- *Corresponding Author:
- S. J. Kim
Department of Biological Sciences, College of Natural Sciences, Kongju National University, Gongju 32588, South Korea
E-mail: ksj85@kongju.ac.kr
| Date of Received | 29 June 2021 |
| Date of Revision | 24 April 2022 |
| Date of Acceptance | 12 September 2022 |
| Indian J Pharm Sci 2022;84(5):1227-1232 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Osteoarthritis is the fourth leading cause of disability affecting more than 300 million people around the globe. In this disease, chondrocytes responsible for the growth and maintenance of articular joints start to deplete due to the rogue involvement of phosphoinositide 3-kinase, protein kinase-B and p38 mitogenactivated protein kinases. Presently, there are medications providing symptomatic treatment, however, they cannot thwart the progression of osteoarthritis. Natural compounds can rejuvenate injured tissues, averting inflammation and can be useful for treating osteoarthritis. The present study was conducted to obtain inhibitors using natural compounds for phosphoinositide 3-kinase/protein kinase-B/mitogenactivated protein kinases implicated in the pathogenesis of osteoarthritis. Results revealed that out of 1541 natural compounds, cordycepin and geraniol formed an inhibitory interaction with these kinases. Moreover, these compounds down regulated important inflammatory markers (DNA methyltransferase 1, cluster of differentiation 74 and ETS proto-oncogene 1) and increased the expression of glutamate-cysteine ligase catalytic, FAM111 trypsin like peptidase A, secreted frizzled related protein 1 and ferritin light chain genes implicated in the survival of chondrocytes. The absorption, distribution, metabolism, excretion, toxicity and lethal dose prediction studies showed that cordycepin has appropriate pharmacokinetics and low acute toxicity level, whereas geraniol has high toxicity level and efficient pharmacokinetic profile. Our study provided a preliminary insight that cordycepin and geraniol are capable inhibitors of phosphoinositide 3-kinase/protein kinase-B/mitogen-activated protein kinases and can be lead compounds in osteoarthritis therapy.
Keywords
Natural compounds, osteoarthritis, kinases, virtual screening, absorption, distribution, metabolism, excretion, toxicity, in silico, gene expression
Osteoarthritis (OA) is a chronic degenerative condition in which joint cartilages cushioning the underlying bones start to break down leading to mobility impairment[1]. The clinical manifestations of OA range from stiffness, numbness or weakness over time in legs and arms, joint instability and deformity, chronic pain and narrowing of joint spaces[2-4]. It is the fourth prevalent musculoskeletal condition and its risk increases with age and commonly affects the elderly (age group >65) [5,6]. According to lancet commission report, about 7 % of people worldwide are affected by this disease[7].
The areas affected by OA are hips, hands, knees and spine, whereas risk factors include joint injury, aging, genetic factors and obesity. The line of therapy exploited for OA include glucosamine and chondroitin sulfate, aerobic exercise, acupuncture, weight loss, anti-inflammatory medications reduce pain, stiffness and replacement surgery, which ameliorates the quality of life[8-10]. The current therapeutic intervention only alleviates symptoms but cannot avert the progression of OA that is causing an economic burden of over 60 billion dollars annually in the United States[8].
The molecular mechanism of OA pathogenesis is still in its infancy stages, but thanks to elaborate signaling pathways, scientists have identified key molecular targets that result in chondrocyte dedifferentiation and apoptosis.
Three molecular targets such as Phosphoinositide 3 Kinase (PI3K), Protein Kinase-B (AKT) and p38 Mitogen-Activated Protein Kinases (MAPK) are a group of kinases which are implicated in cell proliferation, differentiation, growth, survival, intracellular trafficking, metabolism, motility and inflammation[11-13]. In OA, miscommunication between these proteins occurs, which instigates the activation of PI3K/AKT/ MAPK pathway. This pathway is responsible for regulating inflammation and apoptotic activity in chondrocytes. Aberrant cellular signaling activates the expression of PI3K and AKT, which in turn stimulates the activity of apoptotic proteins (B-Cell Lymphoma Protein 2 (BCL-2), Bcl-2-Associated X Protein (BAX) and caspases), whereas inflammatory event conspired by MAP kinases leads to the degeneration and apoptosis of articular cartilages and prevent the repair of wornout cartilages in the joints[11].
