- *Corresponding Author:
- Savani Bhattacharyya
Department of Pharmaceutics, Krupanidhi College of Pharmacy, Bengaluru, Karnataka 560035, India
E-mail: sayanibh@gmail.com
| Date of Received | 10 February 2022 |
| Date of Revision | 11 April 2022 |
| Date of Acceptance | 20 March 2023 |
| Indian J Pharm Sci 2023;85(2):325-337 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Loratadine is an efficacious, non-drowsy antihistaminic molecule prescribed for allergic skin. The conventional oral dosage form is associated with various side effects, limited and variable absorption. The present study attempts to improve the effectiveness of the drug by formulating a niosome loaded hydrogel of loratadine for treating allergic skin. Ether injection method was used to prepare the niosomes of loratadine using Box Behnken design. Particle size analysis, surface charge, surface morphology (atomic force microscopy), compatibility (Fourier transform infrared spectroscopy), thermal (differential scanning calorimetry), and surface characteristics (powder X-ray diffraction) study were done to evaluate the drug-loaded niosomes. The optimized formulation was incorporated into two types of gelling systems. The nanogels were evaluated for rheological and mechanical properties, in vitro, ex vivo permeation and skin irritation studies. The Box Behnken design was found to be productive for the formulation of nano-sized (109.99 nm) and stable -37.5 mV niosomes of loratadine. The atomic force microscopy study revealed the formation of unilamellar niosomes, no incompatibility was detected between drug and excipients through Fourier transform infrared spectroscopy study. The differential scanning calorimetry and powder X-ray diffraction study reported the confinement of drugs in the niosomal core. The nanogels had good rheological and mechanical properties. The ex vivo study revealed the maximum penetration of the drug from the gel base of Xanthan gum, and no skin irritation was reported from the nanogel. The formulation and evaluation of niosomal gel of loratadine were found to have satisfactory permeation and can be a promising alternative approach for the administration of loratadine in the treatment of skin allergy.
Keywords
Loratadine, niosomes, nanogel, transdermal delivery, skin allergy
Insect bites can initiate severe allergic reactions due to the deposition of venom into the skin, especially for insects like bees, wasps, ants, spiders and fleas. Initial allergic reactions are manifested by swelling, redness, pain, skin eruption and blister on the skin associated with pain. Traveling through the endemic countries people may experience these types of allergic reactions. If the reactions are neglected it can lead to fatal incidents[1].
Loratadine (LRTD) is a non-drowsy antihistaminic available as a tablet or mouth dissolving tablet and as a solution, prescribed for the treatment of allergy associated with itching and redness due to insect bites. Oral administration is accompanied by side effects like headaches, dry mouth, nervousness, nose bleeding, stomach pain, etc[2]. In addition, conventional oral administration shows variable absorption[3,4].
Hence an alternative approach of delivering LRTD topically can be beneficial to overcome the difficulties of the conventional dosage form and associated side effects. The drug can be localized at the site of skin allergies, can provide rapid relief and better patient compliance.
Low molecular weight and daily dose partially qualify LRTD as a candidate for dermal delivery[5]. But its high lipophilicity and very poor water solubility can hinder the permeation through the skin[6]. Hence permeability can be improved by incorporation of LRTD into colloidal drug carrier systems, and niosomes can be proposed for that as it is proven by the researchers that it enhances the transdermal penetration[7].
Niosomes are non-ionic surfactant nanovesicles, used to entrap both hydrophilic and hydrophobic drugs. Penetration of encapsulated drugs through niosomal vesicles can happen by adsorption onto the cell surface, followed by fusion with the cell membrane and finally engulfing by the process of endocytosis and thus releasing the entrapped molecule[8]. Niosomes gelling systems are used to modulate the permeability of the horny layer of the skin by reducing trans-epidermal water loss, increasing its smoothness, and replenishing the lipid composition of the skin[8,9]. Thus, the niosome-loaded gel can be a suitable option for efficient topical delivery of LRTD.
The purpose of the present study is to improve the penetrability of LRTD through the skin by entrapping it into vesicles of niosomes and then formulating a hydrogel for application to allergic skin. The formulation was executed using experimental design to have control over particles size for dermal penetration and high entrapment of drug in the formulation. The current study comprises in vitro characterization of the niosomes and ex vivo skin permeation and skin irritation study.
Materials and Methods
LRTD was procured from Strides, Arcolab Bangalore. Tween 60, Span 40, and cholesterol were brought from SD Fine Chem Limited, Bangalore. All other materials and solvents used in the experimental studies were of analytical grade.
