- *Corresponding Author:
- S. D. Vanshiv
Department of Pharmaceutics, S. T. E. S’s Sinhgad Institute of Pharmacy, Narhe, Pune-411 041, India
E-mail: swapnila84@rediffmail.com
| Date of Submission | 19 January 2017 |
| Date of Revision | 06 May 2017 |
| Date of Acceptance | 26 December 2017 |
| Indian J Pharm Sci 2018;80(1):181-191 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The present research was aimed at development and optimization of dipyridamole gastroretentive microspheres. Gastro retention was achieved using swellable polymeric material such as hydroxypropylmethylcellulose K4M, ethyl cellulose as release retardant using solvent diffusion evaporation method. 32 factorial design was applied for the development of best optimized formulation. The design was developed by considering two independent variables, ethyl cellulose, and stirring speed. Their effect on drug release, entrapment efficiency, particle size was analysed using response surface methodology. Formulation B1 among all formulations tested was found to be the best as it demonstrated prolonged floating time, maximum entrapment efficiency and drug release in a controlled manner. Formulation B1 was characterized further for presence of residual solvent by gas chromatography/mass spectrometry and the result showed absence of residual solvent. Gastro retention was assessed using the in vivo gamma scintigraphy technique in Albino rabbits and the results indicated prolonged gastric retention. Pharmacokinetic study comparing pure dipyridamole and dipyridamole microspheres was conducted and Cmax, Tmax and Kel were estimated. In vitro-in vivo correlation was determined using Wagner-Nelson function. Pharmacokinetic study revealed improved bioavailability (30 %) as compared to pure dipyridamole. Good correlation was obtained with a regression coefficient of 0.9704. The in vivo gamma scintigraphy and pharmacokinetic studies confirmed gastric retention of the prepared dipyridamole floating microspheres. Accelerated stability studies indicated integrity of the developed formulation.
Keywords
Gastroretentive, dipyridamole, in vivo gamma scintigraphy, in vivo pharmacokinetic study
Dipyridamole (DIP) is a platelet inhibitor. Clinically it is used as an antithrombotic agent. DIP has a short biological half-life of 2-3 h [1,2]. Oral dosage forms of DIP exhibit variable absorption with limited bioavailability ranging from 11-44 %. DIP exhibits a pH-dependent solubility with good solubility at low pH (37°, 36.5 g/l at pH 1.0) and poor solubility at a higher pH (37°, 0.02 g/l at pH 7.0) [3]. The major absorption sites of DIP are stomach and duodenum [4]. Due to short biological half-life of 2 to 3 h, DIP should be frequently administered or as a sustained-release (SR) preparation. In the present study, a gastroretentive formulation of DIP microspheres (MPs) was attempted to prepare a stable formulation with prolonged gastric retention in the stomach with improved bioavailability.
Previously, researchers have formulated controlled release dosage forms of DIP using various techniques and polymers such as pH-controlled silicon by spray drying [5] and co-evaporates using enteric and insoluble acrylic polymers [6]. DIP was formulated with acidic pH modifiers, SR pellets by extrusion spheronization and also a floating osmotic pump system was developed [4,7].
In the present study preparation of DIP floating MPs using hydroxypropyl methylcellulose (HPMC) K4M and ethyl cellulose by solvent diffusion evaporation method was found to be simple, reproducible and cost effective, which was not reported previously in the literature. Prepared MPs using this method were expected to have lesser floating lag time, longer buoyant time for continuous drug release in the stomach and upper part of gastrointestinal (GI) tract with improvement in bioavailability of DIP.
Materials and Methods
DIP was kindly gifted by Cadila Pharmaceuticals, Ahmadabad, India. Ethyl cellulose, HPMC K4M and polyvinyl alcohol (PVA) were purchased from Research Lab Fine Chem. Industries, Mumbai, India. Dichloromethane (DCM) and ethanol were procured from Loba Chemie Ltd., Mumbai, India.
Preliminary trials and experimental design
In the preliminary trials, floating MPs with central hollow cavity were prepared by solvent diffusion evaporation technique. Trials were taken for the selection of the best combination of polymers (ethyl cellulose and HPMC K4M) to achieve maximum floatation, entrapment efficiency (EE) and drug release retardation. Floating MPs were prepared by dissolving required quantity of DIP, ethyl cellulose and HPMC K4M in a mixture of DCM:ethanol (1:1). This solution was then poured in to aqueous solution of PVA containing 0.02 % Tween 80 as dispersing agent with constant stirring on mechanical stirrer (300 rpm) at 40° (Table 1). The dispersed droplets were solidified in the aqueous phase by evaporation of the solvents. After agitating the system for 1 h at 300 rpm, the resulting polymeric particulate system were washed with water then filtered and dried overnight in desiccators to produce MPs [8].
| Batch code | Drug:ethyl cellulose | Drug:ethyl cellulose:HPMC | Solvent ratio (ethanol:DCM) |
|---|---|---|---|
| P1 | 01:01 | - | 01:01 |
| P2 | 01:02 | - | 01:01 |
| P3 | 01:03 | - | 01:01 |
| P4 | 01:04 | - | 01:01 |
| P5 | - | 01:04:01 | 01:01 |
| P6 | - | 01:04:02 | 01:01 |
| P7 | - | 01:04:03 | 01:01 |
| P8 | - | 01:04:04 | 01:01 |
Table 1: Preliminary trials for polymer selection
Design of experiment
In the pre-optimization studies, the solvent (DCM:ethanol) ratio and the concentration of HPMC were optimized based on the results obtained from particle size, shape and surface morphological studies, floating ability, EE and drug release in 10 h. Formulation development was done by applying 32 randomized full factorial design. In this case, 2 factors were evaluated, each at 3 levels, and experimental trials were performed at all 9 possible combinations, details of study design was given in Table 2.
| Factors | Levels of the factors used in the formulation | |||
| X1 | ||||
| Amount of EC | ||||
| X2 | ||||
| Stirring speed | -1 | 0 | 1 | |
| 150 | 200 | 250 | ||
| 300 | 650 | 1000 | ||
| Responses | ||||
| Y1 | Particle size (μm) | |||
| Y2 | Entrapment efficiency (%) | |||
| Y3 | Drug release (%) | |||
Table 2: 32 Full factorial experimental designs
Statistical analysis of the data and validation of the optimization model
Statistical experimental design was generated and evaluated using a Design Expert 8.0.7.1 software (Stat- Ease, Inc, USA). To demonstrate the influence of each factor and interaction amongst the factors, on each of the response, response surface graphs were constructed.
