- Corresponding Author:
- C. S. Chauhan
Department of Pharmaceutics, B. N. College of Pharmacy, Udaipur-313 001, India
E-mail: chetansinghchauhan2k@rediffmail.com
| Date of Submission | 6 August 2006 |
| Date of Revision | 6 August 2007 |
| Date of Acceptance | 10 November 2007 |
| Indian J Pharm Sci, 2007, 69 (6): 748-752 |
Abstract
Asymmetric membrane-controlled porosity osmotic pumps of cellulose acetate with different pore forming agents such as glycerol, polyethylene glycol and dibutyl phthalate, were fabricated and studied for osmotic release behavior from the system. The effects of various pore-forming agents on the physical characteristics of the polymer film like tensile strength, weight variation, wall thickness, surface area, porosity and void volume were studied as a function of pore forming agent. The asymmetric membrane capsules were fabricated by dip-coating process using stainless steel mold pins of the shape of capsule body and cap and were evaluated for in situ formation of delivery pores. The effect of pore forming agents on the porosity of asymmetric membrane was characterized by scanning electron microscopy, and also by void volume determination of each membrane. Dye-test revealed that the fabricated systems followed osmotic release. Scanning electron microscopy revealed that the porosity depends upon the concentration and type of pore forming agent used. However no significant effect on the thickness and weight variation of asymmetric membrane capsule were observed, but the tensile strength of the film varied with the concentration and type of pore forming agent used. Film with polyethylene glycol-400 as pore forming agent exhibited good tensile strength.
Keywords
Asymmetric membrane, cellulose acetate, controlled porosity, dye-test, fi lm, glycerol
The asymmetric membrane is composed of a thin, dense skin layer supported by a thicker porous substrate layer. The asymmetric membranes first found their use in separation process like demineralization of saline water [1]. Asymmetric membrane function by controlling the rate of transport of various chemical species, allowing some to cross the membrane film more rapidly than others. This fundamental of the asymmetric membrane is utilized in osmotic drug delivery system. Asymmetric membrane coated controlled drug delivery devices consist of osmotic core surrounded by a membrane, which is asymmetric in nature. The release from this type of asymmetric membrane coated system takes place through the in situ pore formation due to the presence of water soluble additives in coating membrane, which in contact with aqueous environment dissolves and results in formation of micro porous membrane. The delivery form these types of system have been shown to follow osmotic release [2,3].
Asymmetric membrane capsule unlike gelatin, are fabricated from water insoluble polymers like cellulose acetate, ethyl cellulose and cellulose acetate butyrate. The asymmetric membrane shells are fabricated by dip-coating process involving phase inversion [4]. The asymmetric membrane capsule shell with varying porosity can be formulated depending on the type and concentration of pore forming agent. The tensile strength can be controlled by the nature of pore forming agent. The advantage of asymmetric membrane capsule is that it is fabricated independent of the core drug, unlike elementary osmotic pump (EOP) no laser [5] or mechanical drilling [6,7] is necessary, as in situ pore formation takes place and the drug is released from these pores through osmotic pumping. Other advantage of asymmetric membrane capsule is the higher rate of water influx allowing the release of drugs with lower osmotic pressure or lower solubility [8]. This system can also serve as a means for screening various drug-excipient compositions. Thus the feasibility of prolonged release of a drug under investigation can be determined in a relatively short time [9]. The process variables like concentration and type of polymer, type of pore forming agent, quenching time, are in control of the formulator, thus the asymmetric membrane capsule shell of desired porosity and physical properties like tensile strength, membrane thickness can be fabricated with ease. The objective of this study was to develop asymmetric membrane capsule shell and show that the prepared system follows osmotic principle. The effect of various pore-forming agent on the physical nature of the prepared system was studied.
Materials and Methods
Cellulose acetate, polyethylene glycol (PEG-400) and dibutyl phthalate (DBT) were obtained from Thomas Baker Chemicals Ltd., Glaxo Laboratories Ltd. and S. D. Fine Chemicals Ltd. All other chemicals used were of analytical grade.
