- *Corresponding Author:
- A. S. Shete
Department of Pharmaceutics,
Krishna Institute of Pharmacy of Krishna Institute of Medical Sciences Deemed To Be University,
Karad,
Maharashtra 415539
India
E-mail: amol.shete@rediffmail.com
| Date of Received | 10 November 2020 |
| Date of Revision | 01 October 2021 |
| Date of Acceptance | 07 May 2022 |
| Indian J Pharm Sci 2022;84(3):560-568 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objectives of present investigation were to evaluate prepared cocrystals of fenofibrate and nicotinamide for pre-compression characteristics, docking studies for target peroxisome proliferator-activated receptor alpha, evaluate performance of cocrystals in tablet dosage form and to carry out in vivo antihyperlipidemic activity. The cocrystals were prepared by antisolvent addition method. Docking studies of cocrystals and pure fenofibrate against of target peroxisome proliferator-activated receptor alpha were carried out by using PyRx (version 0.8) docking tool, AutoDock Vina as docking program. The prepared cocrystals were evaluated for pre-compression properties like angle of repose, bulk density and Carr’s index. Cocrystals were formulated in tablet dosage form and evaluated for official and unofficial quality control tests. The antihyperlipidemic activity carried out in rats by using Triton X-100 induced hyperlipidemia model. Cocrystals binds with peroxisome proliferator-activated receptor alpha with more binding affinity as compared with fenofibrate. Cocrystal shows binding energy of -9.3 kcal/mol while fenofibrate shows -8.5 kcal/mol. The pre-compression properties were within the limits of United States Pharmacopeia guidelines. The pure fenofibrate tablet showed 24.7 % drug release at the end of 90 min and cocrystal based tablet showed 100 % at 45 min. There was no statistically significant difference in values for all biochemical parameters in case of in vivo activity for cocrystals in comparison with pure fenofibrate. From the docking studies we can conclude that cocrystals showed stronger more substantial formed stable complexes with target peroxisome proliferator-activated receptor alpha, but no statistically significant difference in pharmacodynamic studies.
Keywords
Cocrystals, nicotinamide, docking, fenofibrate
Crystal Engineering of Active Pharmaceutical Ingredients (APIs) has become a subject of considerable interest for formulation experts in recent years. The crystalline form of APIs is generally being preferred in pharmaceutical industry for delivery because of the inherent and thermodynamic stability of crystalline API. However, crystalline API can exist in different polymorphic form causing inadequate solubility and dissolution characteristics resulting in limited oral bioavailability especially poorly water soluble compounds. Pharmaceutical cocrystals can be defined as crystalline materials comprised of an API and one or more unique co-crystal formers, which are solids at room temperature. Cocrystals can be constructed through several types of interaction, including hydrogen bonding, p stacking and Vander Waals forces. Cocrystals often rely on hydrogen-bonded assemblies between neutral molecules of API and other component. For nonionizable compounds cocrystals enhance pharmaceutical properties by modification of chemical stability, moisture uptake, mechanical behavior, bulk characteristics, solubility, dissolution rate and bioavailability [1].
Cocrystals are more complex systems than single component molecular systems; prediction of properties with respect to various coformers becomes even more challenging. It is also reported that some physicochemical properties, like acid dissociation constant (pKa) could guide cocrystal design. A more abstract approach is to treat compounds as points in a graph and cocrystals as edges. In this way Devogelaer et al. built an undirected graph from existing cocrystals collected from the Cambridge Structural Database (CSD) and treated cocrystal prediction as a link prediction mission. Other types of methods can be referred as ab initio approach, which is usually based on molecular modeling and energy calculation, Barua et al. also reported the use of molecular dynamics to evaluate the hydrogen bonding tendency between molecules and now Machine Learning (ML) approaches have rapidly emerged as crucial tools for solid-state chemistry and have been successfully applied in crystal structure prediction, solvate formation prediction and crystallization solvent [2].