Polynomial models including interaction terms were generated for all the responses using multi-linear regression analysis. In order to validate the polynomial equations, one optimum formulation composition and two random checkpoints were selected from the entire experimental domain based on the desirability. Formulations corresponding to the selected check points were prepared and evaluated for all the three responses (Y1-Y3). The resultant experimental data of response properties were subsequently compared quantitatively with the predicted values and the best optimized formulation was selected. The in vitro drug release data of the optimized formulation was treated to kinetic models such as zero order, first order, Higuchi model and Peppas model in order to determine the drug release mechanism.
Micrometric properties
Micromeritic properties of the MPs such as particle size, true density, tapped density, compressibility index and flow properties were studied. Tapped density was measured using USP bulk density apparatus and the percent compressibility was determined as Eqns. 1 and 2, tapped density = mass of MPs/volume of MPs after tapping; percent compressibility = 1–V/V0×100, where V and V0= volume of sample after and before the standard tapping, respectively.
True density was determined using a benzene displacement method. Porosity was calculated using the Eqn. 3, porosity (ε) = 1–Pρ/Pt ×100, where Pt and Pρ were the true density and tapped density, respectively.
Angle of repose h of the MPs, which measures the resistance to particle flow, was determined by a fixed funnel method and calculated as Eqn. 4, Tan Ø= 2H/D, where, 2H/D is the surface area of the free-standing height of the MPs heap that is formed on a graph paper after making the MPs flow from the glass funnel. The particle size was measured using calibrated optical microscope and the mean particle size was calculated by measuring 200 to 300 particles.
Percent yield and EE
Percent yield of microsphere formulations were determined for all the formulations based on the dry weight of the drug and the polymers taken. Eqn. 5, percent yield = total weight of floating MPs/total weight of drug and polymer×100. The drug content of MPs was determined by crushing and dispersing 50 mg of formulation in 10 ml methanol followed by agitation on vortex mixer to extract the drug. After filtration through Whatman filter paper, the drug concentration in the methanol phase was determined UV/Vis spectrophotometrically at 283 nm. Each determination was made in triplicate. Percent drug entrapment was calculated as Eqn. 6, % EE = calculated drug content/ theoretical drug content×100.
Scanning electron microscope (SEM)
The external and internal morphology of the MPs was studied using SEM. The samples were prepared by placing the MPs on a double adhesive tape stuck to aluminium stub. The stubs were then coated with gold ion for 5-6 min. These were then observed under different magnifications with an analytical SEM cope (Jeol-JSM 6360A-Japan).
Drug release study
The drug release study was performed using USP type ІI apparatus (Electro lab, Mumbai) at 37°±0.5° and at 50 rpm using dissolution medium 900 ml of 0.1 N HCl (pH 1.2) containing Tween 80 (0.02 % w/v) as a surfactant. MPs equivalent to 50 mg of DIP were used for the study. Five millilitres of sample solution was withdrawn at predetermined time intervals, filtered through a 0.45 μm membrane filter, diluted suitably and analysed UV/Vis spectrophotometrically at 283 nm. An equal amount of fresh dissolution medium was replenished immediately after withdrawal of the test sample. Cumulative percent drug release was determined using PCP Disso v2.08 Software (Poona College of Pharmacy, Pune, India).
In vitro buoyancy study
In vitro buoyancy study was performed to check floating behavior of the prepared MPs using USP type II dissolution apparatus. This dissolution medium consisted of 900 ml of 0.1 N HCl containing Tween 80 (0.02 % w/v). In the above media 100 mg of MPs were spread and rotated at 50 rpm for 10 h. After 10 h, both the settled and floating portions of MPs were collected separately, dried and weighed [9]. The percent floating MPs was calculated using the following Eqn. 7, percent floating MPs = weight of floating MPs/ initial weight of floating MPs×100.
Determination of mechanism of drug release
To understand the mechanism of drug release, in vitro drug release data of the optimized formulation (B1) was treated to kinetic models such as zero order, first order, Higuchi model and Peppa’s model. The most appropriate model was selected based on best goodness-of-fit criteria [10-13].
Residual solvent
According to USP-NF VIII DIP (methylene chloride) belongs to class 2 residual solvent and its limits given in the pharmacopeia are 600 ppm. Residual solvent analysis was qualitatively performed by gas chromatography mass spectrometry (GC/MS) technique. About 50 mg of dried MPs were dissolved in a suitable solvent and subjected to gas chromatography using GC 17A (Shimadzu, Tokyo, Japan) for the detection of presence of DCM in the MPs.
In vivo gamma scintigraphy study, method of radiolabelling
Radio labelling of MPs was achieved by incorporation of an appropriate 99mTc labelled radiopharmaceutical (99mTc–MIBI) into the microsphere formulation. To achieve this 1:4 proportion of HPMC K4M and ethyl cellulose were dissolved in a mixture of DCM and ethanol (1:1) solvent system. To the above organic solution 99mTc–MIBI solution (1 ml) was added. The procedure was then similar to that used for the preparation of MPs (batch B1) [14]. Radiolabel efficiency was checked using thin layer chromatography (TLC) [15]. Instant TLC silica gel plates were used as a stationary phase and 100 % acetone was used as mobile phase. Percent radio labelling was calculated as follows, Eqn. 8, percent radio labelling = radioactivity retained in the lower half of the strip/total count present with the strip×100.