Fabrication of asymmetric membrane capsule
The solution of cellulose acetate (15% w/v) was prepared in acetone:water (90:10 v/v) solvent system. Weighed quantity of cellulose acetate was added to the acetone:water solvent system and the resulting mixture was stirred in a well-closed beaker until a homogenous solution was formed. To this homogeneous solution of cellulose acetate different pore forming agents (glycerol, PEG-400 and DBT) at different levels (50%, 60% and 70% w/w of cellulose acetate) were added respectively, while stirring to ensure proper distribution of pore forming agent in cellulose acetate solution.
The stainless steel mould pins were fabricated with the dimensions so as to form a capsule body and cap. The moulds were dipped in the coating solution of cellulose acetate containing different pore forming agent for 2 min, and then removed carefully so as to form a thin layer of solution on the mould. The pins were taken out of the coating solution and briefly air dried for 30 s followed by quenching in aqueous solution of glycerol (10% w/v) for 3 min. This resulted in phase inversion and formation of asymmetric membrane. The resulting membrane was stripped off and trimmed to desired size and stored for future study. The resulting capsules were white and opaque in appearance.
Osmotic release study
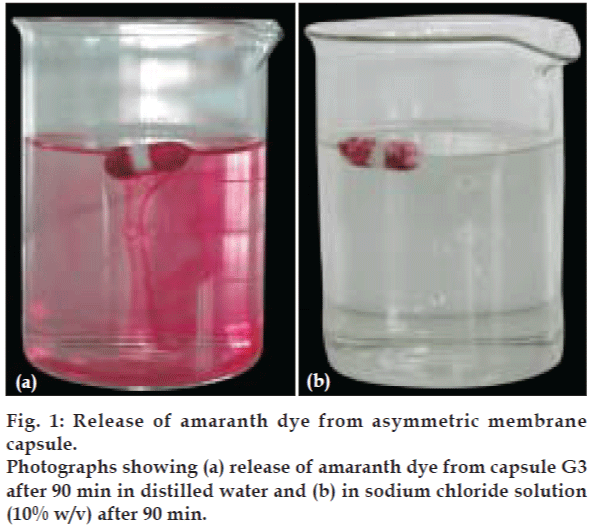
The capsule shell of asymmetric membrane containing different types of pore forming agent were filled with a highly water soluble amaranth dye. The dye was filled in each of the capsule body manually and the cap was snugly fitted to the body of capsule and finally sealed with a sealing solution of cellulose acetate only (16% w/v), to ensure that no release takes place form the seal. The capsules containing the dye were placed in the distilled water and solution of sodium chloride (10% w/v) respectively. The capsules were then observed for release of the colored dye in each of the media. The time taken for initial release of the dye from the capsule was recorded and correlated to the concentration and type of pore forming agent present in the shell of each of the capsule. The release of dye from G3 capsule shell, both in distilled water and in solution of sodium chloride (10% w/v) is shown in fig.1a and 1b.
Physical parameters of prepared asymmetric membrane capsule
The thickness of the wall and the effective surface area of the asymmetric membrane capsule were measured by Mitutoyo digital micrometer. The weight variation of each of the prepared system, after snugly fitting the cap and body of each of the system was determined using Fischer brand digital balance. The porosity and structure of asymmetric membrane was characterized by scanning electron microscopy (SEM, Lyca electron optics - 340)
Tensile strength determination
Tensile strength of each of the prepared asymmetric membrane was determined. The instrument used was designed as described by Agarwal et al [10]. A small strip of the membrane of the capsule of the area 2 cm2 was cut on a glass plate with a sharp blade so it had a smooth margin. One end of the membrane was fixed between adhesive tapes to give support to the membrane when placed in the film holder. Another end of the membrane was fixed between the adhesive tapes with a small pin sandwiched between them to keep the strip straight while stretching. A small hole was made in the adhesive tape near the pin in which hook was inserted. A thread was tied to this hook, passed over the pulley and a small pan attached at other end to hold the weights. A small pointer was attached to the thread, which traveled over the graph paper affixed on the wooden plate. To determine the tensile strength weights were gradually added to the pan to increase the pulley force till the membrane was broken. The weight required to break the membrane was noted as the break force. Tensile strength was determined by using the following formula [11], (Tensile strength= Break force/a×b)(1+ΔL/L), where a, b and L are width, thickness and length of the test membrane, respectively and ΔL is the elongation.