Screening of surfactants:
Non-ionic surfactants comprise the main ingredient in the formulation of niosomes. The most important formulation aspect of niosomes is the selection of a suitable nonionic surfactant or a mixture of surfactants to achieve maximum entrapment and stability of the nano vesicles[10]. The most commonly used surfactants are Spans, Tweens, and Brij. As these surfactants have no charge at their hydrophilic heads, they self-assemble themselves into a bilayer structure in presence of water. Hydrophilic Lipophilic Balance (HLB) of the surfactant or surfactant mix also plays a vital role in the entrapment of the drug in niosomal vesicles. Khan R et al. illustrated that the variation of HLB from 1.7 to 14 can greatly affect the entrapment efficiency of the drug depending on its solubility[11,12]. LRTD being lipophilic, screening of surfactants among Span 20, Span 40, Tween 20, and Tween 60 were done either using them alone or in combination to achieve maximum entrapment of LRTD. Keeping an aim of high entrapment of LRTD in niosomes, the preformulation study suggested the selection of a combination of Span 40 and Tween 60 for the formulation of niosomes. Hence, the study explored the effect of RHLB from 4 to 12 on drug entrapment and vesicle size of the niosomes to determine the optimum Required HLB (RHLB) for the mixture of surfactants.
Cholesterol is used in the preparation as an edge activator. To enhance the entrapment of the drug and to minimize the leakage, a preformulation study was carried out to select the optimum surfactant and cholesterol ratio between 1:1 to 2:1. The study suggested a ratio of surfactant and cholesterol at 1.5:1 to have maximum stability of the vesicles with high entrapment of drug.
Application of design of experiment:
The ether injection method was used for the preparation of LRTD encapsulated niosomal dispersion. Box Behnken design was exercised to evaluate the critical formulation variables like RHLB, rate of injection and organic/aqueous phase ratio on entrapment efficiency and vesicle size of the formulated niosomes. The design yielded 15 runs with three center points to have control over the errors in the formulation[13]. The independent and dependent variables are listed in Table 1. Each of the independent variables is varied from low (-1) to high (+1) level. The dependent variables such as percentage drug entrapment efficiency and particle size were targeted to maximize and minimize respectively during the model optimization process.
| Variables | Ranges | |
|---|---|---|
| Independent Variables | Low (-1) | High (+1) |
| RHLB | 4 | 12 |
| Rate of injection (ml/min) | 0.5 | 1 |
| Org/Aq phase | 0.25 | 0.5 |
| Dependent variables | Target to achieve | |
| Drug Entrapment efficiency (%) | Maximize | |
| Particle size (nm) | Minimize | |
Table 1: List of Dependent and Independent Variables
Methods of preparation of niosomes:
Drug-loaded niosomes were prepared by ethanol injection method, where surfactant and cholesterol ratio was maintained at 1.5:1 in all the trial runs. For all the experimental trials a concentration of 3 % w/w drug was maintained. The weighed amount of cholesterol, span 40, tween 60, and the drug were dissolved in diethyl ether, this dispersion was sonicated for 2 to 3 min. The resulting solution was slowly injected into the aqueous solution of surfactant mix using a microsyringe at a specific rate as per the design. The solution was stirred (500 rpm) continuously using a magnetic stirrer at 60-65° which resulted in the evaporation of the organic solvent, and the formation of niosomal dispersion[14,15].
Evaluation of niosomes:
Particle size distribution: The vesicle size of the formulations was determined by (Horiba SZ-100). A small quantity of the sample was diluted with double distilled water and was sonicated for 30 min. The measurement of the vesicle size was done through the principle of dynamic light scattering, at 25.2°. Three trials were carried out for each sample for the estimation of vesicle size[16].
Determination of entrapment efficiency: The percentage of encapsulation of the formulations was estimated by measuring the amount of unbound drug in the formulation by the ultra-centrifugation method. A known quantity of the formulation was dispersed in an equal volume of methanol, vortexed[17]. A syringe filter of 0.22 μm was used to filter an aliquot of a clear layer of the sample, suitably diluted with the solvent 0.1 N HCL and was analyzed spectrophotometrically at 275 nm to estimate the total drug (DTOTAL). The same volume of the formulation was centrifuged at 5000 rpm for 30 min using Remi centrifuge and the supernatant layer after dilution was analyzed in the same way to determine the unentrapped drug (DFREE). The percentage entrapment efficiency was calculated using the equation. Estimation of each sample was carried out in triplicates[18].
Percent entrapment efficiency=DTOTAL-DFREE/ DTOTAL×100
Freeze drying of optimized niosome formulation: Lyophilization was done for the optimized drugloaded niosomal dispersion. To the dispersion, the lyoprotectants-mannitol (15 % w/w) and dextrose (5 % w/w) were added to yield a sugar:lipid ratio of 3:1.
The suspension was subjected to freezing at -30° for 24 h in a deep freezer followed by freeze-drying at 0.02 bar vacuum pressure and at temperature -60° for 12 h. The freeze-dried product was collected and stored in a desiccator. The lyophilized sample was taken for further analysis[19].