Administration of the radio labelled MPs
MPs were administered orally via feeding tube to each rabbit without anaesthesia. For the study the permission was obtained from Institutional Animal Ethics Committee (SIOP/IAEC/2013/09). Twelve 1 y old male albino rabbits were used to monitor the in vivo transit behaviour of the floating MPs. These rabbits were divided into 2 groups (group I and group II). None of them had symptoms or a past history of GI disease. In order to standardize the conditions of GI motility, the animals were fasted for 12 h prior to the commencement of each experiment.
In vivo gamascintigraphy imaging
After oral administration of DIP floating MPs, gamma scintigraphic imaging was carried out to determine the location and extent of its transit through the GI tract. The location of the formulation in the stomach was monitored by keeping the subject in front of a gamma camera. A specific stomach site (anterior) was imaged by gamma camera after definite time intervals and activity counts were recorded to calculate the counts per minute. All counts were corrected for background and isotope decay. The gamma images were recorded using an online computer system. In between the gamma scanning, the animals were freed and allowed to move and carry out normal activities but were not allowed to take any food or water until the formulation had emptied the stomach completely [16].
Pharmacokinetic study
The pharmacokinetic studies were performed as per the animal ethical committee of Sinhgad Institute of Pharmacy, Narhe and Pune. Protocol approval no. CPCSEA/IAEC/SIOP/2012/62. The study was carried out in Wistar rats of either sex weighing 200- 250 g to determine the concentration of DIP in rat blood. The animals were fasted overnight previous to drug administration with free access to water. The rats were divided into three groups (6 animals each). Out of three groups, one group was kept as a control, administered saline by intragastric tubing and other two were administered with pure DIP and DIP MPs formulation (as suspension), respectively. Pure DIP suspension and DIP MPs dose of 246.6 mg/kg (i.e. 55.5 mg) were ingested orally to rats (DIP suspended in aqueous solution containing 1 % w/v carboxyl methyl cellulose-sodium). The blood samples (200 μl) were withdrawn from retro-orbital plexus region at 0 (pre-dose), 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h in tubes containing anticoagulant, mixed and centrifuged at 3000 rpm for 20 min. The plasma was separated carefully and stored at 2-10° until drug analysis was carried out using reversed-phase high-performance liquid chromatography.
Pharmacokinetic analysis
After collection of data for formulation B1 the pharmacokinetic parameters were derived from the plasma concentration versus time plot. The area under curve (AUC), the peak concentration (Cmax) and the time to attain peak concentration (Tmax) were obtained from such plots. The rate constant was determined using Ke= –2.303 (slope). The total area under the plasma concentration was calculated using the trapezoidal rule.
Bio-analytical method for estimation of DIP in rat plasma
The estimation of DIP in rat plasma was carried out using methanol and 0.5 % acetic acid (pH adjusted to 4.5) in the ratio of 70:30 v/v as the mobile phase at a flow rate of 1.0 ml/min. The analysis was carried out using Kromasil C18 (250×4.6 mm ID, 5 μm) column for the development of a bio-analytical method. The eluents were monitored for peak symmetry and retention time. Diazepam was used as an internal standard (IS) for this study [17]. The eluents were monitored at 284 nm.
In vitro-in vivo correlation (IVIVC)
To execute IVIVC Wagner–Nelson function was used. To obtain IVIVC for DIP MPs (B1) a graph of percent drug dose absorbed in vivo vs. percent drug dose dissolved in vitro was plotted and correlation was established using regression analysis.
Stability study
Stability of DIP floating MPs (batch B1), was performed as per ICH and WHO guidelines [18,19]. Accelerated stability studies were performed at 40±2° and 75±5 % relative humidity for 6 mo to assess their longterm stability and were physico-chemically evaluated. Similarity factor f2, was calculated for comparison of release profiles of initial and stability samples.
Results and Discussion
Preliminary trials were performed for the selection of best combination of ethyl cellulose and HPMC K4M. In the preliminary study total 8 formulations were prepared using ethyl cellulose alone and in combination with HPMC K4M and characterised physico chemically. Formulation containing ethyl cellulose alone shown at higher concentration of ethyl cellulose the particle size was increased with improved sphericity, % yield and % EE of the MPs. This may be due to higher concentration of ethyl cellulose content; more is the probability of drug surrounded by the polymer, which acts as a barrier for the diffusion of the drug to the external phase, thereby increasing the EE. However, at higher concentrations of ethyl cellulose, drug release was found to be greatly retarded, since ethyl cellulose is a hydrophobic and thus acts as a release retardant. Based on the obtained results batch P4 was further used in combination with hydrophilic swellable polymer HPMC K4M in order to improve and achieve the controlled release of DIP. MPs prepared with HPMC K4M could not produce the desired particle size and sphericity at higher levels of HPMC K4M; therefore, the prepared MPs could not float for a longer period. This may be due to greater viscosity imparted by HPMC K4M at this concentration. Whereas HPMC K4M shown significant effect on drug release but only up to certain extent, beyond this concentration it retards the drug release due to increase in the diffusional path length of the formulation. The data obtained from the formulation P1 to P8 indicate that ethyl cellulose has a prominent effect on the buoyancy in comparison to HPMC K4M. The highest buoyancy was observed with the batch P5 i.e., 82.65 %. The decreased in the extent of buoyancy in formulation having higher concentration of HPMC K4M could be due to its hydration effect that favours formation of more pores and channels within the MPs causing increased water diffusion over period of time leading to change in density and subsequent settling of MPs.
