 pmol/ml miR-125a mimics (Shanghai Genechem Co, ltd.) and 50 pmol/ml negative control oligonucleotide were used. According to the manufacturer's instructions, the negative control oligonucleotide and miR-125a mimics were transfected into Hep-2/DDP using Lipofectamine 2000 (Invitrogen).
Experimental grouping: According to the experimental requirements, the cultured cells were randomly divided into three groups: control group (Hep-2 cells cultured under normal conditions), Hep-2/DDP group (Hep-2 cells incubated with DDP at a certain concentration to develop resistance to DDP) and miR-125a mimic group (Hep-2/DDP cells were transfected with miR-125a mimics).
Experimental methods:
Quantitative real-time polymerase chain reaction (qPCR): Total RNA in cell lines was extracted with Trizol reagent (Invitrogen, USA). The miR-125a was reverse transcribed using stem loop RT primer and PrimeScript RT kit (TaKaRa, Japan). The qPCR was performed three times using SYBR Premix Ex Taq (TaKaRa) in the ABI PRISM 7900 sequence detection system (American Applied Biosystems Company). To determine the relative expression of miR-125a, U6 small nuclear RNA (snRNA) was used as an internal reference. The expression of miR-125a was analyzed by 2-ΔΔCT.
Cell IC50 analysis: Hep-2 and Hep-2/DDP were inoculated in a 96 well plate at a density of 1×104/ml and 100 μl DMEM was added to it. After transfection for 24 h, the cells were treated with DDP for 48 h and the cell viability was determined by 3-(4, 5-dimethylthiazol- 2-yl)-2, 5-diphenyltetrazole bromide (MTT). The absorbance of each sample at 570 nm was measured using an enzyme-linked immunoassay (ELISA) microplate reader (Tecan, Switzerland). According to the cell viability curve, IC50 was calculated.
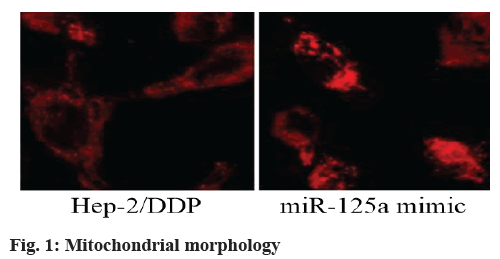
Mitochondrial morphology: Mitochondrial shapes were visualized using mitochondrial specific probe tracker CMXRos51. Mitotracker Red CMXRos is a lipophilic and cationic fluorescent dye. CMXRos binds to the negatively charged matrix side of mitochondrial inner membrane. After treatment in a cell incubator, cells were stained with 200 nM CMXRos at 37° for 15 min. Mitochondrial division inhibitor 1 (Mdivi-1) was purchased from Sigma (Sigma, USA) and used at a concentration of 75 μM for 2 h. Cells were fixed with 4 % paraformaldehyde and stained. The shape of mitochondria was observed with confocal fluorescence microscope (Leica SP8, Germany) at 63 times magnification and the length of mitochondria was observed with Nikon Ti-E microscope. Image was processed and the mitochondrial branch length of nearly 150 cells was measured by Image-J software.