Determination of Zeta potential:
A pilot study was carried out on three volunteers, to The zeta potential of the optimized formulations was determined by (Horiba SZ-100). Samples were diluted in Millipore water and the measurement of the zeta potential was done at 25.2°. The estimation was carried out in triplicates for each sample[16].
Determination of drug content:
A fixed quantity of the freeze-dried optimized formulation was dissolved in methanol using a vortex shaker until the formulation was completely dissolved. From this, an aliquot was pipetted out and was diluted with 0.1 N HCL. The absorbance was measured spectrophotometrically at 275 nm and drug content was calculated. The average LRTD content in the formulation was reported after three determinations[20].
Fourier Transform Infrared (FTIR) spectroscopic study:
The study was performed on a FT-IR using autothermal reforming technology (Nicolet iS50, Thermo Fisher Scientific, USA). The test was carried out for the pure drug, freeze-dried samples of optimized formulation, and optimized blank formulation. The spectra were analyzed for the determination of any incompatibility between the drug and the formulation ingredients[21].
Surface morphology study:
The morphology of the vesicles was analyzed by using Park systems NX-10 Atomic force Microscopy (AFM) at room temperature. A drop of the dispersion after dilution with deionized water and sonication was spread on glass coverslips and dried to remove moisture. The specimen on the coverslip was imaged by scanning 50 μm×50 μm areas in x, y, and z directions using a cantilever[22].
Powder X-ray diffraction (PXRD) Study:
PXRD was done using diffractometer Bruker D8 Advance, Germany. The process was carried out at 40 KV and 30 mA using a Si (Li) PSD detector and Cu X-ray source. The samples were scanned at 0.80 per sec at 2 Theta from 5-60°. PXRD patterns were compared with the pure drug, optimized blank, and drug-loaded optimized formulation[23].
Differential Scanning Calorimetry (DSC) study:
Approximately 1.5 mg of sample was analyzed in SDT Q6 100 TA instruments over a temperature range of 0° to 600° at a heating rate of 20° per min in a nitrogen atmosphere (100 ml /min) for pure drug, optimized and blank formulation[24].
Preparation of LRTD loaded niosomal gel:
Lyophilized niosomes (0.5 % w/w drug equivalent) were used to prepare two different hydrogels with Carbopol and Xanthum gum. The composition of the niosomal loaded gel is listed in Table 2.
| Composition | GEL I | GEL II |
|---|---|---|
| LYO-NIO (% w/w) | 0.5 | 0.5 |
| Carbopol 934 (% w/v) | 1 | - |
| Xanthum Gum (% w/v) | - | 1 |
| HPMC (% w/v) | - | - |
| Glycerol (% w/v) | 3 | - |
| TEA(% w/v) | 1 | - |
| Propylene Glycol (% w/v) | - | 2 |
| Distilled water qs (g) | 10 | 10 |
Table 2: Composition of Niosomal Gel
Gel I was prepared by dispersing Carbopol 934 in distilled water and was allowed to hydrate for 4 h. Glycerol was added to this dispersion under magnetic stirring. Triethanolamine solution was added for adjusting the pH of the gel base to 5.5[25]. The entrapped air in the gel was removed by letting it stand undisturbed overnight, finally distilled water was added to adjust gel weight to 10 g. Finally, the lyophilized drug-loaded niosomes were incorporated under continuous stirring using a magnetic stirrer at a low speed until uniform gel was obtained.
Gel II was prepared by dispersing xanthan gum in distilled water under continuous magnetic stirring. The dispersion was allowed to hydrate for 2 h. To this propylene glycol was added with continuous stirring. The gel was allowed to stand overnight for the removal of air, then adjusted to the weight of 10 g by the addition of distilled water[26]. The lyophilized drug-loaded niosomes were incorporated under continuous stirring using a magnetic stirrer to the gel base.
Employing the same methods of preparation, gels of the pure drug were formulated in both the bases for comparative drug release study.
Evaluation of nano gel:
Appearance of gel: The formulated LRTD loaded niosomal gel was tested for appearance, odor, and feel upon application such as stickiness, stiffness, greasiness and smoothness[27,28].
Determination of drug content:
A specific quantity of gel was taken in an Eppendorf tube mixed with methanol and vortexed for 10 min. An aliquot was withdrawn filtered with a syringe filter and diluted with 0.1 N HCL and analyzed spectrophotometrically at λ-max of 275 nm. Three trials were run to confirm the results[29].
Determination of spreadability:
To determine the spreadability of the gel two glass slides were used. The gel formulation was sandwiched between the two slides. A weight of 100 g was placed on the upper slide for 1 min so that the gel between the slides was pressed to form a thin layer. Later the weight was removed and spreading of the gel in terms of length was noted. Spread ability was calculated by the formula
Spreadability=Mass×Length/Time
The experimentation was done in triplicates[30].