PI3K/AKT/MAPK pathway is considered an attractive target to prevent chondrocytes degradation, prolong their survival and avert dedifferentiation to promote tissue rehabilitation in OA. In this research article we explored the role of natural compounds for this pathway to acquire potential inhibitors for treating OA.
Materials and Methods
Acquisition of ligands and receptors:
The protein targets involved in the degeneration of chondrocytes are PI3K (Protein Data Bank (PDB) ID: 1E7V), AKT (PDB ID: 3O96) and MAPK (PDB ID: 3FMK) were salvaged from PBD and refined with Galaxy Web Database[14]. A total of 1541 medicinal compounds were randomly selected and downloaded from Natural Product Activity and Species Source (NPASS) database[15] and subsequently prepared with discovery studio 2020 software for virtual interaction studies.
Virtual screening and docking validation:
The interaction of ligands with considered receptors was determined by PYRX 0.9 software[16]. The ligands were aimed at the reported active site residues of these receptors for interaction to obtain potential inhibitors to disturb the degenerative pathway governed by these proteins.
The docking parameters were optimized by defining bounded ligands in the receptors as potential inhibitory binding sites for ligand interaction, which was LY2 (active amino acids: TYR867, VAL882, ILE963 and MET953) for PI3K, IQO for AKT (active amino acids: TYR80, THR82, ILE84, SER205, LEU210, LEU264, LYS268, VAL270, TYR272 and ASP292) and FMK for MAPK (Active Amino acids: ALA51, THR106, MET109 and LEU167). The same docking parameters were applied to relock aboriginal ligands pre-bounded to these receptors to validate this docking method.
Lethal dose, adverse effects and Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) prediction:
The lethal concentration of screened ligands were confirmed with GUSAR database[17] that predicts acute toxicity of compounds using canonical Simplified Molecular-Input Line-Entry System (SMILES) of compounds and with ADVERPred database[18], the adverse effects of these compounds were recorded. For ADMET properties, SwissADME[19] was used to determine pharmacokinetics of screened compounds.
Gene expression studies:
Expression studies were conducted with DIGEP-Pred database[20], which checks the activity of compounds by analyzing the canonical SMILES of compounds on the major gene targets. The canonical SMILES of screened compounds were used to determine their chemical behavior on potential gene targets of OA.
Results and Discussion
Structure-based-virtual screening method was exploited in this study to determine the interaction of 1541 medicinal ligands against these receptors implicated in degeneration of chondrocytes to expedite drug discovery for OA. The docking method optimized for this virtual screening successfully fixated aboriginal ligands of these receptors in their original active site, which allowed us to proceed with our virtual screening analysis. From 1541 ligands, cordycepin (NPC229974) and geraniol (NPC255042) established adequate interaction with the reported active site residues of PI3K, AKT and MAPK. The results of ligand interaction with individual receptors are explained below.
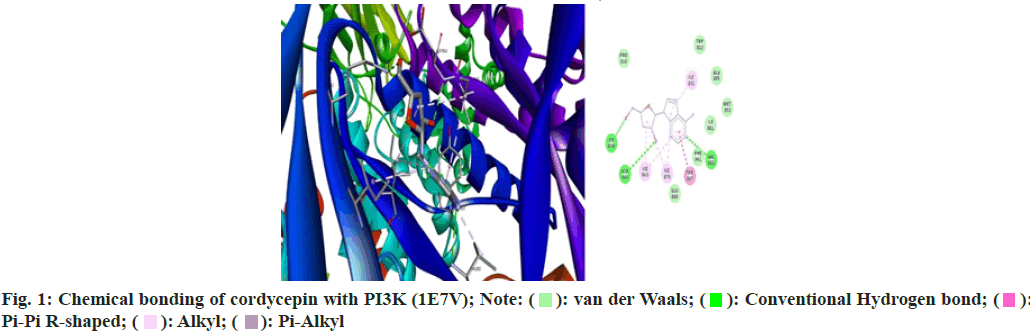
Cordycepin established hydrogen bonds with LYS833, ASP964 using hydroxyl functional groups of deoxyribose sugar and amine group of adenosine participated in forming single hydrogen bond with VAL882 and hydrophobic (pi-alkyl and pi-pi) interactions with TYR867. Geraniol formed a single hydrogen bond with VAL822 using hydroxyl group, whereas methyl group made hydrophobic (pi-alkyl) interactions with PHE961, ILE963, TYR867 and ILE879. The intermolecular energy required by these ligands to establish chemical connections was -5.68 and -6.02 kcal/mol, respectively.