Formulation batch P5 containing combination of ethyl cellulose and HPMC K4M shown an adequate drug release (73.78±0.45), EE (81.95±1.20 %), floating ability (82.65) hence, was further selected for the development of the optimised formulation (Table 3).
| Batch code | Yield (%) | Particle size (μm) | Floating (%) | Entrapment efficiency (%) | Release (% ) up to 10 h |
|---|---|---|---|---|---|
| P1 | 68 | 253.64±9.7 | 60.87 | 68.94±1.34 | 76.85±0.24 |
| P2 | 72 | 313.88±7.7 | 67.12 | 73.17±0.54 | 74.56±0.16 |
| P3 | 80 | 412.59±5.9 | 73.54 | 78.43±0.32 | 71.24±0.89 |
| P4 | 87 | 473.14±3.7 | 80.17 | 80.64±0.62 | 68.56±0.56 |
| P5 | 86.45 | 487.33±7.3 | 82.65 | 81.95±1.20 | 73.78±0.45 |
| P6 | 87.17 | 513.66±4.6 | 78.24 | 78.34±0.89 | 77.16±0.50 |
| P7 | 85.64 | 532.64±5.0 | 72.34 | 74.35±0.76 | 81.56±0.25 |
| P8 | 85.69 | 552.95±4.4 | 71.45 | 71.64±0.34 | 83.71±0.78 |
*Mean±SD (n=3)
Table 3: Effect of ethyl cellulose alone and in combination with HPMC K4m on prepared microspheres
Literature data revealed that stirring speed has significant effect on EE, drug release and particle size. Therefore, stirring speed was kept constant in the preliminary trials and selected as second independent variable. Based on the results obtained in the preliminary trials and their findings ethyl cellulose was selected as independent variable in factorial design since it has significant effect on particle size, EE and drug release. In this preparation though the floating behaviour is one of the important parameters, it was not considered as a dependent variable in the factorial design since at high levels of ethyl cellulose its sphericity improved and ultimately led to improvement in the floating behaviour.
The micromeritic properties showed the tapped density values in the range of 0.32 to 0.62 g/cm3 while their true densities ranged between 0.42 to 0.72 g/cm3 of all formulations, which might be due to the presence of low density MPs. This was less than 1.004 g/cm3, the specific gravity of the gastric fluid, which reflected the floating property of the MPs. The porosity of all the formulations was found to be in the range of 62 to 82 %. The % compressibility index ranged from 15.3 to 23.7 %. All the formulations showed excellent flow pattern as expressed in terms of angle of repose (˂40°). The better flow property indicated that the floating MPs produced were non-aggregated.
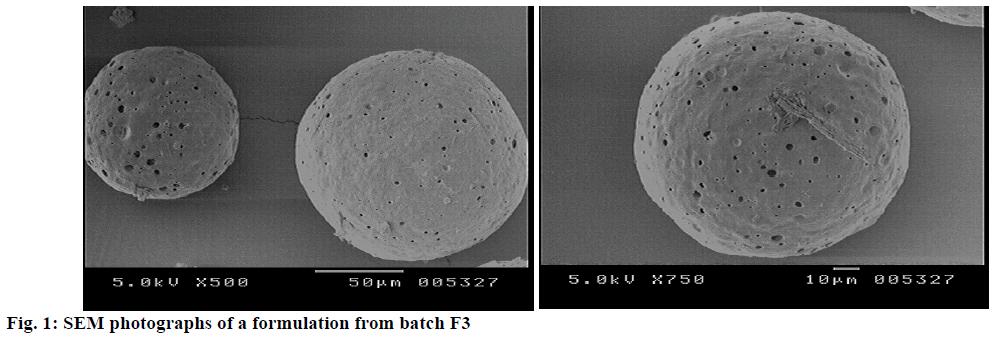
SEM photographs revealed that the developed floating MPs as shown in Figure 1 were predominately spherical in shape and possessed a porous internal surface with smooth and dense outer surface. This implied the solubility of drug in the polymers. The SEM photographs of MPs revealed the absence of drug particles on the surface of the floating MPs, indicating that maximum drug was entrapped in the microspheric wall.
To investigate the floatability, a floating study was performed. The MPs containing ethyl cellulose showed good floating ability (Table 3). Increasing concentration of ethyl cellulose increased the particle size due to insolubility of ethyl cellulose polymer in simulated gastric fluid (SGF, pH 1.2) and increasing particle size resulted in prolonged floating time. Percent drug EE and percent yield of the MPs varied from 62 to 78 % (Table 5) and 64.43 to 84.43 % (Table 4), respectively. These results indicated that upon increasing the concentration of polymer (ethyl cellulose), EE of drug increased.
| Batch code | Yield (%) | Entrapment efficiency (%) | Cumulative drug release (%) 10 h | Particle size (μm) | Floatability (%) |
|---|---|---|---|---|---|
| F1 | 72.4±0.6 | 68.12±1.3 | 74.2±0.04 | 300±3.21 | 76±1.5 |
| F2 | 74.1±1.2 | 64.5±0.8 | 78.12±0.22 | 282 ±2.1 | 72±1.15 |
| F3 | 83±1.3 | 62.3±0.76 | 81.66±0.12 | 270±4.5 | 73±2.4 |
| F4 | 68.9±0.9 | 74.9±1.34 | 68.22±0.21 | 525±1.58 | 81±1.2 |
| F5 | 71±0.8 | 72.3±1.02 | 73.43±0.43 | 510±2.45 | 80±1.5 |
| F6 | 79±1.2 | 70.8±0.89 | 76.6±0.23 | 498±2.1 | 78±2.25 |
| F7 | 68±1.1 | 78±0.56 | 64.23±0.08 | 540±3.15 | 84.25±1.7 |
| F8 | 71.3±0.9 | 75.6±1.09 | 68.38±0.31 | 522±2.0 | 82.3±0.98 |
| F9 | 74.7±0.8 | 73.1±1.78 | 72.39±0.19 | 510±2.4 | 81±1.2 |
*Mean±SD (n=3)
Table 4: Evaluation of floating microspheres subjected to optimization batch F1-F9
| Batch code | Composition | Responses | % Error | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 (mg) | X2 (rpm) | Predicted | Experimental | Y1 | Y2 | Y3 | |||||
| Y1 (μm) |
Y2 (%) |
Y3 (%Rel) |
Y1 (μm) |
Y2 (%) |
Y3 (%Rel) |
||||||
| B1 | 202 | 570 | 520 | 71 | 71 | 518 | 71 | 71.8 | -0.38 | -0.14 | -0.14 |
| B2 | 167.85 | 309.16 | 400 | 70.5 | 72.4 | 404 | 70.4 | 72.1 | 1 | -0.22 | 0.41 |
| B3 | 171.7 | 385 | 418 | 70.1 | 72.3 | 419.5 | 70.2 | 72.2 | 0.35 | 0.14 | 0.13 |
% Error = (predicted value-experimental value/experimental value) × 100. Two random check points for DIP microsphere compositions covering the experimental domain B2 and B3
Table 5: All responses Y1 to Y3 along with percent error for floating microsphere formulations observed for optimal composition B1
The results showed that upon increasing the concentration of ethyl cellulose, the yield of the MPs increased. It was observed that the stirring speed exerted a significant effect on improving percent yield but up to certain extent only. In this case, when stirring speed increased the percent yield increased but decreased with further increment to 1000 rpm. At higher stirring speed of 1000 rpm, the percent yield decreased due to breaking up of MPs and formation of coagulant masses of MPs. Hence, the stirring speed should be optimized to obtain the desired percentage yield.
