- *Corresponding Author:
- S. Lodhi
Sri Sathya Sai Institute of Pharmaceutical Sciences, Ram Krishna Dharmarth Foundation (RKDF) University, Bhopal, Madhya Pradesh 462033, India
E-mail: srlodhi78@gmail.com
| Date of Received | 21 July 2021 |
| Date of Revision | 26 September 2021 |
| Date of Acceptance | 01 July 2022 |
| Indian J Pharm Sci 2022;84(4):821-831 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The main focus of the work was to develop a hydrogel of carboxy methyl cellulose tailored dextran containing piroxicam intended for transdermal delivery. Firstly, hydrogel was formulated by conjugation of carboxy methyl cellulose with amino dextran and then piroxicam was mixed to encapsulation in the hydrogel matrix. The hydrogel characterization was performed in terms of zeta potential, entrapment efficiency, morphology and in vitro drug release. The size of the particles was found 134 nm of carboxy methyl cellulose-dextran hydrogels and entrapment efficiency was observed as 87.36 %±1.23 % and 72.35 %±2.35 % in case of carboxy methyl cellulose-dextran hydrogels and carboxy methyl cellulose hydrogels, respectively. An in vivo study for piroxicam loaded carboxy methyl cellulose-dextran hydrogels formulation was performed according to more (6.22 mg/ml) than carboxy methyl cellulose hydrogels (plain) formulation concentration in plasma (3.21 mg/ml). Carboxy methyl cellulose hydrogels in case of epidermis, the piroxicam retained was 15.61±1.5 mg/ml and in dermis it was 6.24 %±1.2 % as well as in case of carboxy methyl cellulose-dextran hydrogels in epidermis the piroxicam retention was 5.21±1.19 mg/ml and in dermis was 26.34±0.5 mg/ml in 12 h. Piroxicam loaded hydrogel were found effective in wound healing through improved collagen and protein content, and restoration of antioxidants in granuloma tissues.
Keywords
Dextran, carboxy methyl cellulose, hydrogel, piroxicam, wound healing, transdermal
Hydrogels (HGs) are formed of networks of cross linked three dimensional hydrophilic polymers which swells instead of dissolving in contact with water[1]. HGs are widely used in a long range of pharmaceutical and biomedical application because it has rubbery nature with high water content[2]. With these special characters HG materials show resemblance with natural living tissue as compared to other synthetic material of any class. As the gel has water base it produces good patient compliance as it can be washed by water and medication can be blocked at any time. In other hand it is not very sticky as seen in the case of ointment. Now a days HGs are been promising for the researchers over the ointments and conventional creams as it is more efficacious and cost effective than other topical formulations[3,4]. Lipophilic drug molecules can be transported by their dissolution into intercellular lipids near the stratum corneum cell. Hydrophilic molecules can be absorbed with the help of pores like opening of the hair follicles of sebaceous glands, but among the total skin surface comparative surface area of these openings is about 1 %[5]. As drug molecule crosses the barrier of stratum corneal to reach dermal layer, a very fast and smooth systemic uptake occurs relatively. Normally absorption of drug topically occurs by passive diffusion. For any transdermal product the main issue is how the drug is effectively transported along with its prediction of drug performance with other formulations[6].
According to various studies of skin penetration with diffusion cell and excised skin of animal it was noted that the Dextran (DEX) polymer was effective in skin penetration of model permeants, such as 5-Fluorouracil (5-FU) and indomethacin. Permeability of drug is increased in case of DEX in skin with negative charge[7,8]. The current study has been carried out for the development and evaluation of DEX conjugated Carboxy Methyl Cellulose (CMC)-HGs for topical delivery of piroxicam. By covalent coupling method DEX was attached with the HGs of CMC-DEX loaded with piroxicam followed by in vitro and in vivo characterization of HGs was performed.
HGs like Solid Lipid Nanoparticles (SLNs) are best and most commonly used carriers for topical drug delivery because of their lipid components as its excipients are widely used in commercial cosmetics and therapeutic formulations. As lipid particles are smaller in size and provide close contact of drug to the stratum corneum and increase the amount of drug penetrating into the skin. Reduced systemic availability and increased drug stability like prominent considerations can be opted by control release through these carriers due to solid lipid matrix[9].
Piroxicam is widely used antipyretic, analgesic which belongs to category of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and shows promising result in the treatment of osteoarthritis and, chronic and acute rheumatoid arthritis. Due to gastrointestinal mucosa ulceration side effects, it is not given orally, hence better choice in the form of transdermal drug delivery system[10]. The main objective of the research work is to formulate piroxicam loaded CMC-DEX HGs for topical application in order to overcome the adverse effects through oral administration. The prepared formulation of HGs was characterized to validate its predefined properties and anti-inflammatory properties was evaluated and compared with the present standard.
Materials and Methods
Chemicals:
Piroxicam was provided as gift sample from Eros Pharmaceuticals laboratory, Bangalore, India (99 % purity). 1-Ethyl-3,3-Dimethyl Aminopropyl Carbodiimide (EDAC), N-Hydroxy Succinimide (NHS), Dicyclohexyl Carbodiimide (DCC), High Performance Liquid Chromatography (HPLC) grade methanol and water were procured Himedia Lab Pvt. Ltd. Mumbai, India, CMC and DEX were procured from Himedia Lab Pvt. Ltd. Mumbai India, dialysis membrane, 12.000–14.000 molecular weight cut off was procured from Spectrum Laboratories Inc., Rancho Dominguez, Canada. All other chemicals and excipients were of analytical purity and used as purchased.