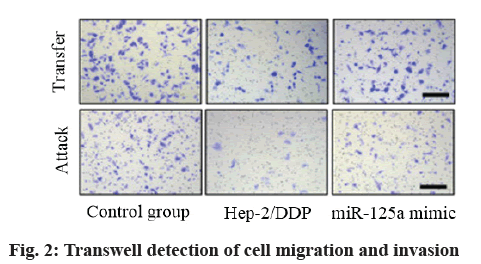
Assay of cell invasion and migration: In a slightly modified Transwell compartment (Corning Costar, mass., USA), an in vitro invasion and migration analysis was carried out in a cell with a polycarbonate filter with a pore size of 8 μm. After treatment, Hep-2 cells suspended in 100 μl serum-free DMEM were placed in the upper transwell chamber at a cell density of 1×105 cells/well and the culture medium added with 10 % FBS was added to the lower chamber as a chemotactic agent. After incubation at 37° for 24 h, the cells on the upper surface of the filter membrane were completely removed with cotton swabs and the cells immersed in the lower side of the membrane were stained with 0.2 % crystal violet dissolved in 2 % ethanol (15 min). These cells migrating to the lower surface of the filter were quantified by counting at least five random fields of view and were photographed under DMI 8 inverted microscope (Leica Microsystems, Wetzlar, Germany). For migration assay, each upper side of the filter membrane was diluted with 100 μl Matrigel (2 mg/ml, in cold serum-free DMEM without chemical attractant; Corning, New York, USA) to form a uniform membrane filter on the top of the membrane. The experiment was carried out under the same conditions as the in vitro invasion test. Each experiment was carried out in triplicate and the data were expressed as migration and invasion rate to represent the average cell number.
Assay of cell viability: Cell viability was measured by MTT reduction assay. Hep-2 cells were inoculated into a 96 well plate for 24 h and treated with DDP. The absorbance at 490 nm was measured by microplate reader. The cell survival rate was calculated using the following formula: cell survival rate (%)= [optical density (OD) 490 of the treatment group/OD 490 of the untreated group]×100.
Apoptosis detection by flow cytometry: The quantitative death of apoptotic cells was analyzed by annexin V-FITC apoptosis detection kit (Becton, Dickinson and Company). The harvested cells were washed twice with pre-cooled phosphate buffered saline (PBS) and stained with annexin V and propidium iodide. Cells were incubated at room temperature for 15 min and then incubated with FACScan flow cytometer (BD Biosciences; Becton, Dickinson and Company).
Western blot analysis: Cell lysate was obtained by using radioimmunoprecipitation assay buffer containing protease inhibitor and protein concentration was determined by Bradford assay. Equal samples were separated on 10 % gels and transferred to polyvinylidene fluoride membrane by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Blocking was performed by incubating with 5 % skim milk at room temperature for 1 h. The membrane was incubated with primary antibody at 4° overnight and then incubated with horseradish peroxidase coupled secondary antibody at room temperature for 1 h. The band intensity was determined and plotted against the total protein amount to produce a standard curve and the protein amount of each blot was determined.
Statistical analysis: All data were expressed as mean±standard deviation (SD). Paired data were compared by t test. Multi-group comparison was conducted by one-way analysis of variance (ANOVA) and Dunnet's t test was carried out. GraphPad 6.0 (GraphPad Software, Inc., La Jolla, California, USA) was used to analyze the data. p<0.05 was considered as a statistically significant difference.
Results and Discussion
MiR-125a inhibited expression in DDP-resistant laryngeal cancer stem cells. The expression of miR- 125a messenger RNA (mRNA) in each group was analyzed by reverse transcription-polymerase chain reaction (RT-PCR). According to results, the expression of miR-125a in Hep-2/DDP group was lower than that in control group (p<0.05) and the expression of miR- 125a in miR-125a mimic group was higher than that in Hep-2/DDP group (p<0.05), indicating that the expression of miR-125a in DDP-resistant laryngeal cancer stem cells decreased as shown in Table 1.
| Group | miR-125a |
|---|---|
| Control group | 1.87±0.24 |
| Hep-2/DDP group | 1.15±0.18 |
| miR-125a mimic group | 2.34±0.31 |
| F value | 11.0385 |
| p value | 0.016 |
Table 1: PCR Analysis of MIR-125A Expression (x?±s)
MiR-125a overexpression reduced DDP resistance. The sensitivity of each group of cells to DDP was determined by MTT assay and the IC50 of DDP was determined according to the cell viability curve. The IC50 of Hep-2/DDP group was higher than that of control group (p<0.05), while that of miR-125a mimic group was lower than that of Hep-2/DDP group (p<0.05) as shown in Table 2.