Hydroxyl group of deoxyribose and amine moiety of adenosine of cordycepin participated in the formation of hydrogen bonds with SER32, ALA51, LEU104, ASP168, ALA34 and LYS53. The same groups also established hydrophobic interactions (p-alkyl) with LEU167, VAL38 and LYS53. Geraniol used hydroxy group to establish two hydrogen bonds with LYS53 and ALA51 whereas methyl groups formed hydrophobic (pi-alkyl and pi-sigma) interactions with LEU75, LEU104, LEU171, ILE84 and PHE161. The intermolecular energy recorded for these ligands was -5.01 and -4.54 kcal/mol, respectively.
Cordycepin utilized hydroxyl and amine moieties to form hydrogen bonds and hydrophobic interactions (pialkyl and pi-anion) with LYS268, ILE290, SER205, THR211, THR291 and TRP80, LEU264 and ASP292. Similarly, geraniol exploited hydroxyl group to make hydrogen bonds with THR291, ASN279 and TYR272, whereas methyl group participated in hydrophobic interactions (pi-alkyl and pi-sigma) with ILE84, TRP80 and TYR272. The binding energy reported by the ligand’s interactions was -6.22 kcal/mol and -6.13 kcal/ mol, respectively (fig. 1 and Table 1).
| Ligands | Receptors (PDB ID) | Reported active site | Recorded hydrogen interaction | Intermolecular energy (Kcal/mol) | Inhibition constant (Ki) | LigRMSD calculation (Å) |
|---|---|---|---|---|---|---|
| Cordycepin | PI3k (1E7V) | TYR867, VAL882, IIE963, MET953 | LYS833, VAL882, ASP964, | -6.02 | 358.59 µM | 1.54 |
| Geraniol | VAL882 | -5.68 | 639.16 µM | 3.88 | ||
| Cordycepin | P38 kinase (3FMK) | ALA51, THR106, MET109, LEU167 | SER32, ASP34, ALA51,LEU104, ASP168, LYS553 | -5.01 | 2.63 mM | 1.3 |
| Geraniol | ALA51, LYS53 | -6.02 | 5.83 mM | 2.7 | ||
| Cordycepin | AKT1 protein (3O96) | TYR80, TYR82, IIE84, SER205, LEU210, LEU264, LYS268, VAL270 TYR272, ASP292 | SER205, THR211, LYS268, ILE290, THR291 | -6.22 | 234.10 µM | 2.95 |
| Geraniol | TYR272, ASN279, THR291 | -6.13 | 398.27 µM | 3.37 |
Table 1: Results of Virtual Screening and Docking Profile of Successful Ligands
The results from GUSAR database revealed that the lethal dose of cordycepin predicted in rodent models were 0,348 log 10 (mmol/kg) for intraperitoneal route, 0,625 log 10 (mmol/kg) for intravenous route, 1,005 and 0,474 log10 (mmol/kg) for oral and subcutaneous route. Whereas for geraniol, the lethal dose was 0,634 log 10 (mmol/kg) for intraperitoneal route, 0,451 log 10 (mmol/kg) for intravenous route, 1,431 and 1,080 log 10 (mmol/kg) for oral and subcutaneous route. Both compounds are class 5 chemicals in accordance with Organization for Economic Cooperation and Development (OECD) chemical classification manual. ADVERPred database showed that cordycepin can cause nephrotoxicity, hepatotoxicity and myocardial infarction on overdose, whereas no such side effects were seen for geraniol. SWISS-ADME analysis reported that cordycepin had lipophilicity of -0.80 Log logarithm of n-octanol/water partition coefficient (log Po/w) and has adequate water solubility. This compound has high gastrointestinal absorption, no Blood Brain Barrier (BBB) penetrative ability and negligible affinity for Permeability Glycoprotein (PGP) substrate and Cytochrome P450 (CYP) variants. It has skin penetration of -8.27 cm/s, drug likeness qualities and bioavailability score of 0.55. Geraniol has the lipophilicity of 2.78 log Po/w and possesses high solubility in water. It has high gastrointestinal absorption and BBB penetration. This compound has no affinity for P-GP substrate and CYP variants and has skin permeability of -4.71 cm/s. Lipinski drug ability criteria was passed by this compound with no violations and has a high bioavailability score of 0.55 (Table 2 and Table 3).