Fenofibrate (FNO) (isopropyl ester of 2-[4-(4-chlorobenzoyl) phenoxy]-2-methylpropanoic acid) is a widely used hypolipidemic drug. Its pharmacological activity consists in reducing triglyceride and cholesterol concentration in plasma. Solubility and permeability are the fundamental parameters controlling the rate and extent of drug absorption. According to the Biopharmaceutics Classification System (BCS), FNO is a Class II drug having low solubility and high permeability. Bioavailability of FNO solely depends on dissolution rate in the gastrointestinal tract. The absolute bioavailability of FNO cannot be determined as the compound is virtually insoluble in aqueous media suitable for injection; bioavailability is optimized when taken with meals. The extent of absorption of one marketed product Triglide (AUC) is comparable between fed and fasted conditions. Food increases the rate of absorption of Triglide approximately 55 %. This drug is used mostly in lipid regulation as it decreases low-Density Lipoprotein (LDL) and Very-Low Density Lipoprotein (VLDL) levels and increases High-Density Lipoprotein (HDL) level. The cocrystals of FNO are reported with tartaric acid[3], saccharin, succinic acid, sucrose[4], Polyethylene Glycol (PEG) 4000 by ball milling technique[5], nicotinamide by different techniques like cogrinding, slurry method, antisolvent precipitation and screening by Hansen solubility parameters[6-8].
The molecular docking approach can be used to model the interaction between a small molecule and a protein at the atomic level, which allow us to characterize the behavior of small molecules in the binding site of target proteins as well as to elucidate fundamental biochemical processes. In present investigation docking studies of cocrystals are carried out. As FNO binds to Peroxisome Proliferator-Activated Receptor Alpha (PPAR-α) and reduces levels of triglycerides, for present investigation so we have selected PPAR-α (Protein Data Bank (PDB) Id: 2ZNN) for molecular docking study [9].
As reported in our previous publication cocrystals prepared by antisolvent addition method showed enhanced dissolution rate of FNO. The objectives of present investigation were to evaluate prepared cocrystals for pre-compression characteristics, incorporate the same in tablet dosage form, evaluate for the performance, to carry out in vivo antihyperlipidemic activity in animal models and molecular docking studies of cocrystals.
Materials and Methods
FNO was obtained as a gift sample from Alembic pharma, Vadodara. Nicotinamide was procured from Loba Chemie Pvt. Ltd. Mumbai. All other chemicals were of analytical grade.
Molecular docking studies and proposed structures of cocrystals:
In order to evaluate pharmacological activity of cocrystals in the form of new chemical entity, we have carried out in vitro screening of cocrystals by using molecular docking and virtual screening. The molecular docking was performed using PyRx (version 0.8) docking tool which uses AutoDock Vina as docking program. The thorough understanding of the structure of API and cocrystal formers is required to correctly locate the hydrogen bonding sites. To get better idea about the binding affinity and interactions we docked FNO, nicotinamide and predicted co-crystal structure. Structures of FNO and nicotinamide were downloaded from PubChem database in Spatial Data File (SDF) file format. The proposed structures of cocrystals were drawn using Chemsketch software and saved in MOL format by using Open Babel GUI 3.1.1 [10-12].
This format was converted into SDF format. FNO binds to PPAR-α and reduces levels of triglycerides, for reported studies we have selected PPAR-α (PDB Id: 2ZNN) for molecular docking study. Further, this protein is processed by using the make macromolecule module provided in PyRx. All water molecules were removed, polar hydrogens were added, also Kollman charges were added to the protein and file was converted into PDBQT file format. Three ligand molecules including cocrystals in SDF format were uploaded in PyRx and energy minimization is carried out by using steepest descent method by using Merck Molecular Force Field (MMFF) [13]. Then SDF files were converted into PDBQT format. Grid box was set to (10.7743446385, 3.89377600387 and -5.46349952579) coordinates based on the active amino acid residues involved in binding with co-crystallized ligand [13].
Preparation of cocrystals:
The cocrystals of FNO and nicotinamide in 1:1 molar ratio were prepared by different techniques like kneading, solvent drop grinding and antisolvent addition and solution crystallization as mentioned in our previous publication [7,8].