Rheological evaluation of gel:
The viscosity of the gels was determined by placing a small amount of gel in Brookfield (DV-E) viscometer using helipath spindle T bar (E) at 100 rpm for 5 min. The measurements were made in triplicates and the readings were recorded[31].
pH measurement:
The pH of the gels was determined by the digital pH meter (Digisun Electronics services, Hyderabad). A small quantity of gel after dilution with distilled water was taken for pH measurements. The measurements were taken in triplicates and an average of the readings was recorded[32].
In vitro diffusion study:
Franz diffusion cell was used for the in vitro diffusion study of the drug from the formulations. The dialysis membrane was soaked in diffusion media for 24 h for activation of the pores. The diffusion medium selected was phosphate buffer of pH 7.4. In the donor compartment formulation equivalent to 1 mg LRTD niosomal gel was taken for the study. The experimentation was conducted at a temperature of 37±1° with a stirring speed of 100 rpm. At a regular interval, 1 ml of the sample was withdrawn and replaced with the same volume of the fresh medium. The experimentation was continued for 6 h. The aliquots were suitably diluted with 0.1 N HCL and analyzed spectrophotometrically at 275 nm. All the readings were taken thrice to avoid errors. The percentage cumulative amount of drug permeated vs. time was plotted[33]. The same experiment was repeated for the gel of pure drug in gel I and gel II base.
Ex vivo permeation study:
Vide the approval No. KCP/IAEC/PCOL/ PCEU/68/2020 the animals were used for the study. Excessive carbon dioxide infusion was given to euthanize the rats. The abdominal skin was collected. After depilation, the skin was washed with saline solution. The fat adhering on the dermis side was removed using a scalpel and washed with isopropyl alcohol[34]. The processed skin was used for the ex vivo permeation study. The permeation studies were carried out in the Franz diffusion apparatus. The receptor chamber was filled with freshly prepared phosphate buffer pH 7.4. The skin was mounted between the donor and receptor compartment with the stratum corneum facing the donor compartment and dermis facing the receiver compartment in the Franz diffusion cell. The temperature in the diffusion cell was maintained at 37±1° and was stirred continuously at a speed of 100 rpm. A weighed quantity of 1 g of nanogel was spread on the surface of the skin in the donor compartment and sealed to provide occlusive conditions. An aliquot was withdrawn at regular time intervals up to 6 h, suitably diluted, and analyzed spectrophotometrically at 275 nm. An equal volume of fresh diffusion medium was replaced after each withdrawal of the sample. The percentage cumulative amount of drug permeated vs. time was plotted[35,36]. The same was repeated for the gel of pure drug in Carbopol and Xanthum gum base.
The data was used to determine the flux at steady state (Jss) and permeability coefficient (Kp)
Skin irritation test:
The study was conducted on eighteen albino Wistar rats distributed in three groups. Group-I served as control where no application was done. A placebo niosomal gel was applied to group II. The best test formulation was applied to group-III animals. After depletion of the hairs from the back of the animals, the areas were marked. The placebo gel formulation, gel of pure drug, and the drug-loaded niosomal gel were applied once daily for 7 consecutive d and observed for any sensitive reaction[37,38].
Results and Discussion
The preparation of niosome by the ether injection method is a multipronged process. The vesicle size and entrapment efficiency are greatly affected by the composition and the process parameters[39,40]. For a lipophilic drug to entrap sufficiently in the vessicles of niosomes, the HLB of the system and the selection of surfactants are critical. Similarly, the phase volume ratio also affects the drug entrapment and the formation of vesicles. The rate of injection influences the formation and size of the vesicles. Box Behnken design can figure out the potential interactions between factors with less experimentation. Hence in the proposed work, all the factors were varied in a wide range using Box Behnken design to observe the effect on responses like vesicle size and entrapment efficiency. The particle size and percentage entrapment efficiency were varied in the range of 89.2±2.76 nm to 494±5.03 nm, and 51.91±1.12 % to 82.60±0.98 % respectively for the fifteen experimental trials as listed in Table 3.
| Formulation code | RHLB | Injection rate | Organic/Aqueous Phase | Entrapment efficiency (%) | Particle Size (nm) |
|---|---|---|---|---|---|
| N1 | 0 | 1 | 1 | 65±0.32 | 110±0.99 |
| N2 | 1 | 0 | 1 | 58±0.12 | 136.4±2.43 |
| N3 | 0 | 0 | 0 | 67±0.56 | 143±4.03 |
| N4 | -1 | -1 | 0 | 74.56±0.43 | 164.82.23 |
| N5 | 1 | 1 | 0 | 68.93±0.78 | 89.2±2.76 |
| N6 | 0 | -1 | -1 | 75±0.89 | 180.2±7.83 |
| N7 | 0 | 1 | -1 | 78±0.24 | 160.2±6.33 |
| N8 | -1 | 0 | -1 | 82.60±0.98 | 245.8±5.62 |
| N9 | 0 | 0 | 0 | 62±1.25 | 158.5±10.25 |
| N10 | 1 | 0 | -1 | 65±3.53 | 261.5±8.36 |
| N11 | -1 | 1 | 0 | 75±2.93 | 338±12.03 |
| N12 | 0 | -1 | 1 | 60±4.32 | 313±9.82 |
| N13 | 0 | 0 | 0 | 51.91±1.12 | 123±11.23 |
| N14 | -1 | 0 | 1 | 74±1.19 | 494±5.03 |
| N15 | 1 | -1 | 0 | 64±1.96 | 310±13.32 |
Note: All the values are mean±SD (n=3)
Table 3: Evaluation of Niosomal Formulation of Lrtd by Box Behnken Design
Model validation was carried out by tailoring the data at a significance level of p<0.05. The model was evaluated for the significant effect of the factors on the responses. The model validation statistics are listed in Table 4.