| Group | IC50 (μM) |
|---|---|
| Control group | 11.35±2.18 |
| Hep-2/DDP group | 37.69±5.72 |
| miR-125a mimic group | 18.41±3.05 |
| F value | 9.372 |
| p value | 0.024 |
Table 2: IC50 Analysis of Cell DDP (x?±s)
Overexpression of miR-125a changed mitochondrial morphology and promoted mitochondrial fission. Mitochondrial morphology is the key factor to determine its activity. The Mitotracker Red CMXRos has been used to examine the morphology of organelles. Compared with Hep-2/DDP group, miR-125a mimic group had more mitochondrial fragments and the average branch length of mitochondria decreased (p<0.05) as shown in fig. 1 and Table 3.
| Group | Branching length (μm) |
|---|---|
| Hep-2/DDP group | 12.57±0.14 |
| miR-125a mimic group | 7.83±0.15 |
| F value | 6.296 |
| p value | 0.011 |
Table 3: Average Branching Length of Mitochondria (x?±s)
MiR-125a overexpression inhibited cell migration and invasion. Transwell assay showed that the migration and invasion of cells in Hep-2/DDP group increased compared with control group (p<0.05) and the migration and invasion of cells in miR-125a mimic group decreased compared with Hep-2/DDP group and control group (p<0.05), indicating that DDP-resistant cells migrated with enhanced invasion ability, miR- 125a could inhibit migration and invasion of cells after transfection and overexpression as shown in fig. 2 and Table 4.
| Group | Cell migration | Cell invasion |
|---|---|---|
| Control group | 148.61±24.57 | 128.76±15.88 |
| Hep-2/DDP group | 205.66±37.25 | 179.42±21.04 |
| miR-125a mimic group | 102.37±11.15 | 95.23±8.19 |
| F value | 11.827 | 13.604 |
| p value | 0.016 | 0.022 |
Table 4: Detection of Cell Migration and Invasion (x?±s)
Overexpression of miR-125a inhibited cell viability and promoted apoptosis. The activity and apoptosis of cells in different groups were assayed by MTT reduction assay and flow cytometry. It was found that the activity of cells in Hep-2/DDP group was higher than that in control group (p<0.05) and the activity of cells in miR-125a mimic group was lower than that in Hep-2/DDP group (p<0.05). Compared with control group, the apoptosis rate in Hep-2/DDP group decreased (p<0.05), while that in miR-125a mimic group increased (p<0.05), which indicated that miR- 125a reversed the drug resistance of laryngeal cancer, inhibited the activity of laryngeal cells and promoted apoptosis as shown in Table 5.
| Group | Cell vitality (%) | Cell apoptosis (%) |
|---|---|---|
| Control group | 81.37±10.24 | 18.28±4.12 |
| Hep-2/DDP group | 94.18±12.05 | 7.05±1.68 |
| miR-125a mimic group | 62.69±17.32 | 34.42±9.85 |
| F value | 9.301 | 12.465 |
| p value | 0.024 | 0.035 |
Table 5: Detection of Apoptosis and Vitality (x?±s)
Overexpression of miR-125a promoted autophagy. The expression of autophagy markers B-cell lymphoma 2 (Bcl-2), microtubule-associated protein 1A/1Blight chain 3 (LC3), LC3-phosphatidylethanolamine conjugate (LC3-II)/cytosolic form of LC3 (LC3-I) and autophagy related 5 (ATG5) was evaluated by western blot analysis. The expression of Bcl-2, LC3-II/I and ATG5 in Hep-2/DDP group was lower than that in control group (p<0.05) and that in miR-125a mimic group was higher than that in Hep-2/DDP group as shown in Table 6.