Preparation of piroxicam loaded CMC-DEX HGs:
In 10 ml of hot distilled water, 50 mg CMC was dissolved and 250 mg of EDAC was added for the activation of CMC (polymeric dispersion) and with continuous stirring for 3 h at 5000 rpm utilizing magnetic stirrer (Remi, India). 175 mg of DCC was added finally to obtain the activated polymeric CMC dispersion formation. In the dispersion of CMC, piroxicam drug was added to get piroxicam loaded CMC HGs. On another side AminoDEX (50 mg) (AminoDEX were synthesized as per method reported by Zalipsky et al., was dissolved in 100 ml of distilled water and added in drop wise manner into activated CMC dispersion under constant stirring[11]. Piroxicam (50 mg) was dissolved in ethanol (10 ml) and then the drug dispersion was added in drop wise manner onto CMC-DEX dispersion and magnetically stirred (Remi, Mumbai, India) for 10 h. For the separation of CMC-DEX with unreacted DEX and CMC the reaction mixture was gently dialyzed (MWCO 12–14 kDa). Finally the copolymer obtained was named CMC-DEX HGs and for further use, it was dried under vacuum lyophillizer (Tanco Laboratory Equipment, PG-302).
Characterization of Piroxicam HG
Morphology:
The structure and surface characteristics of CMC-DEX HGs was determined by Atomic Force Microscope (AFM) (AIST-NT Smart SPM 1000, CA). AFM of the HG and surface characteristics of the selected CMC-DEX HGs formulation was done at glass substrate in Assist-control (AC) mode and by Scanning Electron Microscopy (SEM) (JELO 5400, Japan), respectively. For the sample of scanning electron microscopy in a double adhesive tape the HG powder was lightly sprinkled and was attached with aluminum stub then with the help of sputter coater, stub were coated with gold to a thickness of about 300 Å.
Determination of entrapment efficiency:
Entrapped drug determination was performed by pre weighed piece of totally dry HGs. 10 mg of CMC-DEX HGs was added to aqueous solution and for a period of 24 h it was equilibrated to confirm equilibrium loading. To determine the quantity of drug entrapped HPLC (Agilent Technologies, 1220 infinity LC, United Kingdom (UK)) was performed. Various wavelength detectors were used in HPLC for analysis of various samples, Zorbax, C18 column (250×4.60 mm) at 334 nm. Water:methanol (95:5 v/v) was taken as mobile phase it was pumped at a flow rate of 1 ml/min at 25º. With the following formula drug entrapment efficiency was calculated.
Percentage encapsulation efficiency=(Practical drug content/theoretical drug content]×100
Particle size analysis:
CMC-DEX HGs particle size was measured by laser diffraction particle size analyzer (DTS Ver. 4.10, Malvern Instruments, WR14 1XZ, UK) by filling the dilute dispersion of HGs in the chamber with this the average particle size was examined. Further with laser doppler anemometry the zeta potentials of the HGs were noted by using Malvern Zetasizer (DTS Ver. 4.10; Malvern Instruments, WR14 1XZ, UK).
Percentage cumulative drug release:
Drug loaded HGs formulation was placed in a basket with 50 ml of physiological fluid at 37º±0.5º .After 1-6, 8, 10, 12 and 24 h, 1 ml of sample was withdrawn maintaining the sink condition by replacing the same volume of fresh medium. For the examination of drug released HPLC was done at different intervals. For the analysis of the amount of drug released concentration the calibration curve was made for drug solution.
In vitro permeation study:
Piroxicam loaded CMC-DEX HGs in vitro permeation was performed with using full thickness dorsal skin taken from albino rat (male, 120±20 g) after approval of ethics committee as per the protocol (Ethical Committee registration no. is 147/PO/a/11/CPCSEA). Before using rats they were kept fast. With the help of razors dorsal skin hair were removed. Further with 8 % Sodium sulfide (Na2S) solution rest of fur were removed. After harvesting the skin after 24 h the rat were sacrificed. Gently subcutaneous connective tissues and fat were removed[12]. After that skin samples were stored on Franz diffusion cells with the stratum corneum side facing in the upward direction into the donor compartment. Receptor compartment was filled with 20 ml of physiological saline having 1% tween 80, at 32º±0.5º in a water bath. About 250 ml solution of HGs was applied on the stratum corneum of the skin. For keeping constant volume 0.5 ml of the acceptor fluid is collected at fixed interval and replaced by fresh medium of equal volume. HPLC was further employed to know the cumulative amount of piroxicam permeated through excised skin by the sample with plotted function of time. In addition to that piroxicam fluxes (Js, µg cm-2 h-1) through the skin, from the piroxicam loaded formulation and plain formulation is calculated from the slope of linear portion of the cumulative amounts permeated with in the skin per unit surface area vs. time plot.
In vivo permeation study:
The condition and feeding management of rat is same as mentioned above in in vitro permeation study. Before using rats they were kept fast. With the help of razors dorsal skin hair were removed. Further with 8 % Na2S solution rest of fur were removed approximately before 24 h. Initially 0.6 ml of HGs loaded with piroxicam was applied to skin of rat and along with it plain formulation was also applied to skin of other rat. At the fix interval of 0.5, 1, 2, 3, 6, 9 and 12 h, 1 ml of blood sample was collected by retro orbital puncture and to achieve plasma from supernatant blood sample were centrifuged (4000 rpm for 5 min). After this the rat was sacrificed and administered skin was stripped properly. The skin was frequently washed with alcohol water solution to eliminate the piroxicam remains on the surface of the excised skin. Later with heat separation method epidermis and dermis was separated[13]. Further sample of skin was kept in a watch glass for 2 min at 60º±1º and then epidermis is scraped from the skin with dull scalpel blade. In 1 ml of physiological saline the dermis and epidermis was thoroughly mixed and homogenized for 3 min. And then by HPLC analysis, the amount of piroxicam in the blood, epidermis or dermis was observed.