| Responses | Model | Statistical significance | p-value | Model Equation |
|---|---|---|---|---|
| Entrapment efficiency | Linear | Model | 0.0191* | Entrapment Efficiency=68.0275+-6.20348*A+1.67152* B+-5.375*C |
| A-RHLB | 0.0148* | |||
| B-Rate of injection | 0.453 | |||
| C-org/aq phase | 0.0294* | |||
| Particle Size | Quadratic | Model | 0.0193* | Particle size=141.5+-55.6875*A+-33.825*B+25.7125*C+-98.5*AB+-93.325*AC+-45.75*BC+88.7875*A22+-4.7875*B22+54.1375*C22 |
| A-RHLB | 0.0228* | |||
| B-Rate of injection | 0.0105* | |||
| C-org/aq phase | 0.0194* |
Table 4: Statistical Model Validation for the Responses
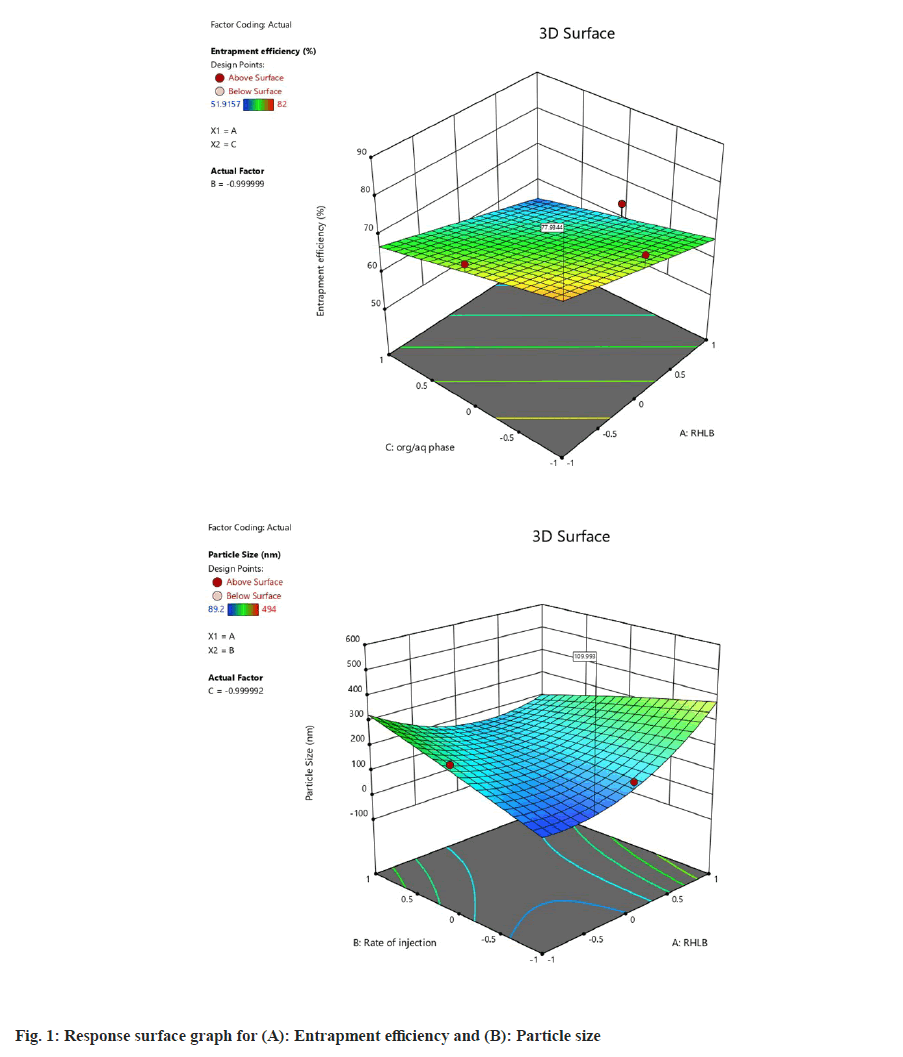
The model validation statistics revealed that entrapment efficiency was significantly affected by RHLB, and phase volume ratio, where the response and the variables yielded a linear relation, as evidenced from the response surface diagram shown in fig. 1. All the factors were found significant for particle size as revealed from the statistics in Table 5. The curvature in the response surface fig. 1B shows a quadratic relation between particle size and the factors.