| Group | Bcl-2 | LC3-II/I | ATG5 |
|---|---|---|---|
| Control group | 1.76±0.25 | 1.64±0.27 | 1.95±0.31 |
| Hep-2/DDP group | 1.14±0.11 | 1.08±0.09 | 1.24±0.15 |
| miR-125a mimic group | 1.99±0.35 | 2.07±0.36 | 2.24±.35 |
| F value | 12.538 | 13.427 | 11.729 |
| p value | 0.034 | 0.028 | 0.019 |
Table 6: Western Blot Analysis of Autophagy Related Protein Expression (x?±s)
MiR-125a overexpression inhibited PI3K/Akt/mTOR pathway. The expression of PI3K, Akt and mTOR in Hep-2/DDP group was higher than that in control group (p<0.05), while that in miR-125a mimic group was lower than that in Hep-2/DDP group (p<0.05) as shown in Table 7.
| Group | PI3K | Akt | mTOR |
|---|---|---|---|
| Control group | 1.37±0.16 | 1.16±0.12 | 1.32±0.19 |
| Hep-2/DDP group | 2.16±0.28 | 1.91±0.15 | 2.07±0.25 |
| miR-125a mimic group | 1.12±0.09 | 1.05±0.13 | 1.14±0.10 |
| F value | 10.725 | 12.664 | 13.549 |
| p value | 0.037 | 0.018 | 0.004 |
Table 7: Western Blot Analysis of PI3K/AKT/mTOR Expression (x?±s)
DDP has been widely used as a chemotherapeutic agent in clinic and has broad-spectrum anti-tumor effect. However, side effects, especially intrinsic or acquired drug resistance, have seriously affected the clinical efficacy of DDP [10]. Therefore, it is necessary to determine the mechanism of DDP resistance and seek potential co-treatment measures to improve its efficacy on laryngeal cancer. In this study, it is proved that the down-regulation of miR-125a contributes to the acquired DDP resistance of laryngeal cancer cells.
As a common cytotoxic anti-tumor drug, DDP has been used as the first-line chemotherapy drug for laryngeal cancer in the past decades [11]. DDP promotes apoptosis through DNA damage and changes in cell metabolism. However, DDP resistance seriously affects the effectiveness of chemotherapy. In addition, DDP will inevitably cause common adverse reactions, including alopecia, nausea, vomiting, bone marrow suppression and liver function damage [12]. Chemical resistance includes primary resistance and acquired resistance. Primary drug resistance is a manifestation of low sensitivity or insensitivity when some chemotherapy drugs are used for the first time. This chemical resistance may be attributed to the characteristics of tumor cells [13]. Acquired drug resistance is described as a common type of drug resistance in clinic, which means that the sensitivity of tumor cells to certain chemotherapeutic drugs decreases after repeated application. In clinical practice, radiotherapy and chemotherapy are considered as effective methods to treat laryngeal cancer. Ionizing radiation and chemotherapy drugs may induce laryngeal cancer cell death through various signal pathways. Progress has been made in the research on the role of type I programmed cell death (apoptosis) in the combination of radiotherapy and chemotherapy [14]. However, our understanding of the role of type II programmed cell death (autophagy) is still limited. In normal cells, autophagy can promote the metabolism of intracellular proteins and eliminate the damage of organelles and other activities to maintain the homeostasis of intracellular environment [15]. However, autophagy can be considered as a mechanism to promote survival by autophagy and phagocytosis of cytoplasmic components for reuse, so that cells can continue to grow under unfavorable conditions [16]. Under physiological conditions, autophagy is the degradation process of autologous longevity proteins and waste organelles through the formation of autophagosomes and the participation of lysosomes. In addition, autophagy can also recover amino acids and proteins to maintain cell homeostasis. Excessive cell stimulation, long stimulation duration and large-scale autophagy degradation products can promote programmed cell death. In this study, we found that the down-regulation of miR-125a could promote the growth and metastasis of Hep-2/DDP cells and inhibit apoptosis, while the overexpression of miR-125a could inhibit the growth and metastasis of cells and induce apoptosis.