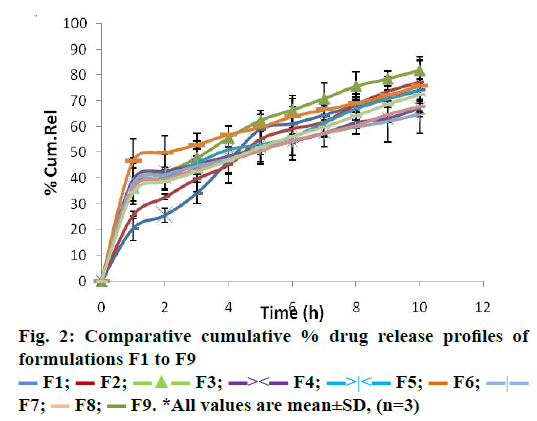
The in vitro dissolution study was performed by using USP type II apparatus containing SGF i.e. 0.1 N HCl (pH 1.2) for 10 h. It was observed that formulations F1 to F3 showed 74.2±0.04 to 81.66±0.12 % of cumulative drug release at 10 h. With formulation F4, F5 and F6, retarded release was observed i.e. 68.22±0.21 to 76.6±0.23 %. This could be because of increasing concentration of release retarding polymer (ethyl cellulose) in the formulations. Furthermore, it was reduced to 64.23±0.08 to 72.39±0.19 % for F7 to F9. The data obtained in the dissolution study revealed that, as the concentration of ethyl cellulose increased, the percent cumulative release of drug was decreased (Figure 2).
The investigation contained a total of 9 trial batches as proposed by the 32 factorial design. In which, two independent variables, amount of ethyl cellulose (X1 mg) and stirring speed (X2 rpm), were varied at three different levels, high, low and medium. The effect of these independent variables on particle size (μm), EE (%) and drug release (%) were investigated. Overview of the experimental and observed responses is given in Table 4. Design-Expert 8.0.7.1. software provided suitable polynomial equations involving main factors and interaction factor after fitting this data. The model Eqn. related to particle size in μm as a response became Eqn. p, Y1 (μm) = +509.33+120×X1– 14.50×X2+3.55E–0.15×X1X2–107×X1+2.50×X2; R2= 0.9990; F value= 285.31; p= ˂0.0001. In the Eqn. the concentration of ethyl cellulose X1 showed the positive effect on particle size. The particles size was in the range of 270±4.5 to 540±3.15 μm (Table 5). This increase in particle size may be due to at high concentration viscosity of the medium increased resulting in enhanced interfacial tension and shearing efficiency is also diminished at higher viscosities [20]. Whereas, stirring speed (X2) shown negative effect on particle size.
The model Eqn. relating EE (%) as a response is Eqn. 10, Y2 (% EE) = +71.67+5.33×X1–2.50× +0.25×X1X2– 2.00×X1+0.50×X2; R2= 0.9950; F value= 31; p= ˂0.0001. The independent factors X1 (amount of ethyl cellulose) and X2 (stirring speed) significantly affected the EE. At higher levels of ethyl cellulose EE was improved, this could be due to increase in the ethyl cellulose concentration made organic phase viscous and thus, increased the diffusional resistance of drug molecules to diffuse from organic to aqueous phase. As a result, more and more drug gets entrapped in polymer matrix. In the above Eqn. the stirring speed X2 exerted negative effect on EE. Model Eqn. relating to drug release (%) is Eqn. 11, Y3 (%Rel) = +72.67–4.83×X1+3.83×X2; R2= 0.9928; F value=56.96; p= ˂0.0001.
In this case, negative coefficient of X1 indicated that as the concentration of ethyl cellulose increased the percent release decreased whereas in the above equation, coefficient of X2 had positive effect on the percent release. Increasing stirring speed caused increase in drug release and this could be due to reduction in particle size, which attributed increase in surface area, so due to the surface area particle size relationship, the contact area between MPs and dissolution medium increased leading to an increase in the rate of dissolution.
In addition, the three-dimensional response surface plots were generated and represented in Figure 3A, which showed nearly linear decreasing patterns for the particle size values as the stirring speed increased. But at higher levels of the polymers, the response surface showed a slightly linear shape. Maximum particle size is observed at high levels of ethyl cellulose concentration. It shows nearly linear decreasing pattern for the values of particle size as the stirring speed increased. Figure 3B showed quite a linearly increasing trend in the values of drug EE with increased value of ethyl cellulose and stirring speed. However, the influence of stirring speed is more significant than that of the ethyl cellulose concentration. Hence, lower levels of ethyl cellulose to maintain drug entrapment at a constant level. Figure 3C revealed a decline in the values of drug release (at 10 h) with increasing in concentration of ethyl cellulose. A linear increasing pattern is seen with the increase in stirring speed. It also elucidated that the variation in the real10 h is a complex function of the concentration of ethyl cellulose and the effect of stirring speed being less significant.