| Responses | Predicted | Experimented | % Bias |
|---|---|---|---|
| Entrapment Efficiency (EE) (%) | 77.93 | 70.67±1.15 | 9.74 |
| Particle size (PS) (nm) | 109.99 | 101.33±8.65 | 7.89 |
Note: All the values are mean±SD (n=3)
Table 5: Evaluation of Responses for Optimized Niosome Formulation
The model optimization was carried out to maximize entrapment of drug and to obtain the vesicle size <150 nm for effective dermal penetration. The optimization yielded desirability of 0.844 and yielded a coded ratio of RHLB:Rate of injection:Org/aq phase at (-0.449):(-1):(-1) with a prediction of entrapment efficiency (77.93 %) and particle size (109.99 nm) as listed in Table 6. The optimized formulation was prepared at an RHLB of 6.2, rate of injection at 0.5 ml/min, and an organic/aqueous phase 0.25.
| Gel composition | Appearance | pH | Spreadability (g cm/sec ) | Viscosity (cps) | % Drug content |
|---|---|---|---|---|---|
| GEL I | Clear, translucent | 5.5±0.10 | 1.66±0.15 | 4148.5±0.04 | 89.23±0.05 |
| GEL II | Clear, translucent | 5.5±0.0 | 2.25±1.03 | 3633±0.13 | 83.9±0.02 |
Note: All the values are mean±SD (n=3)
Table 6: Evaluation of Lrtd Loaded Niosomal Gel
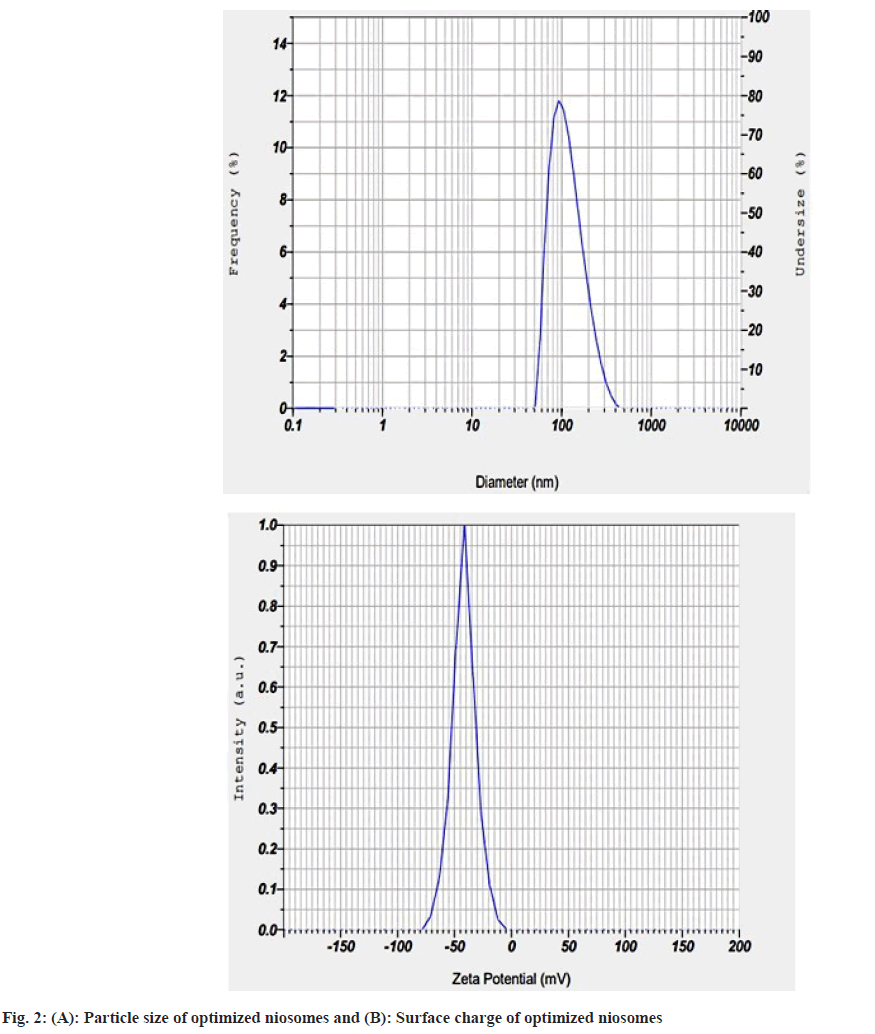
The experimentation yielded a stable nanosized formulation (101.33 nm) as shown in fig. 2A and high drug entrapment efficiency (70.67 %). The percentage bias between the prediction and experimentation of the optimized formulation was calculated and listed in Table 5.