Void Volume determination
The void volume of each of the asymmetric membrane as the function of pore forming agents present at different level was determined. The weight of the empty capsule (Wo) was obtained. The weighed capsule was put into a vial filled with distilled water and left overnight to effect complete quenching of the pore-forming agent present in the wall of the capsule shell. It was made sure that the capsule was completely immersed in the water. The capsule was taken out of the vial, wiped with tissue paper and immediately weighed (Ww). The capsule was then placed into an oven at 50ο; it was periodically weighed until a constant weight was obtained (Wd). The volume of the pore forming agent (Vp) present in the capsule wall was measured by (Wo-Wd)/ρ, where ρ is the density of pore forming agent used. The total volume of water (Vw) present in the dry film was measured by (Ww-Wd)/1 (density of water = 1 g/cm2). The void volume of the polymer per unit weight of polymer was determined by (Vw-Vp)/Wd.
Results and Discussion
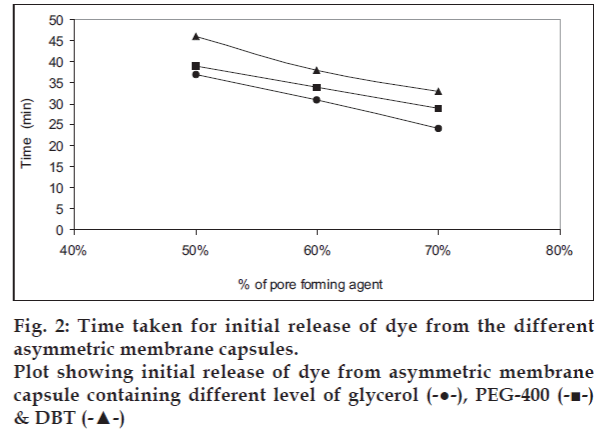
The asymmetric membrane capsules prepared with various type of pore forming agent appeared to be white and opaque with no visible physical defects. In situ pore formation for delivery was demonstrated by dye-test shown in fig. 1. The effect of pore forming agent on onset of dye release is shown in fig. 2. The onset of dye release is shown to depend upon the concentration and type of pore forming agent used. It was observed that the onset of dye release decreased as the concentration of pore forming was increased. The onset of dye release was shown to decrease in the order of pore forming agent: Glycerol>PEG-400>DBT. Thus onset of dye release depends upon the time taken for the in situ pore formation in the asymmetric membrane of each of the system. The observation suggests that when glycerol is used as a pore-forming agent the lag time for release can be reduced.
Dye-test also revealed that all the prepared systems followed osmotic release. All the asymmetric membrane capsules released dye after a lag time when suspended in distilled water, but none of the system showed the release of the dye when suspended in sodium chloride solution (10% w/v) as shown in fig.1b. This may be attributed to the fact that the osmotic release from the system was inactivated by the higher osmotic pressure of the surrounding medium i.e.10% w/v sodium chloride solution, which did not allowed the system to osmotically release the dye. Thus the system was shown to follow the osmotic release.
Membrane thickness and surface area (Table 1) were found to be almost same for all the asymmetric membrane capsule with slight variation. However the weight of the capsules increased as the concentration of the pore forming agent was increased the capsules with glycerol as pore forming agent weighed heavier followed by PEG-400 and DBT.