Our understanding of the importance of autophagy and apoptosis in the development and prevention of human diseases has been improved. In addition, the relationship between them has also been studied. It is worth noting that it has been observed that apoptosisregulating genes, such as Bcl-2 family members, can also regulate autophagy, while autophagy-related proteins (such as ATG5, Beclin 1 and autophagy related 4D cysteine peptidase (ATG4D)) are also involved in apoptosis [17]. In addition, activation of LC3-II may promote apoptosis, while autophagy degradation of LC3-II is helpful to inhibit apoptosis of mammalian cells. The effect of autophagy on pre-death cells is also related to apoptosis. In other words, autophagy may be an upstream event before apoptosis, which may be necessary for the initiation of apoptosis. In this study, the down-regulation of miR-125a inhibited the expression of Bcl-2, LC3-II/I and ATG5, while the overexpression of miR-125a restored the expression of Bcl-2, LC3-II/I and ATG5 in Hep-2/DDP cells.
PI3K is one of the main downstream effectors mediating receptor tyrosine kinase signal transduction. Activation of Akt can activate the downstream mTOR, thereby reducing the expression of Beclin-1 and LC3 and inhibiting autophagy. Studies have shown that DDP can activate Akt/mTOR pathway and cause drug resistance in bladder cancer, ovarian cancer and nasopharyngeal carcinoma [18]. PI3K/Akt/mTOR signaling pathway can stimulate cell growth in multiple stages of cell cycle. In addition, it can control cell processes through its downstream targets, thus initiating and regulating the development of cancer. The activation of this signaling pathway eventually leads to the inhibition of autophagy of cancer cells and promotes the development of cancer cells [19]. In addition, PI3K/Akt/mTOR signaling pathway makes cancer cells resistant to various cancer treatments, resulting in poor prognosis of various cancers. PI3K/Akt/mTOR signaling pathway is activated in many cancers and the activated signal can promote the differentiation, growth and survival of tumor cells [20]. The mechanisms of activating PI3K/ Akt/mTOR signaling include the loss of phosphatase and tensin homolog (PTEN), the proliferation and mutation of PI3K and/or Akt, the activation of growth factor receptor and the stimulation of carcinogens. The signal is transmitted to mTOR, which can regulate protein translation after the signal pathway is activated. mTOR is the downstream target gene of Akt, which is generally considered to regulate autophagy by regulating a series of autophagy-related genes. In addition, the overexpression of miR-125a promotes PI3K/Akt/mTOR signaling, while the down-regulation of miR-125a reduces PI3K/Akt/mTOR signaling in Hep-2/DDP cells.
To sum up, this study shows that the imbalance of miR-125a is related to DDP resistance in laryngeal cancer and DDP resistance is induced by autophagy inhibition mediated by miR-125a through PI3K/Akt/ mTOR pathway. Overexpression of miR-125a inhibits the growth and metastasis of Hep-2/DDP cells through PI3K/Akt/mTOR pathway and induces apoptosis and autophagy. Therefore, miR-125a may be helpful to elucidate the potential molecular mechanism of chemotherapy resistance of laryngeal cancer, thus providing a basis for new treatment strategies for laryngeal cancer in clinic practices.
Conflicts of interest:
The authors declared no conflict of interest.
References
- Huang W, Zeng C, Hu S, Wang L, Liu J. ATG3, a target of miR-431-5p, promotes proliferation and invasion of colon cancer via promoting autophagy. Cancer Manag Res 2019;11:10275-85.
- Sun WU, Li J, Zhou L, Han J, Liu R, Zhang H, et al. The c-Myc/miR-27b-3p/ATG10 regulatory axis regulates chemoresistance in colorectal cancer. Theranostics 2020;10(5):1981-96.
- Li H, Liu J, Cao W, Xiao X, Liang L, Liu-Smith F, et al. C-myc/miR-150/EPG5 axis mediated dysfunction of autophagy promotes development of non-small cell lung cancer. Theranostics 2019;9(18):5134-48.
- Zhang Y, Li C, Liu X, Wang Y, Zhao R, Yang Y, et al. circHIPK3 promotes oxaliplatin-resistance in colorectal cancer through autophagy by sponging miR-637. EBioMedicine 2019;48:277-88.