| No | Compounds | Chemical classification | Side effects on overdose | LD50 dose for intraperitoneal route log 10 (mmol/kg) | LD50 dose for intra venous route log 10 (mmol/kg) | LD50 dose for oral route log 10 (mmol/kg) | LD50 dose for subcutaneous route log 10 (mmol/kg) |
|---|---|---|---|---|---|---|---|
| 1 | Cordycepin | Class 5 | Nephrotoxicity, hepatotoxicity and myocardial infarction | 0,348 | 0,625 | 1,005 | 0,474 |
| 2 | Geraniol | Class 5 | No side effect | 0,634 | 0,451 | 1,431 | 1,080 |
Table 2: Lethal Dose and Adverse Effects Prediction of Screened Compounds in In Silico Mouse Model
| No | Compound | Consensus Log Po/w | Water solubility Log S (ESOL) | Pharmacokinetics | Druglikeness | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI absorption | BBB permeant | P-GP substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor | Log Kp (skin permeation) | Lipinski’s criteria | Bioavailability score | ||||
| 1 | Cordycepin | -0.8 | -1.25 (very soluble) | High | No | No | No | No | No | No | No | -8.27 cm/s | Yes: No violations | 0.55 |
| 2 | Geraniol | 2.75 | -2.78 (soluble) | High | Yes | No | No | No | No | No | No | -4.71 cm/s | Yes: No violations | 0.55 |
Table 3: Admet Results of Screened Compounds
In order to explore the induced effect of these screened compounds on major gene targets of OA, in silico-based gene expression studies were performed with DIGEPPred database. It was observed that cordycepin down regulated the expression of DNA Methyltransferase 1 (DNMT1) gene which is involved in provoking the activity of Interleukin-1 beta (IL-1β) in human articular chondrocytes[21] whereas up regulated the expression of Secreted Frizzled Related Protein 1 (SFRP1) and FAM111 Trypsin Like Peptidase A (FAM111A), which prevents premature aging development of OA by inhibiting WNT/Beta (β)-catenin pathway and reduces Endoplasmic Reticulum (ER) stress on articular chondrocytes[22,23]. Alternatively, geraniol had a down regulatory effect on Cluster of Differentiation 74 (CD74)[24] and ETS Proto-Oncogene 1 (ETS1) genes[25], as both of them are implicated in instigating exaggerated inflammatory response in OA and up regulated Glutamate-Cysteine Ligase Catalytic (GCLC) and Ferritin Light Chain (FTL) involved in reducing Reactive Oxygen Species (ROS) and iron accumulation in these cells[26,27] (Table 4).
| No | Compounds | % Age down regulation/up regulation | Gene | Role | Reference |
|---|---|---|---|---|---|
| 1 | Cordycepin | 64.5 | DNMT1 ↓ | Promotes the activity of IL-1β in human articular chondrocytes | [21] |
| 93.7 | SFRP1 ↑ | Prevents premature aging and development of osteoarthritis by inhibiting WNT/b-catenin pathway | [22] | ||
| 68.1 | FAM111A ↑ | Reduces cartilage endoplasmic reticulum stress | [23] | ||
| 2 | Geraniol | 64.4 | CD74 ↓ | Exacerbates inflammation in cartilage end plates | [24] |
| 55.3 | ETS1 ↓ | Modulate the activation of proinflammatory cytokines. | [25] | ||
| 89.8 | GCLC ↑ | Regulation of reactive oxygen species | [26] | ||
| 75.8 | FTL ↑ | Iron detoxification in chondrocyte tissues | [27] |
Table 4: Expression Profile of Screened Compounds on Gene Targets
Chondrocytes are highly specialized cells in particular cartilages and are responsible for regulating tissue homeostasis and extracellular matrix to sustain the structure and function of major joints[28]. These cells have cellular differentiation properties, as they convert their cellular phenotypes back and forth to assist the growth and maintenance of cartilages and bone formation[29,30]. These differentiation properties depend upon several key molecular factors which govern these changes between cell types. However, in OA, exposure of chondrocytes to some cellular agents such as IL- 1β, catenin, nitric oxide, transforming growth factorbeta, WNT3A, ROS, stress on ER and mitochondria, PI3K, AKT and MAPK destroy their cellular integrity by inducing dedifferentiation and then lead them to apoptosis[11,31-33]. Following this event, causes degradation of joint spaces which instigates friction between bones, subsequently pain and reduced mobility of joints. Presently, there are no concrete strategies to reverse the chondrocytes dedifferentiation and prevent their degradation to retard the OA progression.