In vivo wound healing evaluation:
Animal protocol: Wistar albino rats 150-200 g were chosen for in vivo examination. The animals were taken care of with standard pellet diet (Hindustan switch Ltd. Bangalore) and they were held under standard natural states of research facility temperature and water. The animals kept up interchange patterns of haziness and light at 12 h. They were acclimatized to the research center condition for multi-week before beginning the test. The animals were fasted for in any event 12 h before the beginning of the investigation. The test conventions were endorsed by the Institutional Animal Ethics Committee. Six animals were taken in each group and complete three groups were made. The group I was control and treated with vehicle, while group II was treated with piroxicam stacked CMC-DEX HGs and group III was treated with standard 5 % w/w povidone iodine ointment USP (Zenith Drugs Pvt Ltd, India) salve was utilized.
Dead space wound creation: For granuloma tissue formation study, dead space wound model was used with weight measurement of granuloma weight along with biochemical measurement. Before wound creation, animals were anaesthetized with light ether. With proper implantation of sterile polypropylene tube (2.0×0.5) in a wound that was made in the lumber region, at the dorsal portion of rats. On the 9th d of post wounding, granuloma tissue formed on implanted tube was dissected out and the weight of dry granuloma tissue from each animal was noted and kept in 10 % formalin for the biochemical assay[14-17].
Hydroxyproline content measurement:
A wound tissue was examined for hydroxypyroline content on 9th post wounding day which is a basic content of collagen. Collected wound tissues were dried at 60º-70º followed by hydrolyzing with 6 N hydrochloric acid for 4 h at 130°. After neutralization of hydrolysate it was treated with Chloramine-T oxidation for 20 min. An Ehrlich reagent was added with maintaining temperature 60° and color formed was observed at 557 nm. With the help of standard hydroxyproline, content values reported as g/mg dry weight of tissue[18].
Protein content and granuloma weight determination:
On the 9th d of post wounding, one part of the skin tissues was used for protein content determination by reported method[19]. After usual processing of skin tissues to produce lysate was treated with the mixture of copper sulphate, sodium tartrate and sodium carbonate and left for 10 min for reaction with Folin-Ciocalteau reagent that produces a bluish color. After 20 min, mixture was used for taking absorbance at 650 nm by using spectrophotometer.
Enzymatic and non-enzymatic antioxidant assay:
In the dead space wound model, one part of granuloma tissue was used for antioxidant assay. Catalase (CAT) activity was evaluated through the degradation of hydrogen peroxide as indicated by the standard method[20]. Activity of Superoxide Dismutase (SOD) was determined through stoppage of epinephrine autoxidation by the protein as per reported method[21]. Level of reduced Glutathione (GSH) in granuloma tissue was determined by the technique for Moron et al.[22].
Histopathology:
For this study, firstly animals were anaesthetized by diethyl ether before taking skin sample. From each group sample of wound tissue were collected on 18th d. 10 % buffered formalin was used to fix the sample and after processing it was blocked with paraffin and finally cut into 6 m thickness and stained with Hematoxylin and Eosin (HE) stains. The tissues were observed by light microscope[23].
Statistical analysis:
Results are shown as the mean±Standard Deviation (SD). Treated groups are examined with standard group. Results were determined statistically with student’s T-test. The information was found significant at p<0.01.
Results and Discussion
The aim of present study was to evaluate the potential of CMC attached DEX HG as an effective delivery of piroxicam via topical route for potential wound healing activity. The synthesized HG formulation was characterized by various evaluation parameters to explain their potential features and properties, like morphological assessment (by SEM and AFM), entrapment proficiency, drug release characteristics and also investigate in vitro and in vivo permeation analysis to find out the rate of drug transportation between skin layers. At the end we investigated the in vivo wound healing profiling of CMC-DEX HGs and validate its predefined properties and anti-inflammatory properties was evaluated and compared with the present standard.
There are various variables which can affect release profile as well as drug entrapment in the HG. Results show that with increased particle size of system (Table 1), decrement in the drug encapsulation efficiency of HGs was noted. Due to increase in viscosity and particle size emulsion bigger droplets were formed[24]. The amount of drug can alter the entrapment efficiency of its own and particle size of the HGs. In this regards the particle size of piroxicam HGs was found increased with respect to increasing amount of drug. Initially the entrapment efficiency of drug increases with gradual increase in amount of drug. As the ratio of drug to HGs matrix increases the available space for the drug to be entrapped is reduced significant decreases the entrapment efficiency[25]. Thus the poor water solubility of a drug is helpful for its increase entrapment proficiency in hydrophobic cores[26].
| S. No. | Formulations | Particle size (nm) | % Entrapment efficiency | Zeta potential (mV) |
|---|---|---|---|---|
| 1 | CMC HGs | 153.67±1.50 | 72.35±2.35 | -7.69 mV. |
| 2 | CMC-DEX HGs | 134.98±1.10 | 87.36±1.23 | -13.5 mV |
Table 1: Percentage Entrapment Efficiency, Particle Size and Zeta Potential of different HG Systems.