- Tang Q, Chen Z, Zhao L. Circular RNA hsa_circ_0000515 acts as a miR-326 sponge to promote cervical cancer progression through up-regulation of ELK1. Aging 2019;11(22):9982.
- Ju S, Liang Z, Li C, Ding C, Xu C, Song X, et al. The effect and mechanism of miR-210 in down-regulating the autophagy of lung cancer cells. Pathol Res Pract 2019;215(3):453-8.
- Wang B, Mao JH, Wang BY, Wang LX, Wen HY, Xu LJ, et al. Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-κB signaling pathway. Cancer Lett 2020;489:87-99.
- Kolenda T, Guglas K, Kopczynska M, Sobocinska J, Teresiak A, Blizniak R, et al. Good or not good: Role of miR-18a in cancer biology. Rep Pract Oncol Radiother 2020;25(5):808-19.
- Yang Z, Sun Q, Guo J, Wang S, Song G, Liu W, et al. GRSF1-mediated MIR-G-1 promotes malignant behavior and nuclear autophagy by directly upregulating TMED5 and LMNB1 in cervical cancer cells. Autophagy 2019;15(4):668-85.
- Feng J, Li Z, Li L, Xie H, Lu Q, He X. Hypoxia?induced circCCDC66 promotes the tumorigenesis of colorectal cancer via the miR?3140/autophagy pathway. Int J Mol Med 2020;46(6):1973-82.
- Zhang XH, Li BF, Ding J, Shi L, Ren HM, Liu K, et al. LncRNA DANCR-miR-758-3p-PAX6 molecular network regulates apoptosis and autophagy of breast cancer cells. Cancer Manag Res 2020;12:4073-84.
- Liu F, Ai FY, Zhang DC, Tian L, Yang ZY, Liu SJ. LncRNA NEAT1 knockdown attenuates autophagy to elevate 5?FU sensitivity in colorectal cancer via targeting miR?34a. Cancer Med 2020;9(3):1079-91.
- Li H, He C, Wang X, Wang H, Nan G, Fang L. MicroRNA-183 affects the development of gastric cancer by regulating autophagy via MALAT1-miR-183-SIRT1 axis and PI3K/AKT/mTOR signals. Artif Cells Nanomed Biotechnol 2019;47(1):3163-71.
- Shi YP, Liu GL, Li S, Liu XL. miR-17-5p knockdown inhibits proliferation, autophagy and promotes apoptosis in thyroid cancer via targeting PTEN. Neoplasma 2020;67(2):249-58.
- Che J, Wang W, Huang Y, Zhang L, Zhao J, Zhang P, et al. miR?20a inhibits hypoxia?induced autophagy by targeting ATG5/FIP200 in colorectal cancer. Mol Carcinog 2019;58(7):1234-47.
- Gu Y, Fei Z, Zhu R. miR-21 modulates cisplatin resistance of gastric cancer cells by inhibiting autophagy via the PI3K/Akt/mTOR pathway. Anticancer Drugs 2020;31(4):385-93.
- Hu Z, Cai M, Zhang Y, Tao L, Guo R. miR-29c-3p inhibits autophagy and cisplatin resistance in ovarian cancer by regulating FOXP1/ATG14 pathway. Cell Cycle 2020;19(2):193-206.
- Cui X, Wang X, Zhou X, Jia J, Chen H, Zhao W. miR-106a Regulates Cell Proliferation and Autophagy by Targeting LKB1 in HPV-16–Associated Cervical Cancer. Mol Cancer Res 2020;18(8):1129-41.
- Li JP, Zhang HM, Liu MJ, Xiang Y, Li H, Huang F, et al. miR?133a?3p/FOXP3 axis regulates cell proliferation and autophagy in gastric cancer. J Cell Biochem 2020;121:3392-405.
- Nam RK, Benatar T, Amemiya Y, Sherman C, Seth A. Mir-139 regulates autophagy in prostate cancer cells through Beclin-1 and mTOR signaling proteins. Anticancer Res 2020;40(12):6649-63.