The percentage bias for both the responses of the optimized formulation was found to be less than 10 %. This indicates the successful implementation of Box Behnken's design to fabricate nano-sized LRTD loaded niosomes. The surface charge of the optimized formulation was found to be -32 mV as shown in fig. 2B. This stipulates the formation of a stable niosomal dispersion.
The freeze-dried optimized formulation was subjected to particle size analysis and estimation of drug entrapment. The particle size and entrapment efficiency of the freeze-dried optimized formulation were found to be 125±16.36 nm and 68±1.09 % respectively. A slight increase in the particle size of the freeze-dried product was observed and that might be due to the formation of agglomerates. However, the ratio of particle size of pre and post lyophilization of the product was 0.8 (<1). This is an indication of the conservation of particle size of the formulation post lyophilization. Leakage of the drug during the lyophilization process might have resulted in the lower entrapment of the drug. Hence it can be concluded that the freeze-drying condition was effective for the preservation of the wholeness of niosomal LRTD. The drug content of the optimized freeze-dried product was found to be 76±0.55 %.
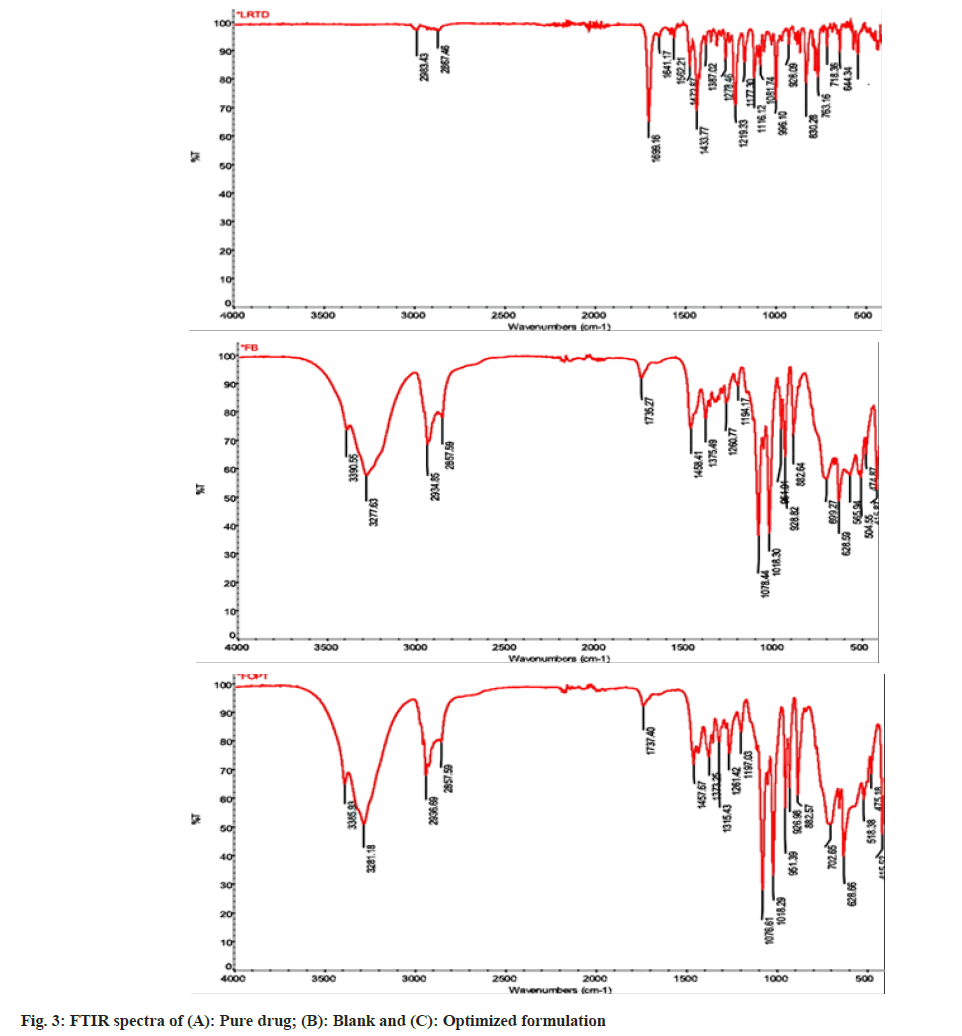
The FTIR spectra for LRTD (drug), blank, and the optimized freeze-dried formulation are shown in fig. 3. The peaks of functional groups of the optimized formulation were detected at the wavelength as follows. The C-H stretch at 2936.69 cm-1, carboxyl O-H stretch at 2857.59 cm-1, C=O stretch at 1737.4 cm-1, aromatic C=C stretch at 1457.67 cm-1, tertiary aromatic amine C-N=C stretch at 1315.43 cm-1, ether C-O stretch at 1197.03 cm-1, aryl chloride stretches at 1018.29 cm-1, C-H bending at 702.65 cm-1 and stretching vibrations of benzene ring at 1457.67 cm-1 of the drugs were preserved in the optimized formulation. The optimized formulation could retain the major peaks of the blank also. The spectra disseminated the overall compatibility of the drug and excipients.