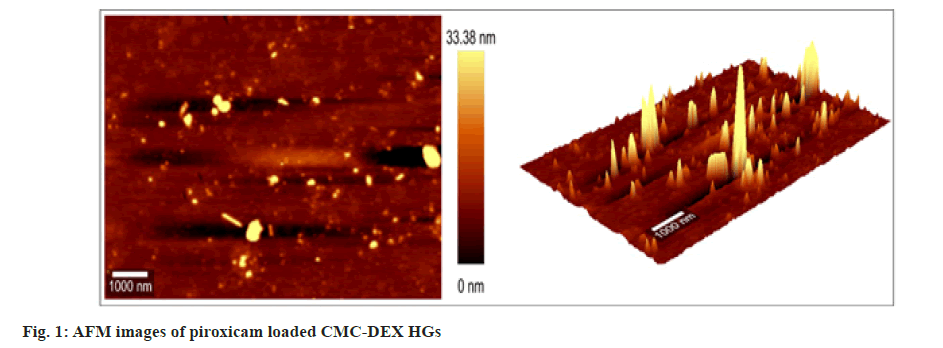
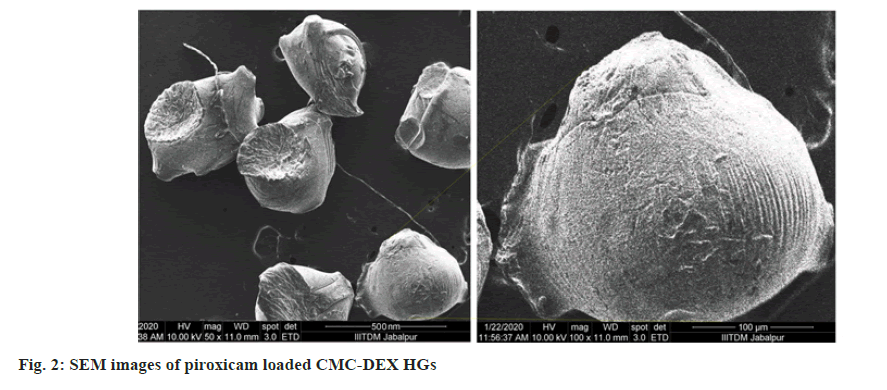
With utilizing of AFM and SEM, the structure and surface characteristics of CMC-DEX HGs was examined. The CMC-DEX HGs was noted in uniformed size distribution by AFM depicted in fig. 1. The spherical shape and nanometric size range characterized by SEM and image shown in fig. 2.
The drug entrapment efficiency of optimized CMC-DEX HGs and CMC HGs formulation was found to be 87.36 %±1.23 % and 72.35 %±2.35 %, respectively (Table 1). As the drug concentration, increased the drug entrapment efficiencies was also enhanced which results in drug entrapment in dense and high amount. It can be considered that as the quantity of piroxicam increased there was cross linking between amino group of DEX and carboxyl moiety of CMC. Further as the compactness in insoluble dense matrix with crosslinking of the polymer was increased, it results in extra drug entrapment in the CMC-DEX HGs.
The average particle size of CMC-DEX HGs and CMC HGs were obtained to be 134.98±1.10 nm and 153.67±1.50 respectively (Table 1), whereas particles size distribution of CMC-DEX HGs was examined to be 72.3 % of 136.31 nm, 14.5 % of 134.67 nm and 13.2 % of 132.5 nm. The zeta potential of synthesized CMC-DEX HGs and CMC HGs was observed -13.5 mV and -7.69 mV respectively.
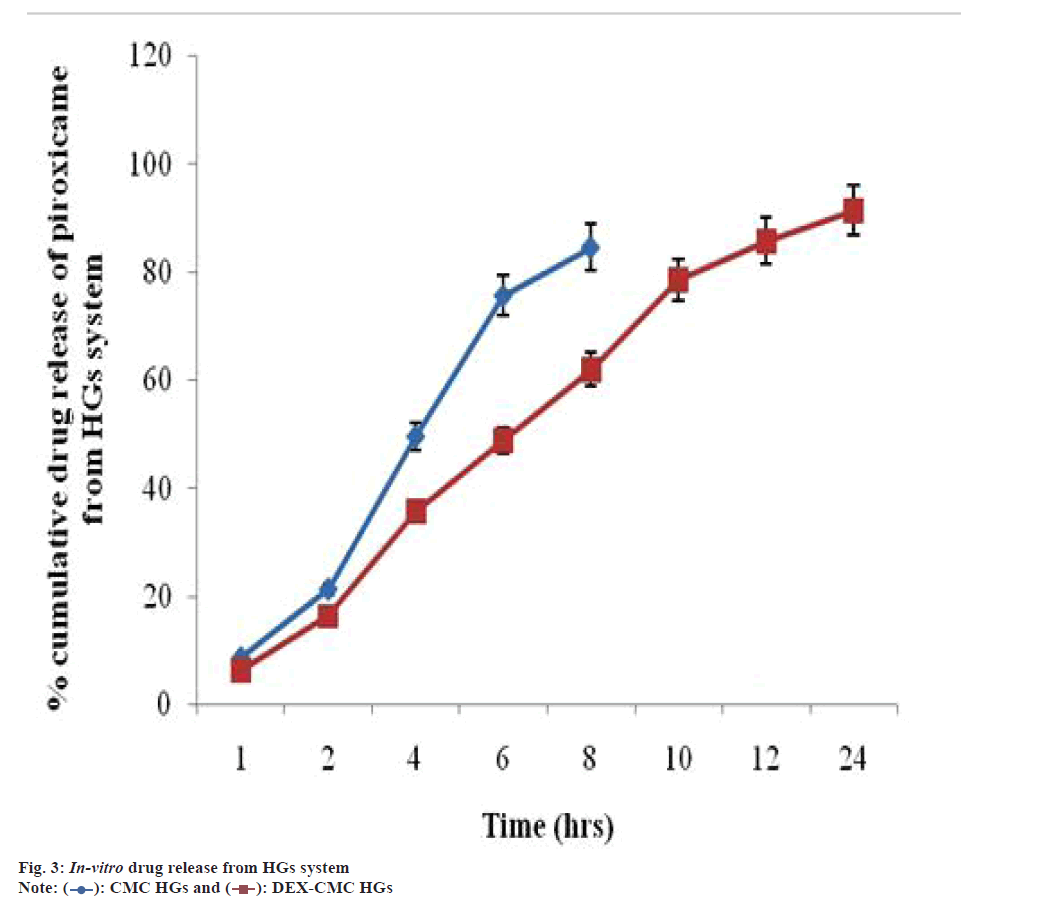
Among current available methods for sustained and controlled drug release show various merits for skin. The desired constant drug concentration at the site of delivery offered by systems for the controlled local release, reduced potential and lower systemic drug concentrations for deleterious side effect[27]. After the treatment with biodegradable polymer there is no need for the removal of delivery system from skin and HGs have promising ingredients for healing of wounds as it has physical and mechanical property. Drug release curve shows initially rapid release of piroxicam and in longer time shows constant release. A time prolongation release was noted for the drug from the CMC-DEX HGs. In 24 h, release percent of drug from CMC-DEX HGs was noted around 91.3 %. Further percent release of CMC HGs was noted in 8 h as 84.51 % respectively, which was represented in fig. 3. Due to the breakdown of the strong hydrogen bonding among CMC-DEX and CMC-water molecules and to release carboxyl groups for the crosslinking reaction with the amine group of DEX (CMC-Carboxyl (COOH) crosslinking with Amine (NH2)-DEX). After the consumption of carboxyl group by NH2 moiety the crosslinking chains become less soluble[19].
For study in vitro skin permeation in Franz diffusion cells for an effective diffusion area of 3.12 cm2 is taken. Initially piroxicam loaded CMC-DEX HGs amount in the donor rooms is the same and flux rates resulted from permeation of piroxicam experiments is given in Table 2. Further examined the flux rate of piroxicam HG formulation is increase in quantity than plain formulation. No lag time seen in the permeation of piroxicam loaded HG but in the control has shown a lag time of 1 