Natural compounds provide effective solution to this problem as they possess astounding capability to calm the inflammation, articulate cellular behavior and promote tissue rehabilitation[34-37]. Previously, some reports indicated that natural compounds cause chondrocytes differentiation and regulate molecular targets involved in causing chondrocytes apoptosis[13,38-40]. In this study, we made medicinal compounds as a possible lead to discover potential inhibitors for OA. The targets chosen for this study were PI3K, AKT and MAPK. These kinases are involved in regulating differentiation, apoptosis, proliferation and chondrocytes survival[11].
In OA, during inflammatory surge in particular cartilages cause stress on ER and mitochondrial machinery which collapses cellular functioning and provoke ROS production[31]. Up regulation of ROS activates PI3K, AKT and MAPK which stimulates the activity of p53 to stop the proliferation of chondrocytes and induce apoptosis[41]. For this particular reason, we randomly selected 1541 medicinal compounds from NPASS database to determine the chemical nature of these compounds against these kinases.
Our virtual screening analysis showed that cordycepin and geraniol are promising ligands as they established hydrogen bonds with the reported active site residues of these receptors. Hydrogen bonding of compounds brings disturbance in the structure and function of protein target which ultimately leads to their inhibition as evident from these studies[16,42-45]. Moreover, we also determined the toxic side effects and lethal dose of these compounds to ensure safety and dose calibration during in vitro experimentations to procure maximal affectivity against OA. These compounds are class-5 chemicals and possess adequate lethal dose level to be exploited whereas ADMET studies were also performed to know more about pharmacokinetics of these compounds. These compounds have efficient pharmacokinetic and drug likeness properties with no bioavailability issue which usually majority of natural compounds face after administration[46,47].
Further gene expression studies were also conducted to unravel any other essential pathway in the treatment of OA. These compounds down regulate the expression of inflammatory markers and promote the expression of important genes such as SFRP1, GCLC and FTS in inhibiting WNT/β-catenin pathway, ER stress and ROS, and detoxify the effects of iron on chondrocyte’s tissue[22,25-27].
Cordycepin and geraniol are promising natural compounds, which efficiently inhibited PI3K AKT and MAPK by binding to their active site. This provides a clear indication that these compounds possess affinity for these proteins and can reverse the OA pathogenesis. Having efficient toxicity and ADMET properties combined with appreciable induced gene expression activity, we believe that these compounds can prevent dedifferentiation of chondrocytes and would be able to regulate the inflammatory and other pathogenic molecular markers in OA. In vitro experimentation and animal-based models are currently being explored to confirm the activity of these compounds. However, our study provides a preliminary insight that these compounds hold a promise in the OA therapeutics and should be further explored as lead compounds in OA.
Acknowledgements:
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean Government (MEST) (2020R1I1B306969912).
Conflict of interests:
The authors declared no conflict of interest.
References
- Abramoff B, Caldera FE. Osteoarthritis: Pathology, diagnosis and treatment options. Med Clin 2020;104(2):293-311.
[Crossref] [Google Scholar] [PubMed]
- Hawker GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol 2019;37(120):3-6.
[Google Scholar] [PubMed]
- Nelson AE. Osteoarthritis year in review 2017: Clinical. Osteoarthr Cartil 2018;26(3):319-25.
[Crossref] [Google Scholar] [PubMed]
- Mandl LA. Osteoarthritis year in review 2018: Clinical. Osteoarthr Cartil 2019;27(3):359-64.
[Crossref] [Google Scholar] [PubMed]
- Woolf AD. Global burden of osteoarthritis and musculoskeletal diseases. BMC Musculoskelet Disord 2015;16(1):S1-3.
- Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr Cartil 2020;28(3):242-8.
[Crossref] [Google Scholar] [PubMed]
- Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond: A lancet commission. Lancet 2020;396(10264):1711-2.
[Crossref] [Google Scholar] [PubMed]
- Gu YT, Chen J, Meng ZL, Ge WY, Bian YY, Cheng SW, et al. Research progress on osteoarthritis treatment mechanisms. Biomed Pharmacother 2017;93:1246-52.
- Hermann W, Lambova S, Muller-Ladner U. Current treatment options for osteoarthritis. Curr Rheumatol Rev 2018;14(2):108-16.
[Crossref] [Google Scholar] [PubMed]
- Grässel S, Muschter D. Recent advances in the treatment of osteoarthritis. F1000Research 2020;9:F1000.