Bulk characterization:
The prepared cocrystals were evaluated for angle of repose, bulk density, tap density, Carr’s index and Hausner’s ratio [14].
Formulation of tablet dosage form:
The tables were prepared by direct compression technique by using Potassium bromide (KBr) press for pure FNO and cocrystals of FNO prepared by antisolvent addition method containing 40 mg of the dose. Microcrystalline cellulose, croscarmellose cellulose, talc and magnesium stearate were used as diluent, disintegrant, glidant and lubricant respectively to get final weight of 80 mg.
Evaluation of tablets:
The prepared tablets were evaluated for official and unofficial tests like thickness, diameter, hardness, weight variation, friability, drug content and in vitro dissolution studies [15].
In vitro dissolution studies of tablets:
In vitro dissolution studies of tablets of FNO were performed using 8 station United States Pharmacopeia (USP) type II DBK dissolution rate test apparatus determined in 900 ml of 0.5 % Sodium Lauryl Sulfate (SLS) solution. Dissolution medium was kept in a thermostatically controlled water bath, maintained at 37°±0.5° at rotation speed of 100 rpm. Samples were withdrawn periodically and fresh equal volume of dissolution media was introduced in vessels to maintain the sink condition. Samples were filtered through Whatman filter paper, diluted and analyzed at 290 nm by using Shimadzu UV-1601 PharmaSpec, spectrophotometer (Tokyo, Japan).
In vivo antihyperlipidemic activity:
Wistar albino male rats weighing 200-250 gm were selected and housed in polypropylene cages in room where the temperature was 27° for 12 h light and dark cycles were maintained (Protocol approval number- IAEC/TKCP/2015/02).
Standardisation of hyperlipidemic dose of Triton X-100:
To induce the hyperlipidemia rats were kept in fasting for 18 h with excess of water and subjected to Triton X-100 at the dose of 300, 400, 500, 600 and 700 mg/kg orally (p.o.) and the different lipoproteins is evaluated at 24, 48 and 72 h. It was observed that Triton X-100 in the dose 400 mg/kg p.o. can induces maximum hyperlipidemia after 48 h. Hence 400 mg/kg p.o. was considered as the ideal dose for induction of hyperlipidemia [16].
Evaluation of anti-hyperlipidemic activity:
Animals were divided into four groups containing 3 animals in each group. Animals were kept fasting for 18 h and injected Triton X-100 at a dose of 400 mg/ kg p.o. prepared in saline solution. According to treatment protocol, the first dose of the drug treatment was given immediately after Triton administration to animals from group 2 to 5. Second and third dose was administrated after 24 h and 44 h respectively. After 4 h of third dose the animals were used for the study of various biochemical parameters. Blood was collected by retro orbital plexus of the rat under anaesthesia and centrifuged at 2000 rpm for 30 min to get serum and analysed for biochemical parameter.
Biochemical estimation:
Blood sample were collected after 48 h of Triton injection, blood was immediately centrifuged (2500 rpm for 10 min) and serum is analysed for Total Cholesterol (TC), Triglyceride (TG), HDL and LDL.
Statistical analysis:
All results are expressed as mean±Standard Error of the Mean (SEM). The data is analysed using one ways of Analysis of Variance (ANOVA). The statically significance of the different of means is evaluated.