h, therefore it clearly shows that permeation followed the zero order release kinetics and the steady-state flux (Js) of plain formulation 4.80 mg cm-2 h-1 where as that of piroxicam loaded HG formulation was 7.95 mg cm-2 h-1 respectively. Then the significant permeation difference also depends on the nature and property of the lipid. The skin surface was covered by a film of piroxicam loaded HG after application. The cover makes drug carrier instantly accessible to the skin, which helps drug permeation across the stratum corneum.
| S. No. | Formulation | Transdermal flux | Enhancement ratio | Permeation coefficient |
|---|---|---|---|---|
| 1 | Plain drug formulation | 4.8 | 1.13 | 4.80×10-4 |
| 2 | Piroxicam loaded CMC-DEX HGs. | 7.95 | 1.48 | 7.95×10-4 |
Table 2: Skin Permeation Parameters of Plain Formulation and Piroxicam Loaded Formulation.
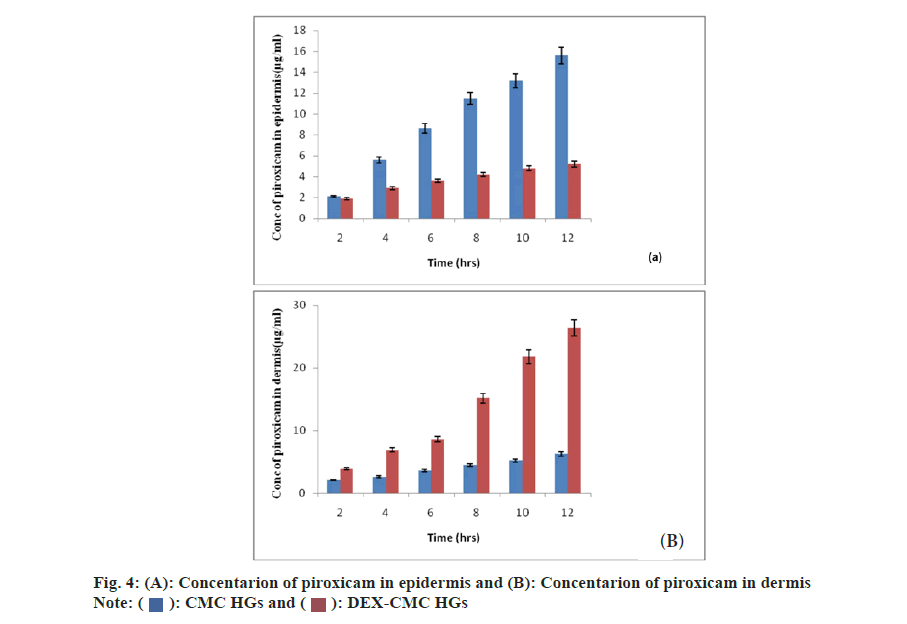
The objective of in vivo permeation study of piroxicam loaded HG is to know how feasibly piroxicam can be delivered into blood through skin. From the dispersion of the HG formulation it is cleared that piroxicam concentration has proven much lower concentration for the plain. In Table 3, for in vivo permeation study pharmacokinetic parameter is given. Further it is demonstrated that the piroxicam loaded HGs formulation has shown maximum concentration (Cmax) (6.22 mg/l) in comparison to the piroxicam loaded CMC HGs (plain) formulation (3.21 mg/l). The plasma Area Under the Curve (AUC) (0–t) of piroxicam loaded HGs formulation was 21.35 h mg/l, which is considerably superior in quantity in comparison to of the plain formulation 8.36 h mg/l (p<0.05). Due to the close contact between HGs formulation and stratum corneum, above results has been found. The long term hydration of skin is maintained by forming an occlusive film on skin by HGs formulation, hence provides the stratum corneum a compact structure helps in penetration of piroxicam into blood through the skin[28]. The amounts of piroxicam in dermis and epidermis is being examined the residual piroxicam within skin (fig. 4). The piroxicam content of CMC HGs has been deposited in epidermis enhanced frequently, even up to 15.61±1.5 mg/ml at 12 h, in other hand in dermis; the piroxicam examined was 6.24±1.2 mg/ml in 12 h. Later in CMC-DEX HGs, in epidermis the release of piroxicam from HGs system was comparatively reduced, then CMC HGs was 5.21±1.19 mg/ml in 12 h, and the level of drug in dermis is found comparatively increased level in dermis was 26.34±0.5 mg/ml in 12 h. It is cleared that the different function mode may also be responsible for difference in permeability between test groups. For increase piroxicam penetrating by HGs into the viable skin is due to closed interaction between HGs formulation and the stratum corneum in blood circulation. Hence piroxicam HGs formed in small size increased its penetrating power when were in close contact with skin. Finally it is proved that when cross-linking is done between DEX and CMC HGs, it increase the penetration of piroxicam from prepared HGs system.