| Capsule shell code | G1 | G2 | G3 | P1 | P2 | P3 | D1 | D2 | D3 |
|---|---|---|---|---|---|---|---|---|---|
| Membrane | 0.273 ± 0.33 | 0.244 ± 0.21 | 0.224 ± 0.4 | 0.291 ± 0.24 | 0.262 ± 0.21 | 0.276 ± 0.14 | 0.272 ± 0.19 | 0.278 ± 0.23 | 0.260 ± 0.28 |
| thickness (mm) | |||||||||
| Surface area | 639.21 ± 1.5 | 641.04 ± 1.57 | 641.32 ± 1.4 | 640.25 ± 0.23 | 640.64 ± 1.16 | 640.33 ± 0.8 | 638 ± 0.32 | 640.64 ± 1.71 | 640.52 ± 0.8 |
| (mm2) | |||||||||
| Capsule shell | 79.28 ± 1.96 | 84.37 ± 1.64 | 87.31 ± 1.73 | 57.3 ± 1.13 | 64.75 ± 1.18 | 71.92 ± 1.27 | 55.59 ± 1.27 | 60.4 ± 1.28 | 62.63 ± 1.34 |
| weight (mg) | |||||||||
| Tensile strength | 0.109 ± 0.24 | 0.137 ± 0.83 | 0.178 ± 0.32 | 0.127 ± 0.59 | 0.164 ± 0.31 | 0.215 ± 0.74 | 0.052 ± 0.91 | 0.064 ± 0.15 | 0.082 ± 0.18 |
| (kg/cm2) | |||||||||
| Void volume | 2.604 ± 0.32 | 3.939 ± 0.71 | 4.935 ± 0.17 | 1.815 ± 0.28 | 2.579 ± 0.02 | 3.496 ± 0.07 | 0.889 ± 0.22 | 1.267 ± 0.39 | 1.479 ± 0.87 |
Each listed value is mean ± standard deviation of three repetitions. Twenty capsules were used for determination of average capsule shell weight. G1, G2 and G3 represent 50%, 60% and 70% glycerol; P1, P2 and P3 represent 50%, 60% and 70% PEG-400; D1, D2 and D3 represent 50%, 60% and 70% DBT in asymmetric membrane respectively.
Table 1: Average physical characteristics of asymmetric membrane containing different pore forming agent
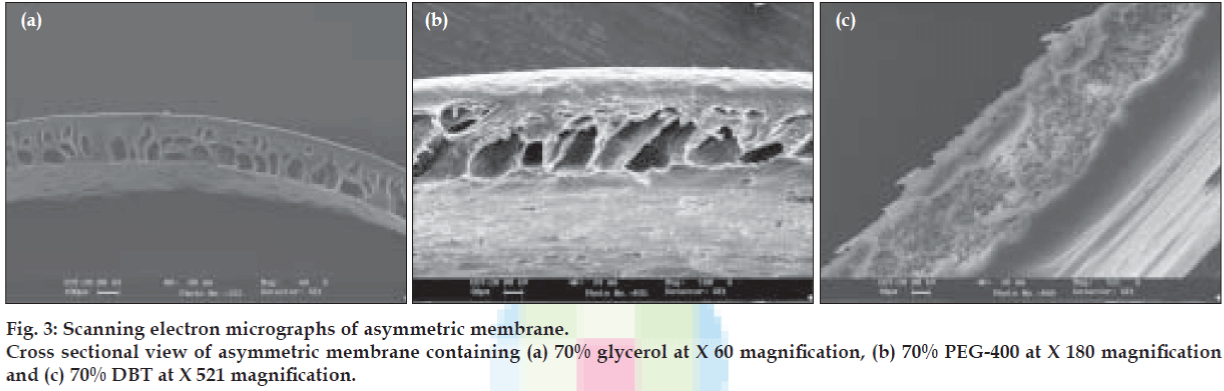
SEM showed these films to be asymmetric in nature. The porosity of cellulose acetate was shown to increase with the increase in the concentration of the pore forming agent. But the porosity was found to significantly alter with the type of pore agent used as shown in fig. 3c. The porosity of cellulose acetate films containing glycerol were found to be higher followed by PEG-400 and DBT. The size of the pores of asymmetric membrane also increased in the following order; glycerol>PEG-400>DBT.