Results and Discussion
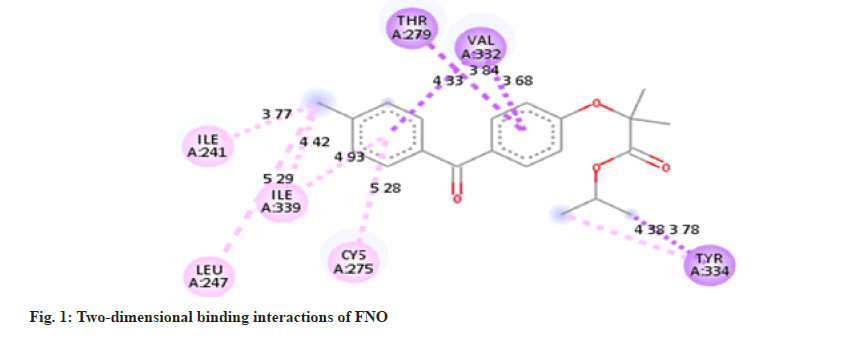
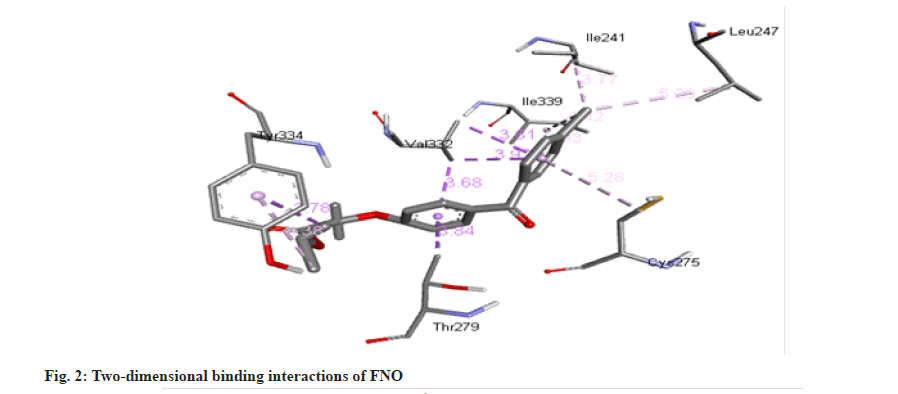
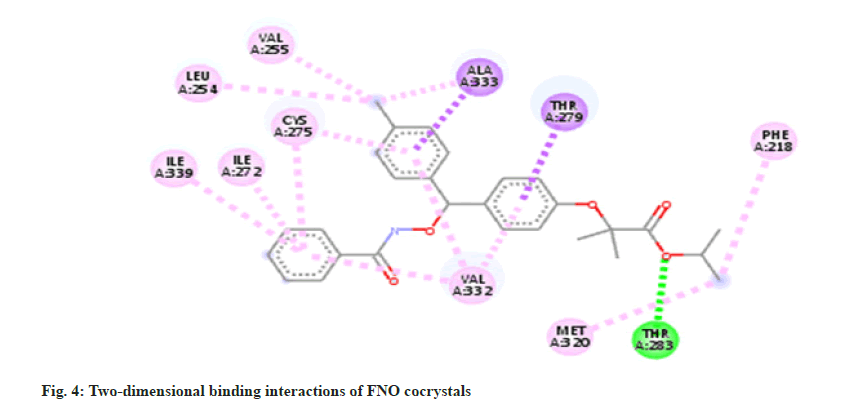
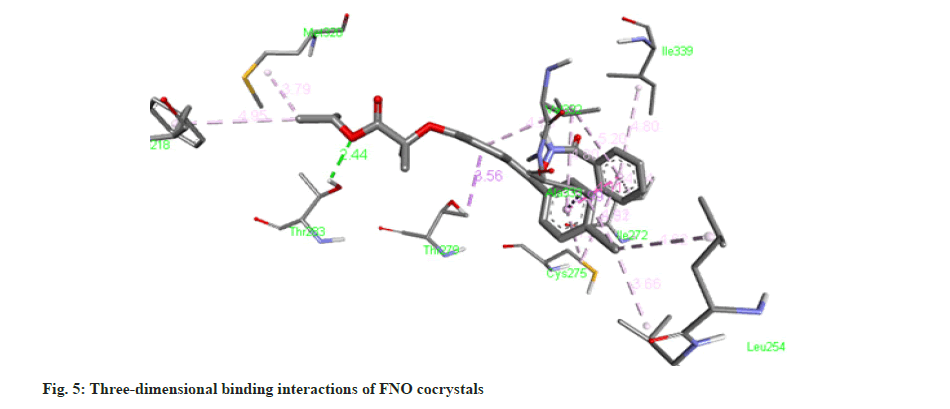
The molecule of FNO structure consists of two aromatic rings, two carbonyl ether and chlorine groups. FNO molecule has three hydrogen bond acceptors, seven rotatable bond count [17]. It can easily form cocrystals with nicotinamide. The -CONH bonding is more robust and strong about to be present in between -NH2 from nicotinamide and C=O from FNO. As the nicotinamide possess aromatic -N- it has affinity for the carboxylic acid moiety, which forms the intermolecular hydrogen bonding as docking results are shown in fig. 1-fig. 6.