| Pharmacokinetic parameter |
Unit | Piroxicam loaded CMC-DEX HGs |
CMC HGs |
|---|---|---|---|
| AUC0-12 | h mg/l | 21.35 | 8.36 |
| AUC0-∞ | h mg/l | 22.98 | 5.87 |
| Tmax | h | 5.2 | 8.6 |
| Cmax | mg/l | 6.22 | 3.21 |
Table 3: Pharmacokinetic Parameters for in vivo Permeation Study.
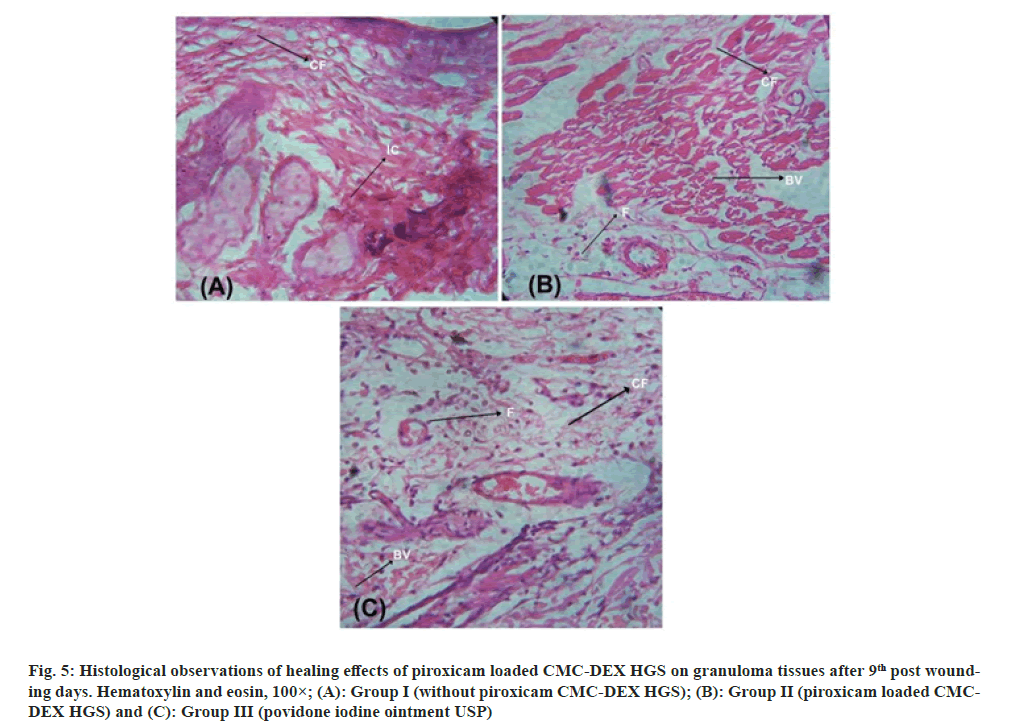
The data shows promising results of HG formulation for enhancing healing of wounds (fig. 5). The result provided that the regeneration of original tissue was efficient in wound treated with piroxicam loaded CMC-DEX as compared to reference group.
Fig. 5:Histological observations of healing effects of piroxicam loaded CMC-DEX HGS on granuloma tissues after 9th post wounding days. Hematoxylin and eosin, 100×; (A): Group I (without piroxicam CMC-DEX HGS); (B): Group II (piroxicam loaded CMCDEX HGS) and (C): Group III (povidone iodine ointment USP).
According to reports active oxygen are cytotoxic and inactivating cellular components may damage cells. Biological molecules like natural antioxidants can act readily or indirectly by quenching a free radical scavenging. In current study piroxicam loaded CMC-DEX HG was formulated and wound healing activity was investigated. The promising result was found as wound contraction rate was enhanced and healing time was reduced in animals treated with piroxicam loaded CMC-DEX HGs.