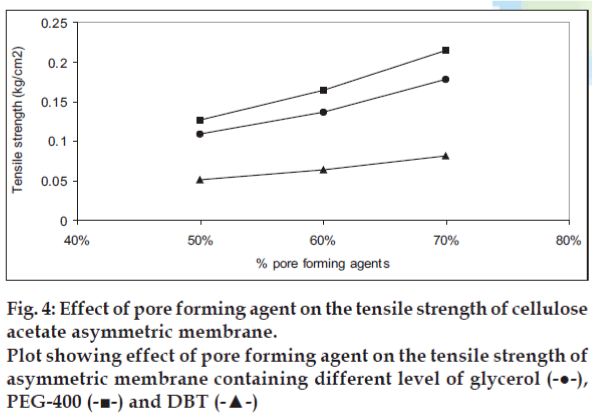
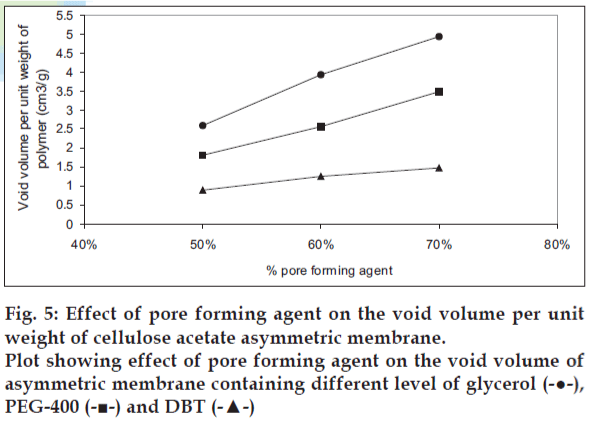
The effect of pore forming agent on the tensile strength is presented in fig. 4. The cellulose acetate membrane containing PEG-400 as pore forming agent showed best tensile strength while cellulose acetate containing DBT showed poor tensile strength. The effect of pore forming agent on void volume of the cellulose acetate films is shown in fig. 5. The void volume of each of the film was determined as function of the volume of the pore-forming agent leached out of the membrane during overnight dip in the vial resulting in void formation. The void volume per unit weight of polymer was found to be highest in case of glycerol followed by PEG-400 and DBT. A liner relationship between the concentration of pore forming agent and void volume per weight of polymer was observed. As a statistically significant correlation was demonstrated: for glycerol, r2 = 0.9929; for PEG- 400, r2 = 0.9973 and for DBT, r2 = 0.9871.
The prepared system can be extended to controlled osmotic delivery of pharmaceutical agents as the dye test suggests osmotic release and in situ pore formation. By the proper choice of pore forming agent the lag time for initial release of drug can be controlled. The porosity and void volume per unit weight of the asymmetric membrane depends on the type and concentration of pore forming agent incorporated; the desired porosity can be achieved to suit the osmotic delivery of a particular drug i.e. higher porosity may be desired for a poorly water soluble drug and vise versa.
References
- Loeb S, Sourirajan S. Sea water demineralization by means of an osmotic membrane. Adv Chem Ser 1963;38:117-32.
- Zentner GN, McClelland GA, Sutton SC. Controlled porosity solubility and resin modulated osmotic drug delivery. J Control Release 1991;16:237-44.
- Lin Y, Ho H. Investigation on the drug releasing mechanism from an asymmetric membrane coated capsule with an in situ formed delivery orifice. J Control Release 2003;89:57-69.
- Ende MT, Herbig SM, Korsemeyer RW, Chidlaw MB. Osmotic drug delivery from asymmetric membrane film coated dosage form. In: Wise DL, editor. Handbook of Pharmaceutical Release Technology. 1st ed. New York: Marcel Dekker; 2000. p. 751-85.
- Theeuwes F, Saunders RJ, Mefford WS. Process for forming outlet passageway in pills using a laser. US patent No., US4088864, 1978.
- Verma RK, Mishra B. Studies on formulation and evaluation of oral osmotic pump of nimesulide. Pharmazie 1999;54:74-5.
- Theeuwes F, Swanson D, Wong P, Bonsen P, Place V, Heimlich K, et al. Elementary osmotic pump for endomethacin. J Pharm Sci 1983;72:253-8.
- Thombre AG, DeNoto AR, Gibbes DC. Delivery of glipizide from asymmetric membrane capsule using encapsulated excipients. J Control Release 1999;60:333-41.
- Thombre AG, Cardinal JR, DeNoto AR, Herbig SM, Smith KL. Asymmetric membrane capsule for osmotic drug delivery i. development of manufacturing process. J Control Release 1999;57:55-64.
- Agarwal AK, Seth AK, Saini TR. Evaluation of free films. Indian Drugs 1985;23:45-7.
- Allen DJ, DeMarco JD, Kwen, KC. Free films I: Apparatus and preliminary evaluation. J Pharm Sci 1972;61:106-10.