Molecular docking is a theoretical simulation method used to study binding interactions between protein and ligands. Based on docking study we observed that complex between cocrystal with PPAR receptor has less binding energy as compared to complex between individual FNO and the receptor. Cocrystals binds with PPAR-α receptor with more binding affinity as compared with FNO. Cocrystal shows binding energy of -9.3 kcal/ mol while FNO shows -8.5 kcal/mol indicating more binding affinity of cocrystals with receptor. The binding interaction study confirms that cocrystal complex is involved in sigma-pi stacked interaction with ALA333 and THR279. Amino acids LEU254, VAL255, ILE272, CYS275, ILE339, VAL332, MET320 and PHE218 are associated with hydrophobic interaction while THR283 is involved in Hydrogen bonding. FNO is involved in sigma-pi stacked interaction with THR279, VAL332 and TYR334. ILE241, LEU247, CYS275 and ILE339 are showing hydrophobic interaction with ligand. FNO is not involved in any hydrogen bonding. The cocrystals showed the bond length of hydrogen bonds <5 Å, which indicating stronger more substantial formed stable complexes [18]. Binding interaction study clearly indicates newly formed cocrystal entity is also showing interaction with PPAR-α receptor exhibiting more activity towards the receptor. Binding energy and interactions are shown in Table 1.
| Ligand name | Binding energy (kcal/mol) | Hydrogen bonds | Sigma-pi stacked | Alkyl hydrophobic/Pi-alkyl | |||
|---|---|---|---|---|---|---|---|
| Residue | Distance | Residue | Distance | Residue | Distance | ||
| Co-crystal complex | -9.3 | THR283 | 2.44 | ALA333 | 3.99 | LEU254, | 4.62 |
| THR279 | 3.56 | VAL255, | 3.66 | ||||
| ILE272, | 4.8 | ||||||
| CYS275, | 4.92 | ||||||
| ILE339, | 4.8 | ||||||
| VAL332, | 4.79, 5.20 | ||||||
| MET320, | 3.79 | ||||||
| PHE218 | 4.95 | ||||||
| FNO | -8.5 | -- | -- | THR279, | 4.33 | ILE241, | 3.77 |
| VAL332 | 3.84, 3.68 | LEU247, | 5.29 | ||||
| TYR334 | 3.78 | CYS275 | 5.28 | ||||
| ILE339 | 4.93 | ||||||
Table 1: Table Showing Binding Energy and Binding Interactions.
The FNO showed good flow properties while the prepared cocrystals showed excellent flow properties. The pre-compression properties which were within the limits of USP guidelines are shown in Table 2. This indicates that the cocrystals improved the flow property and compressibility of FNO this might be due to change in crystal habits [19]. The results of evaluation parameters of tablets as shown in Table 3 found in the limits specified in the USP.