[Crossref] [Google Scholar] [PubMed]
- Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A narrative review. Osteoarthr Cartil 2020;28(4):400-9.
[Crossref] [Google Scholar] [PubMed]
- Xie J, Lin J, Wei M, Teng Y, He Q, Yang G, et al. Sustained Akt signaling in articular chondrocytes causes osteoarthritis viaoxidative stress-induced senescence in mice. Bone Res 2019;7(1):1-9.
- Ju SH, Tan LR, Liu PW, Tan YL, Zhang YT, Li XH, et al. Scutellarin regulates osteoarthritis in vitro by inhibiting the PI3K/AKT/mTOR signaling pathway. Mol Med Rep 2021;23(1):83.
[Crossref] [Google Scholar] [PubMed]
- Ko J, Park H, Heo L, Seok C. GalaxyWEB server for protein structure prediction and refinement. Nucleic Acids Res 2012;40(W1):W294-7.
[Crossref] [Google Scholar] [PubMed]
- Zeng X, Zhang P, He W, Qin C, Chen S, Tao L, et al. NPASS: Natural product activity and species source database for natural product research, discovery and tool development. Nucleic Acids Res 2018;46(D1):D1217-22.
[Crossref] [Google Scholar] [PubMed]
- Shah FH, Kim SJ. Targeting FGL2, a molecular drug target for glioblastoma, with natural compounds through virtual screening method. Future Med Chem 2021;13(9):805-16.
[Crossref] [Google Scholar] [PubMed]
- Lagunin A, Zakharov A, Filimonov D, Poroikov V. QSAR modelling of rat acute toxicity on the basis of PASS prediction. Mol Inform 2011;30(3):241-50.
[Crossref] [Google Scholar] [PubMed]
- Ivanov SM, Lagunin AA, Rudik AV, Filimonov DA, Poroikov VV. ADVERPred–Web service for prediction of adverse effects of drugs. J Chem Inform Model 2018;58(1):8-11.
[Crossref] [Google Scholar] [PubMed]
- Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 2017;7(1):42717-3.
[Crossref] [Google Scholar] [PubMed]
- Lagunin A, Ivanov S, Rudik A, Filimonov D, Poroikov V. DIGEP-Pred: Web service for in silico prediction of drug-induced gene expression profiles based on structural formula. Bioinformatics 2013;29(16):2062-3.
[Crossref] [Google Scholar] [PubMed]
- Nakano K, Boyle DL, Firestein GS. Regulation of DNA methylation in rheumatoid arthritis synoviocytes. J Immunol 201;190(3):1297-303.
[Crossref] [Google Scholar] [PubMed]
- Pasold J, Osterberg A, Peters K, Taipaleenmäki H, Säämänen AM, Vollmar B, et al. Reduced expression of Sfrp1 during chondrogenesis and in articular chondrocytes correlates with osteoarthritis in STR/ort mice. Exp Cell Res 2013;319(5):649-59.
[Crossref] [Google Scholar] [PubMed]
- Kung LH, Mullan L, Soul J, Wang P, Mori K, Bateman JF, et al. Cartilage endoplasmic reticulum stress may influence the onset but not the progression of experimental osteoarthritis. Arthritis Res Ther 2019;21(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Xiong C, Huang B, Cun Y, Aghdasi BG, Zhou Y. Migration inhibitory factor enhances inflammation via CD74 in cartilage end plates with Modic type 1 changes on MRI. Clin Orthop Relat Res 2014;472(6):1943-54.
[Crossref] [Google Scholar] [PubMed]
- Redlich K, Kiener HP, Schett G, Tohidast-Akrad M, Selzer E, Radda I, et al. Overexpression of transcription factor Ets-1 in rheumatoid arthritis synovial membrane: Regulation of expression and activation by interleukin-1 and tumor necrosis factor α. Arthritis Rheum 2001;44(2):266-74.
[Crossref] [Google Scholar] [PubMed]
- Khan NM, Haseeb A, Ansari MY, Devarapalli P, Haynie S, Haqqi TM. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic Biol Med 2017;106:288-301.
[Crossref] [Google Scholar] [PubMed]
- Ashwell MS, O'Nan AT, Gonda MG, Mente PL. Gene expression profiling of chondrocytes from a porcine impact injury model. Osteoarthr Cartil 2008;16(8):936-46.