The noteworthy increment was seen in hydroxyproline and protein content in the benchmark group of animals. These perceptions were an impression of expanded collagen content that was additionally supported by histopathology and increase granuloma weight. This demonstrated improved collagen development by expanded cross-connecting while an expansion in dry granuloma weight arraigned higher protein content. Increase in hydroxyproline content likewise leads to the better development of collagen which dynamically prompts shrinkage of granulation tissue. Beginning expanded in protein content in the medication treated animals demonstrates that the medication act at each cell level. The expansion in explicit action in the medication treated animal in the entire days in the wake of injuring is reminiscent of the improved union of chemical protein. For dead space wound model, the hydroxylproline level of piroxicam loaded CMC-DEX group was increased in comparison to control group (Table 4). The piroxicam loaded CMC-DEX, protein content was 67.51±2.60 and reference ointment group was 75.84±2.61 and in group of animals found significant higher than control group (48.72±2.71). The piroxicam loaded CMC-DEX granuloma weight was 95.42±4.79, 94.26±4.29 and 61.74±3.17 mg in reference ointment, treated and in control group of animals simultaneously. The protein content of granulation tissues shows the degree of protein synthesis that reflects to cellular proliferation. The treated wounds increase in protein contents in comparison to control group show that piroxicam, stimulates cellular proliferation by an unknown mechanism. For granuloma tissue formation protein synthesis is important. On 9th d granuloma tissues were gathered from every single animal from each group and their dry granuloma weight was calculated (Table 4). Piroxicam loaded CMC-DEX treated group was found higher granuloma mass like the reference gathering of animal.
| Groups | Hydroxyproline (mg/g tissue) | Protein content (mg/g tissue) | Granuloma dry weight (mg) |
|---|---|---|---|
| I (Without piroxicam CMC-DEX HGS) | 32.82±2.54 | 48.72±2.71 | 61.74±3.17 |
| II (Piroxicam loaded CMC-DEX HGS) | 54.26±2.13** | 67.51±2.60* | 94.26±4.29* |
| III (Povidone iodine ointment USP) | 56.21±2.46** | 75.14±2.61* | 95.42±4.79* |
Table 4: Effect of Piroxicam Loaded CMC-DEX HGS on Various Wound Parameters of Dead Space Wound Tissues in Rats.
Inflammation phase involves, neutrophils and fibroblasts proliferation, infiltration of macrophages which are the basic sources for granuloma formation. Higher granuloma weight can related to higher protein content and indication of proliferative phase progression. Collagen is a major component of extracellular matrix. It contributes to the wound strength through healing process. After breakdown of collagen, it produces hydroxyproline and its peptides. For collagen turnover hydroxyproline is estimated as index. The newly formed collagen molecules are deposited at the wound surface and occurring cross linking with fibers. By remodeling of collagen along with formation of intra and inter-molecular cross links provided wound strength. Further when hydroxyproline level increased in piroxicam loaded CMC-DEX treated tissues it shows improved collagen turnover which results in fast curing of the treated wounds.
The degree of antioxidant enzymes and non-enzyme in skin tissues are given in Table 5. Critical increment in cell reinforcement level was seen in piroxicam loaded CMC-DEX HGs treated animals in contrasted and control group. Noteworthy increment in SOD, CAT and GSH were found correspondingly in a reference group of animals too. A huge increment in the cell reinforcement level was seen in granuloma tissues of dead space twisted after 9th d of treatment. The antioxidant plays a crucial job in postponed wound treatment. These cancer prevention agents are known to extinguish the superoxide radical and consequently control the harm of cells brought about by free radicals. In this way, the current examination uncovers that one of the systems of the improved mending by the piroxicam can be because of its ability to upgrade tissue antioxidant levels. These discoveries could legitimize in any event mostly, the consideration of piroxicam in the administration of wound healing.
| Groups | Enzymatic and non-enzymatic assay | ||
|---|---|---|---|
| SOD (μg/50 mg tissue) | CAT (μmol/50 mg tissue) | GSH (μmol/50 mg tissue) | |
| I (Without piroxicam CMC-DEX HGS) | 34.28±2.76 | 52.16±2.64 | 46.72±2.75 |
| II (Piroxicam loaded CMC-DEX HGS) | 47.62±2.47* | 76.42±3.58* | 64.23±3.21* |
| III (Povidone iodine ointment USP) | 51.64±2.84* | 77.84±3.64* | 67.94±3.26* |
Table 5: Effect of Piroxicam Loaded CMC-DEX HGS on Enzymes and Non-Enzymatic Antioxidants Level of Dead Space Wound Tissues in Rats.
In different treatment group, histopathological study of stained sections was showed various healing conditions of the wounded tissues. The histopathological data shows increased collagen fibers, fibroblast cells growth and presence of new blood vessels. The covering of wound site with newly formed epithelial tissue in both treated groups was observed. The dermal tailoring process in vehicle control group was found very slow and was confirmed by lower epithelialization time. Fibrosis and aggregation of macrophages were also observed in control group which shows incomplete wounds healing.
From the above investigations, it was concluded that piroxicam drug delivery can be appropriate via HGs system and it is also considered that CMC-DEX HGs system is a promising delivery tool for this drug. The larger amount of the drug can be entrapped in HGs and sustained released of drug was achieved for extended time. HGs possess a perfect size range for transdermal route and can be prepared with non-toxic and non-irritative characteristics, and can effectively treat inflamed or damaged skin. The schematic representation depicted in fig. 6.
Acknowledgments:
The author recognizes to the IIITDM, Jabalpur, Madhya Pradesh, for allowing AFM and SEM facility. The author is earnestly appreciative to SAIL SOPS-RGPV, Bhopal, India for providing particle size and zeta potential facility.