| Formulation code | Angle of repose | Bulk density (gm/cm3) | Tapped density (gm/cm3) | Carr’s index (%) | Hausner’s ratio |
|---|---|---|---|---|---|
| FNO | 33.15±0.830 (Good) | 0.4168±0.0012 | 0.4759±0.0016 | 12.42±0.123 (Good) | 1.13±0.02 (Good) |
| PM | 32.22±0.723 (Good) | 0.4089±0.0022 | 0.4623±0.024 | 11.85 ±0.154 (Good) | 1.12±0.03 (Good) |
| SC | 30.62±0.812 (Excellent) | 0.4202±0.0023 | 0.4664±0.0022 | 9.907±0.125 (Excellent) | 1.10±0.022 (Excellent) |
| SD | 29.74±0.786 (Excellent) | 0.4110±0.0016 | 0.4545±0.0012 | 9.570±0.174 (Excellent) | 1.105±0.029 (Excellent) |
| KN | 28.92±0.789 (Excellent) | 0.4000±0.0019 | 0.4347±0.0022 | 7.9825±0.146 (Excellent) | 1.08±0.027 (Excellent) |
| AN | 28.65±0.810 (Excellent) | 0.4347±0.024 | 0.4505±0.0019 | 3.5072±0.136 (Excellent) | 1.03±0.013 (Excellent) |
Table 2: Micromeritic Properties of FNO and Prepared Cocrystals (Mean±Sem).
| Formulation code | Diameter | Hardness | Thickness | Weight variation | Friability | Disintegration time | Drug content |
|---|---|---|---|---|---|---|---|
| Pure FNO tablets | 4 mm | 4-5 kg/cm3 | 2-2.5 mm | 1.50 % | 0.84 % | 13 min | 99.07 % |
| Cocrystal based tablets | 4 mm | 4-4.5 kg/cm3 | 2-2.4 mm | 1.70 % | 0.74 % | 15 min | 99.05 % |
Table 3: Evaluation Parameters of Tablets (Mean Values).
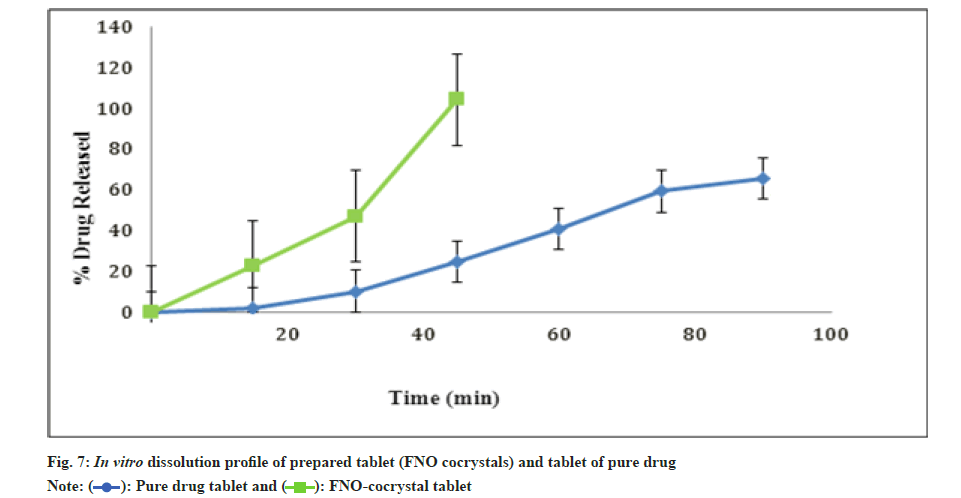
Fig. 7 shows the results of in vitro dissolution profile of prepared tablet (FNO cocrystals) and tablet of pure FNO. The pure FNO tablet showed 24.7 % controlled drug release at the end of 90 min and cocrystal tablet showed 100 % at 45 min.
This might be due to an important feature of cocrystals is the increase of the saturation solubility also, maintain super saturation level over a period of time without any phase transformation or conversion to pure drug and dissolution velocity which may further enhance the bioavailability [20]. The cocrystals were prepared by antisolvent addition method which produces small, uniform and stable FNO cocrystals with markedly enhanced dissolution rate because of large specific surface area of small particles. Also cocrystallization with nicotinamide shows volume expansion correlates with the shortening and strengthening of hydrogen bonds upon cocrystallization, analogous to water freezing and which minimizes the energy and enhances the rate of dissolution. The spring and parachute concept of amorphous drugs was considered to better understand the advantage of higher solubility of cocrystals [21]. Dissociation of cocrystal within a few minutes of oral administration gives amorphous or nanocrystalline drug clusters, which are responsible for peak solubility in the aqueous medium (spring). The transformation of such metastable states to stable crystalline form in stages is capable of releasing high drug concentration for several hours (parachute) [22].