[Crossref] [Google Scholar] [PubMed]
- Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, et al. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol 2019;165:49-65.
[Crossref] [Google Scholar] [PubMed]
- Akkiraju H, Nohe A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J Dev Biol 2015;3(4):177-92.
- Hall AC. The role of chondrocyte morphology and volume in controlling phenotype-implications for osteoarthritis, cartilage repair and cartilage engineering. Curr Rheumatol Rep 2019;21(8):1-38.
[Crossref] [Google Scholar] [PubMed]
- Xia B, Chen D, Zhang J, Hu S, Jin H, Tong P. Osteoarthritis pathogenesis: A review of molecular mechanisms. Calcif Tissue Int 2014;95(6):495-505.
[Crossref] [Google Scholar] [PubMed]
- Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, et al. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res 2009;24(1):12-21.
[Crossref] [Google Scholar] [PubMed]
- Van Der Kraan PM. Differential role of transforming growth factor-beta in an osteoarthritic or a healthy joint. J Bone Metabol 2018;25(2):65-72.
[Crossref] [Google Scholar] [PubMed]
- Shedoeva A, Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evid Based Complement Altern Med 2019;2019:2684108.
[Crossref] [Google Scholar] [PubMed]
- Singh V. Medicinal plants and bone healing. Natl J Maxillofac Surg 2017;8(1):4-11.
[Crossref] [Google Scholar] [PubMed]
- Tasneem S, Liu B, Li B, Choudhary MI, Wang W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol Res 2019;139:126-40.
[Crossref] [Google Scholar] [PubMed]
- Liu X, Zhu R, Zhang Y, Xue Y, Fan H. Effects of drugs and organics on dedifferentiation of chondrocytes. J Biomater Tissue Eng 2017;7(1):1-8.
- Yu SM, Cho H, Kim GH, Chung KW, Seo SY, Kim SJ. Berberine induces dedifferentiation by actin cytoskeleton reorganization via phosphoinositide 3-kinase/Akt and p38 kinase pathways in rabbit articular chondrocytes. Exp Biol Med 2016;241(8):800-7.
[Crossref] [Google Scholar] [PubMed]
- Phull AR, Eo SH, Kim SJ. Oleanolic acid (OA) regulates inflammation and cellular dedifferentiation of chondrocytes via MAPK signaling pathways. Cell Mol Biol 2017;63(3):12-7.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Wu H, Wang L, Zheng L, Zhao J. Protective effects of baicalin on rabbit articular chondrocytes in vitro. Exp Ther Med 2017;13(4):1267-74.
[Crossref] [Google Scholar] [PubMed]
- Yu SM, Kim SJ. Thymoquinone-induced reactive oxygen species causes apoptosis of chondrocytes via PI3K/Akt and p38kinase pathway. Exp Biol Med 2013;238(7):811-20.
[Crossref] [Google Scholar] [PubMed]
- Pandey SK, Yadav S, Goel Y, Temre MK, Singh VK, Singh SM. Molecular docking of anti-inflammatory drug diclofenac with metabolic targets: Potential applications in cancer therapeutics. J Theor Biol 2019;465:117-25.
[Crossref] [Google Scholar] [PubMed]
- Shah FH, Salman S, Idrees J, Idrees F, Akbar MY. In silico study of thymohydroquinone interaction with blood–brain barrier disrupting proteins. Future Sci OA 2020;6(10):FSO632.
[Crossref] [Google Scholar] [PubMed]
- Schena A, Griss R, Johnsson K. Modulating protein activity using tethered ligands with mutually exclusive binding sites. Nat Commun 2015;6(1):7830.
[Crossref] [Google Scholar] [PubMed]
- Kojetin DJ, Burris TP. Small molecule modulation of nuclear receptor conformational dynamics: Implications for function and drug discovery. Mol Pharmacol 2013;83(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Souza JE, Casanova LM, Costa SS. Bioavailability of phenolic compounds: A major challenge for drug development? Fitos Magazine 2015;9(1):55-67.
- Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, et al. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch Toxicol 2014;88(10):1803-53.
[Crossref] [Google Scholar] [PubMed]
 : van der Waals;
: van der Waals;  : Conventional Hydrogen bond;
: Conventional Hydrogen bond;  :
Pi-Pi R-shaped;
:
Pi-Pi R-shaped; 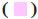 : Alkyl;
: Alkyl; 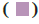 : Pi-Alkyl
: Pi-Alkyl