The aim of the optimization of pharmaceutical formulations is to determine the levels of the variable from which a robust product with high quality characteristics may be produced. In order to assess the reliability of the developed mathematical model, formulations corresponding to optimum composition and two additional random compositions covering the entire range of experimental domain were performed and all the three responses Y1, Y2 and Y3 were estimated using developed mathematical model Eqns. 9-11 and also by the experimental procedures. The formulation composition of the optimum and the random check points, their experimental and predicted values for all the three responses variables are tabularized (Table 5). The low magnitudes of the bias values indicated a reasonable agreement between the predicted and the experimental values. It also indicated the robustness and high degree of predictive power of the generated quantitative mathematical models [21-23]. From the results obtained, it could be concluded that the generated Eqns. described adequately the influence of the selected formulation components on the responses under study and indicated the robustness of the model and high prognostic ability of multi variate optimization technique and also proved that 32 full factorial designs was thus validated. The calculated desirability factor for optimized formulation (B1) was close to one, indicating suitability of the designed factorial model. The results of dependent variables from the software were found to be 71 % for percent ethyl cellulose and 518 µm for particle size and 71.1% cumulative release at these levels.
Determination of mechanism of drug release,optimized batch B1, was subjected to different kinetic models to understand in vitro drug release mechanism. Correlation coefficient of different kinetic models is shown in Table 6. Based on the regression coefficient (R2) values Higuchi’s model shown highest R2 value of 0.989 this indicates the release occurs by diffusion mechanism and when it was analysed according to Peppa’s model the release exponent n was 0.489. In this model n value indicates diffusional (controlled) release and found to be non Fickian transport since exponent n was less than 0.5. The non Fickian mechanism of drug release demonstrates both diffusion controlled, and swelling controlled drug release from buoyant MPs containing DIP [24].
| Kinetic model | Parameters | Values |
|---|---|---|
| Zero order | R2 | 0.8183 |
| Higuchi | R2 | 0.9892 |
| Hixon | R2 | 0.9889 |
| Korsmeyer–Peppas | R2 | 0.9778 |
| n* | 0.488 |
*Release exponent
Table 6: The correlation coefficient (R2) values for B1 formulation
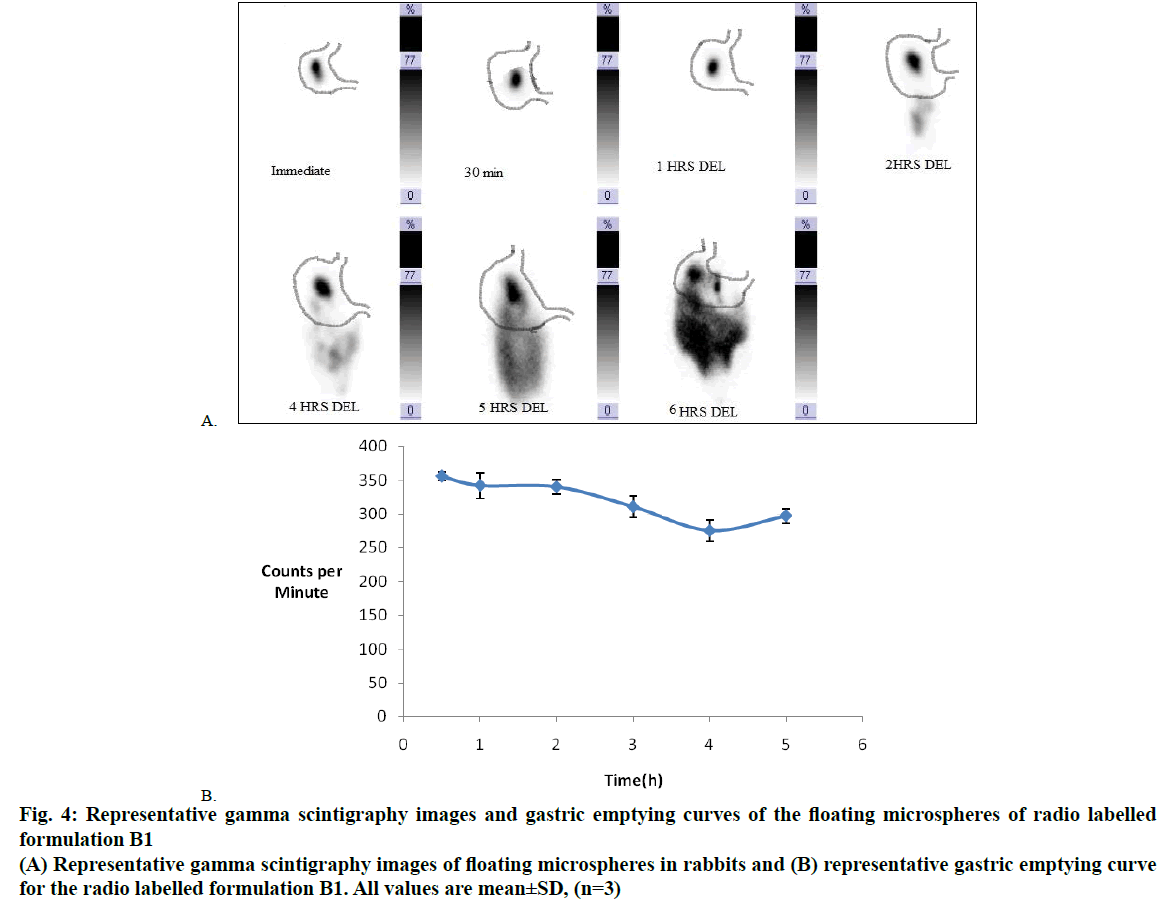
Floating DIP MPs (batch B1) were prepared using the stirring time of 1 h at 40°. The GC/MS results of this batch shown absence of DCM. This indicated 1 h stirring at 40° is sufficient to remove DCM and to form the hollow cavity in the MPs. In the present study in vivo gamma scintigraphy study was done to observe the in vivo floating behaviour of optimized floating MPs. Radiolabelling efficiency for radiolabelled formulation (B1) was found more than 90 %. Examination of the sequential gamma scintigraphy images during the study clearly indicated that the formulation B1 remained buoyant and uniformly distributed in the gastric contents for the study period of 6 h (Figure 4A). This might be due to after swallowing; the floating MPs adopted a floating position on top of the stomach content. A measurable number of counts of 99mTc-tagged B1 for the 6 h study period showed very good gastro-retentive propensity as the administered MPs remained floating and distributed in the stomach contents for the 6 h study period. Gamma scintigraphy was performed for 6 h, corresponding to the half-life of 99mTc (Figure 4B).