Triton acts as a surfactant and suppresses the action of lipase to block the uptake of lipoproteins from circulation by extra hepatic tissues, resulting into increased blood lipid concentration. The biphasic nature of Triton X-100 induced hyperlipidemia is helpful in understanding the mode of action of hypolipidemc agents. Drugs interfering with lipid biosynthesis of uptake will be active in the synthesis phase and metabolism will be active in the excretory phase. In the present study, the FNO reduced the cholesterol and triglyceride. FNO inhibits the biosynthesis of cholesterol and triglyceride and therefore can be used for the prevention of hyperlipidemia.
The results for antihyperlipidemic activity in animals were expressed as mean±SEM. The statistical significance between mean was analyzed by using One way ANOVA followed by Dunnet’s multiple comparison posttest by using GraphPad software. As shown in Table 4, it has been concluded that there was no statistically significant difference in values for all biochemical parameters for cocrystals in comparison with pure FNO.
| Treatment | Serum total cholesterol | Serum HDL | Serum LDL | Serum VLDL | Serum TG |
|---|---|---|---|---|---|
| Normal | 162.83±3.49 | 47.83±2.75 | 87.97±2.77 | 25.25±2.22 | 125±4.22 |
| Control | 213±7.59 | 58.08±2.67 | 126.05**± 7.66 | 37.33±2.04 | 184.83±5.02 |
| Pure FNO | 144.67**±7.65 | 53.16±3.23 | 67.27**±4.18 | 21.0**±1.41 | 104.67**±5.76 |
| Cocrystals | 162**±2.55 | 51.33*±2.03 | 87.48**±2.49 | 25.55±1.74 | 125.83**±4.5 |
Note: Values are expressed as mean±SEM, n=3 in each group. **p<0.01, *p<0.01 compared with Triton control group
Table 4: Antihyperlipidemic Activity of FNO in Triton X-100 Induced Hyperlipidemia.
As per regulatory requirement for cocrystals, API should be substantially dissociated from its cocrystal form before the drug reaching the site and without change in pharmacological activity and which has been obeyed by prepared cocrystals [23].
From the docking studies we can conclude that cocrystals showed stronger more substantial formed stable complexes with target PPAR-α, but no statistically significant difference in pharmacodynamic studies and this might be due to dissociation of FNO from its cocrystal before reaching the target and which is regulatory requirement of filling cocrystal based NDA and ANDAs. The further investigation of behavior of cocrystals in blood sample is necessary. The use of artificial intelligence and ML will be the future of development of robust, scalable corystals of API to give new life to old drugs.
Acknowledgements
The authors are grateful to the Board of management of Krishna Institute of Medical Sciences Deemed To Be University, Karad for providing research facilities.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Yadav AV, Shete AS, Dabke AP, Kulkarni PV, Sakhare SS. Co-crystals: A novel approach to modify physicochemical properties of active pharmaceutical ingredients. Indian J Pharm Sci 2009;71(4):359-70.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Yang Z, Zhu B, Mei X, Luo X. Machine-learning-guided cocrystal prediction based on large data base. Cryst Growth Des 2020;20(10):6610-21.
- Bhalekar M, Pradhan SB. Scientific coformer screening, preparation and evaluation of fenofibrate tartaric acid cocrystal. J Drug Deliv Technol 2019;9(4):406-10.
- Payghan SA. Preparation and characterization of molecular complexes of fenofibrate cocrystal. Asian J Pharm 2017;11(4):S745-59.
- Joshi R, Raje S, Akram W, Garud N. Particle engineering of fenofibrate for advanced drug delivery system. Future J Pharm Sci 2019;5(1):1.