Conflict of interests:
The authors report no conflict of interests.
References
- Pooley SA, Rivas BL, Lillo FE, Pizarro GDC. Hydrogels from acrylic acid with N, N dimethyl acrylamide: Synthesis, characterization and water absorption properties. J Chil Chem Soc 2010;55(1);19-24.
- Kadajji VG, Betageri GV. Water soluble polymers for pharmaceutical applications. Polymers 2011;3(4):1972-2009.
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deli Rev 2002;54(1):3-12.
- Kim SJ, Shin SR, Lee YM, Kim SI. Swelling characterizations of chitosan and polyacrylonitrile semi interpenetrating polymer network hydrogels. J Appl Poly Sci 2003;87(12):2011-15.
- Surber C. Optimization of topical therapy: Partitioning of drugs into stratum corneum. Pharm Res 1990;7(12):1320-4.
[Crossref] [Google Scholar] [PubMed]
- Ranade VV, Hollinger MA. Drug delivery systems. 2nd ed. Boca Raton (FL): CRC Press; 1996.
- Fukushima S, Kishimoto S, Horai S, Miyawaki K, Kamiyabu S, Kamata Y, et al. Transdermal drug delivery by electroporation applied on the stratum corneum of rat using stamp-type electrode and frog-type electrode in vitro. Biol Pharm Bull 2001;24(9):1027-31.
[Crossref] [Google Scholar] [PubMed]
- Akimoto T, Nagase Y. Novel transdermal drug penetration enhancer: Synthesis and enhancing effect of alkyldisiloxane compounds containing glucopyranosyl group. J Control Release 2003;88(2):243-52.
[Crossref] [Google Scholar] [PubMed]
- Souto EB, Wissing SA, Barbosa CM, Muller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm 2004;278(1):71-7.
[Crossref] [Google Scholar] [PubMed]
- Cordero JA, Alarcon L, Ecribano F, Obach R, Domenech J. Comparative study of transdermal penetration of series of nonsteroidal anti-inflammatory drugs. J Pharm Sci 1997;86(4):503-8.
[Crossref] [Google Scholar] [PubMed]
- Zalipsky S, Gilon C, Zilkha A. Attachment of drugs to polyethylene glycols. Eur J Polym 1983;19(12):1177-83.
- Chen S, Liu W, Wan J. Preparation of coenzyme Q10 nanostructured lipid carriers for epidermal targeting with high-pressure microfluidics technique. Drug Dev Ind Pharm 2013;39(1):20-8.
[Crossref] [Google Scholar] [PubMed]
- Kligman AM, Christophers E. Preparation of isolated sheets of human stratum corneum. Arch Dermatol 1963;88:702-5.
[Crossref] [Google Scholar] [PubMed]
- Azeez S, Amudhan S, Adiga S, Rao N, Udupa LA. Wound healing profile of Areca catechu extracts on different wound models in Wistar rats. Kuwait Med J 2007;39(1):48-52.
- Asif A, Kakub G, Mehmood S, Khunum R, Gulfraz M. Wound healing activity of root extracts of Berberis lyceum Royle in rats. Phytother Res 2007;21(6):589-91.
[Crossref] [Google Scholar] [PubMed]
- Tajik H, Jalali FSS. Influence of aqueous extract of Yarrow on healing process of experimental burn wound in rabbit: Clinical and microbiological study. J Anim Vet Adv 2007;6(12):1464-8.
- Lodhi S, Pawar RS, Jain AP, Singhai AK. Wound healing potential of Tephrosiapurpurea (Linn.) Pers. in rats. J Ethnopharmacol 2006;108(2):204-10.
[Crossref] [Google Scholar] [PubMed]
- Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961;93(2):440-7.
[Crossref] [Google Scholar] [PubMed]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193(1):265-75.
[Crossref] [Google Scholar] [PubMed]
- Beers RF, Sizer IW. A Spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 1952;195(1):133-40.
[Crossref] [Google Scholar] [PubMed]
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170-5.
[Crossref] [Google Scholar] [PubMed]
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 1979;582(1):67-78.
[Crossref] [Google Scholar] [PubMed]
- Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. Philadelphia (PA), Churchill Livingstone: Elsevier; 2008.
- Quintanar-Guerrero D, Fessi H, All’emann E, Doelker E. Influence of stabilizing agents and preparative variables on the formation of poly (d,l-lactic acid) nanoparticles by an emulsification diffusion technique. Int J Pharm 1996;143(2):133-41.
- Kim BD, Na K, Choi HK. Preparation and characterization of solid lipid nanoparticles (SLN) made of cacao butter and curdlan. Eur J Pharm Sci 2005;24(2-3):199-205.
[Crossref] [Google Scholar] [PubMed]
- Barakat NS, Yassin AEB. In vitro characterization of carbamazepine-loaded precifac hydrogels. Drug Deliv 2006;13(2):95-104.
[Crossref] [Google Scholar] [PubMed]
- Stoica Guzun A, Stroescu M, Tache F, Zaharescu T, Grosu E. Effect of electron beam irradiation on bacterial cellulose membranes used as transdermal drug delivery systems. Nucl Instrum Methods Phys Res 2007;265(1):434-8.
- Kawadkar J, Pathak A, Kishore R. Formulation, characterization and in vitro–in vivo evaluation of flurbiprofen-loaded nanostructured lipid carriers for transdermal delivery. Drug Dev Ind Pharm 2013;39(4):569-78.
[Crossref] [Google Scholar] [PubMed]
 .
.
 .
.