Figure 4: Representative gamma scintigraphy images and gastric emptying curves of the floating microspheres of radio labelled
formulation B1
(A) Representative gamma scintigraphy images of floating microspheres in rabbits and (B) representative gastric emptying curve
for the radio labelled formulation B1. All values are mean±SD, (n=3)
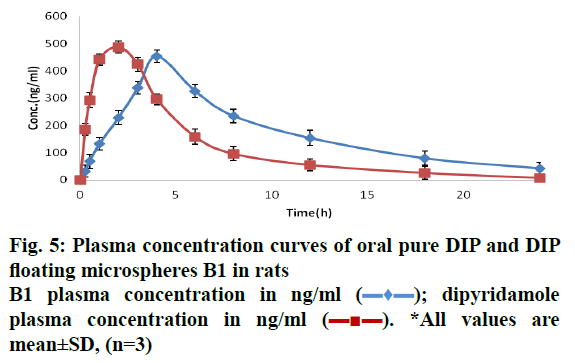
The proof of concept of gastro retention of floating MPs was further confirmed by performing the in vivo pharmacokinetic study. The pharmacokinetic parameters of batch B1 and pure DIP are shown in Table 7. B1 formulation showed slower absorption and elimination compared to pure DIP (Figure 5). The plasma level of pure DIP was increased quickly and the maximum concentration was reached at 2 h after administration. The maximum concentration of floating MPs was reached at 4 h and the concentration slowly decreased even at 24 h after administration. In case of floating MPs, Tmax was shifted towards higher side (4 h) indicating sustained release properties. Elimination rate constant (Kel) and AUCinf of floating DIP formulation was greater than the pure DIP. The bioavailability was improved by 1.41 folds. In the present study IVIVC relationship was studied by Wagner Nelson method. The relationship between percent DIP released in vitro vs. percent DIP absorbed in vivo is described by the regression Eqn. as follows, Eqn. 12, Y = 0.0002x3+0.0256x2+0.0166x.
| Pharmacokinetic parameters | Pure DIP | B1 formulation |
|---|---|---|
| Cmax (ng/ml) | 487.2±1.25 | 453.6±1.85 |
| Tmax (h) | 2 | 4 |
| Ke (h-1) | 158.2 | 37.14 |
| AUC0-inf (ng.h/ml) | 2891.3 | 4105.25 |
| BA (in folds) | - | 1.41 (30 %) |
*Mean±SD (n=3)
Table 7: Pharmacokinetic parameters of pure dip and dip microspheres formulation (B1)
The second order polynomial relationship was found to be the best fit for IVIVC data of DIP MPs. An acceptable correlation was obtained with regression coefficient of 0.9744. This improvement in the bioavailability confirms the gastric retention of the prepared floating DIP MPs in the stomach. Accelerated stability study shown no significant changes by characterizing samples for appearance, % drug release, % buoyancy and drug content for a period of 6 mo (Table 7). Similarity factor (f2) was found to be more than 50 for batch B1 kept at 0 mo and 6 mo and evaluated dissolution profile. Results shown no significant change in the release profile indicating two dissolution profiles are similar (Figure 6).
The non-effervescent floating MPs of DIP were successfully developed using ethyl cellulose and HPMC K4M by solvent diffusion evaporation method with the help of 32 factorial design, the in vitro results indicate that they are potentially useful. The optimized buoyant MPs (B1) containing DIP combining excellent drug entrapment, good buoyant ability with a minimum buoyant lag-time and suitable prolonged drug release pattern with improved bioavailability of DIP. In vivo gammascintigraphy study provided the confirmation of gastroretentive behaviour of optimized formulation. The method used in the preparation of DIP floating MPs was found to be simple, reproducible, easily controllable, economical and consistent process. The use of concept of formulation by design using experimental design might be useful to develop an effective GRDF with desired floating behaviour and drug release profile with minimum efforts in shortest time.
Acknowledgements
Authors wish to thank STES’s Sinhgad Institute of Pharmacy, Narhe, Pune for providing facilities for completion of this project.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Chen S, Zhu J, Ma F. Preparation and characterization of solid dispersions of dipyridamole with a carrier ‘‘copolyvidonum Plasdone S-630’’. Drug Dev Ind Pharm 2007;33:888-99.
- Patel VF, Patel NM. Statistical evaluation of influence of xanthan gum and guar gum blends on dipyridamole release from floating matrix tablets. Drug Dev Ind Pharm 2007;33:327-34.
- Xu L, Luo Y, Feng J. Preparation and in vitro-in vivo evaluation of non-gastric resident dipyridamole (DIP) sustained release pellets with enhanced bioavailability. Int J Pharm 2012;422:9-16.
- Zhan Z, Peng B, Yang X. Design and evaluation of a novel floating osmotic pump system. J Pharm Sci 2009;12:129-37.
- Sutinen R, Laasanen V, Paronen P, Urtti A. pH controlled silicon microspheres for controlled drug delivery. J Control Release1995;33:163-71.
- Beten DB, Moes AS. Controlled release co evaporates of dipyridamole prepared with acrylic polymers. Int J Pharm 1994;103:243-51.