- Yadav VB, Yadav AV. Enhancement of solubility and dissolution rate of fenofibrate by melt granulation technique. Int J Pharm Tech Res 2009;2(1):256-63.
- Shete AS, Khandagale VV, Murthy SM, Vyankatrao A, Yadav SS, Doijad RC. Solid State characterization and tableting studies of ethanol based cocrystals of fenofibrate with nicotinamide. Indian J Pharm Educ Res 2018;51(2):71-7.
- Shewale S, Shete AS, Doijad RC, Kadam SS, Patil VA, Yadav AV. Formulation and solid state characterization of nicotinamide-based co-crystals of fenofibrate. Indian J Pharm Sci 2015;77(3):328-34.
[Crossref] [Google Scholar] [PubMed]
- Meng XY, Zhang HX, Mezei M, Cui M. Molecular docking: A powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des 2011;7(2):146-57.
[Crossref] [Google Scholar] [PubMed]
- Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol 2015;1263:243-50.
[Crossref] [Google Scholar] [PubMed]
- Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 2010;31(2):455-61.
[Crossref] [Google Scholar] [PubMed]
- O'Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: An open chemical toolbox. J Cheminform 2011;3(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Halgren TA. Merck molecular force field. I. Basis, form, scope, parameterization and performance of MMFF94. J Comput Chem 1996;17(5?6):490-519.
- Eraga SO, Arhewoh MI, Akpan FE, Iwuagwu MA. Evaluation of fast disintegrating tablets of paracetamol prepared from a blend of croscarmellose sodium and Pleurotus tuber-regium powder. Pak J Pharm Sci 2018;31(6):2503-8.
[Google Scholar] [PubMed]
- Shoaib MH, Tazeen J, Merchant HA, Yousuf RI. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak J Pharm Sci 2006;19(2):119-24.
[Google Scholar] [PubMed]
- Parwin A, Najmi AK, Ismail MV, Kaundal M, Akhtar M. Protective effects of alendronate in Triton X-100-induced hyperlipidemia in rats. Turk J Gastroenterol 2019;30(6):557-64.
[Crossref] [Google Scholar] [PubMed]
- Bhalekar M, Pradhan SB. Scientific coformer screening, preparation and evaluation of fenofibrate tartaric acid cocrystal. J Drug Deliv Ther 2019;9(4):406-10.
- Prasanth DS, Panda SP, Rao AL, Chakravarti G, Teja N, Vani VN, et al. In silico strategies of some selected phytoconstituents from Zingiber officinale as sars cov-2 main protease (COVID-19) inhibitors. Indian J Pharm Educ Res 2020;54(3):s552-9.
- Serrano DR, O'Connell P, Paluch KJ, Walsh D, Healy AM. Cocrystal habit engineering to improve drug dissolution and alter derived powder properties. J Pharm Pharmacol 2016;68(5):665-77.
[Crossref] [Google Scholar] [PubMed]
- Salas-Zúñiga R, Rodríguez-Ruiz C, Höpfl H, Morales-Rojas H, Sánchez-Guadarrama O, Rodríguez-Cuamatzi P, et al. Dissolution advantage of nitazoxanide cocrystals in the presence of cellulosic polymers. Pharmaceutics 2019;12(1):23.
[Crossref] [Google Scholar] [PubMed]
- Zhang SW, Harasimowicz MT, de Villiers MM, Yu L. Cocrystals of nicotinamide and (R)-mandelic acid in many ratios with anomalous formation properties. J Am Chem Soc 2013;135(50):18981-9.
[Crossref] [Google Scholar] [PubMed]
- Koteswari P, Sunium S, Srinivasababu P, Babu GK, Nithya PD. Formulation development and evaluation of fast disintegrating tablets of Lamotrigine using liqui-solid technique. Int J Pharm Investig 2014;4(4):207-14.
[Crossref] [Google Scholar] [PubMed]
- United States Food and Drug Administration. Regulatory classification of pharmaceutical co-crystals: Guidance for industry. Silver Spring, US: Center for Drug Evaluation and Research (CDER); 2018.
 .
.