- Tang X, Xu L, Luo Y, Feng J, Xu M, Tao X, et al. Preparation and in vitro-in vivo evaluation of none gastric resident dipyridamole (DIP) sustained-release pellets with enhanced bioavailability. Int J Pharm 2012;422:9-16.
- Rao MRP, Borate SG, Thanki KC, Ranpise AA, Parikh GN. Development and in vitro evaluation of floating rosiglitazone maleate microspheres.Drug Dev Ind Pharm 2009;35(7):834-42.
- Naggar VF, E-Kamel AH, Sokar MS, Al-Gamal SS. Preparation and evaluation of Ketoprofen floating oral delivery system. Int J Pharm 2001;220:13-21.
- Korsmeyer R, Gurny W, Doelker R. Mechanisms of solute from porous hydrophilic polymers. Int J Pharm 1983;25-35.
- Peppas NA. Analysis of Fickian and non Fickian drug release from polymers. Pharm Acta Helv 1985;60:110-11.
- Costa P, Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 2001;13:23-33.
- Jose S, Angueiro JF, Smiths J. Predictive modeling of Insulin release profile from cross linked chitosan microspheres. Eur J Med Chem 2013;60:249-53.
- Sato Y, Kawashima Y, Takeuchi H, Yamamoto H. Pharmacoscintigraphic evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. J Control Release 2004;98:75-85.
- Pund S, Joshi A, Vasu K, Nivsarkar M, Shishoo C. Gastroretentive delivery of rifampicin: In vitro mucoadhesion and in vivo gamma scintigraphy. Int J Pharm 2011;411:106-12.
- Jain S, Agrawal G, Jain N. Evaluation of porous carrier-based floating orlistat microspheres for gastric delivery. AAPS Pharm SciTech 2006;4:E54-E62.
- Feng G, Haijun Z, Jing H, Baogang X, Fen L, Helin X, et al. Self-microemulsifying drug delivery system for improved oral bioavailability of dipyridamole: preparation and evaluation. Arch Pharm Res 2011(34):1113-23.
- Ritger PL, Peppas NA. A simple equation for description of solute release. I Fickian and non-Fickian release from non swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release 1987;5:23-36.
- Mathews BR. Regulatory aspects of stability testing in Europe. Drug Dev Ind Pharm 1999;25:831-56.
- Nila MV, Sudhir MR, Chinu TA, Aleykutty N, Jose S. Floating microspheres of carvedilol as gastroretentive drug delivery system: 32 full factorial design and in vitro evaluation. Drug Delivery 2013;1-8.
- Singh B, Chakkal SK, Ahuja N. Formulation and optimization of controlled release mucoadhesive tablets of atenolol using response surface methodology. AAPS Pharm Sci Tech 2006;7:E1-E10.
- Pund S, Joshi A, Vasu K, Nivsarkar M, Shishoo C. Multivariate optimization of formulation and process variables influencing physico-mechanical characteristics of site specific release isoniazid pellets. Int J Pharm 2010;388:64-72.
- Patil S, Pund S, Joshi A, Shishoo C, Shahiwala A. Chronomodulated press-coated pulsatile therapeutic system for aceclofenac: optimization of factors influencing drug release and lag time. J Chronophysiol Ther 2011;1:1-10.
- Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 2010;67:217-23.
 F1;
F1;  F2;
F2;  F3;
F3;  F4;
F4;  F5;
F5;  F6;
F6;  F7;
F7;  F8;
F8;  F9. *All values are mean±SD, (n=3)
F9. *All values are mean±SD, (n=3)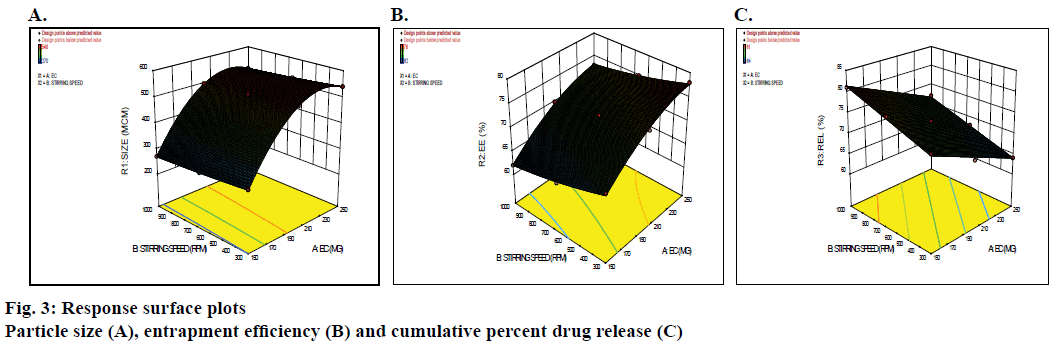
 ); dipyridamole
plasma concentration in ng/ml (
); dipyridamole
plasma concentration in ng/ml ( ). *All values are
mean±SD, (n=3)
). *All values are
mean±SD, (n=3)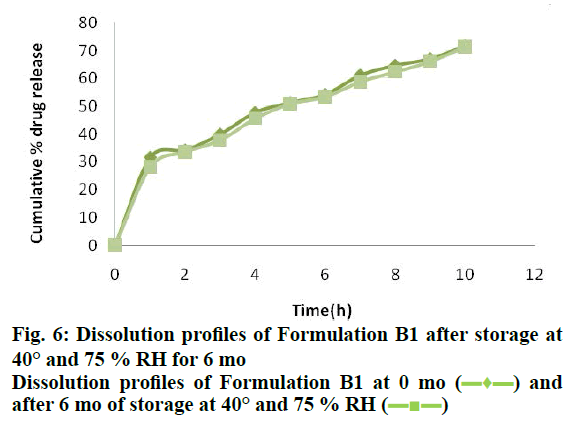
 ) and
after 6 mo of storage at 40° and 75 % RH (
) and
after 6 mo of storage at 40° and 75 % RH ( )
)