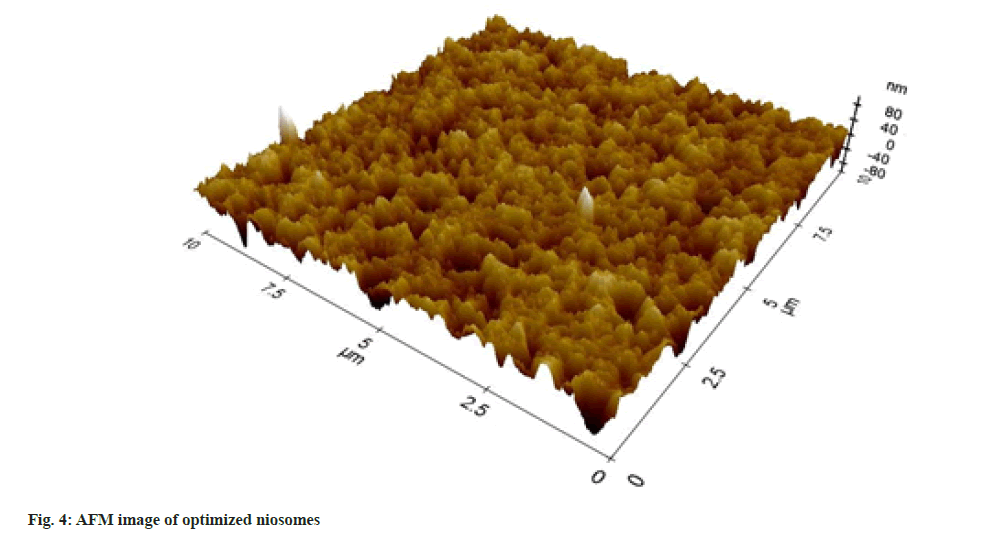
The surface morphology study by AFM revealed the formation of nano-sized vesicles as presented in fig. 4. The 3D image in the nanometric scale further proved the capability of the design prediction to form nanosized niosomal vesicles.
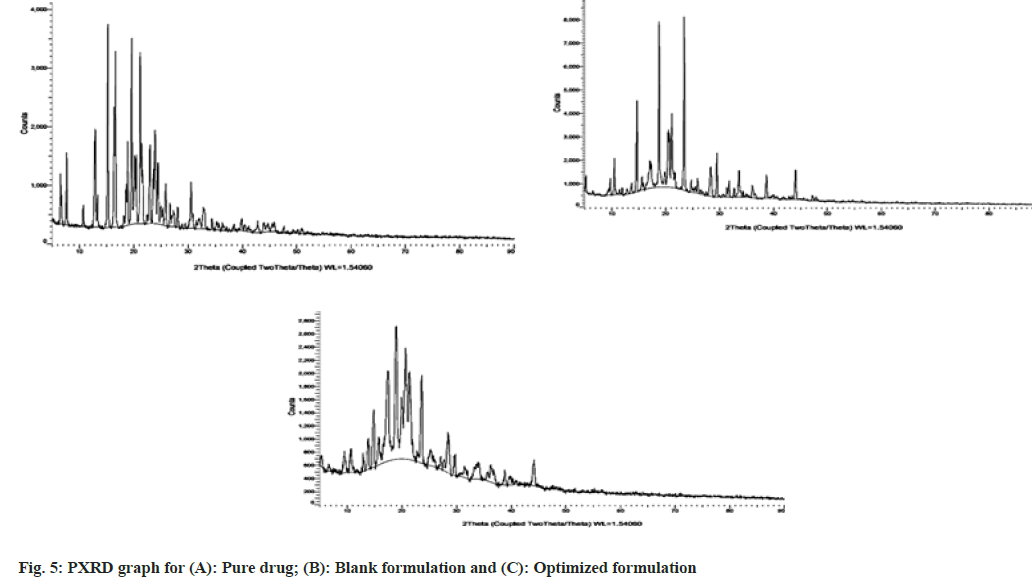
Comparing the PXRD results from fig. 5, the pure drug exhibited high-intensity peaks at 2 Theta 6.56, 7.65, 12.92, 15.23, 16.63, 19.03, 21.19 and 23.62. These peaks represented the crystallinity of the drug. The intensity of these peaks was reduced in the optimized formulation and depicting the entrapment of the amorphous drug in the niosomal core. The characteristic peaks of the blank optimized formulations were retained in the optimized formulation further confirming the compatibility of the drug and excipients as obtained from the FTIR studies.
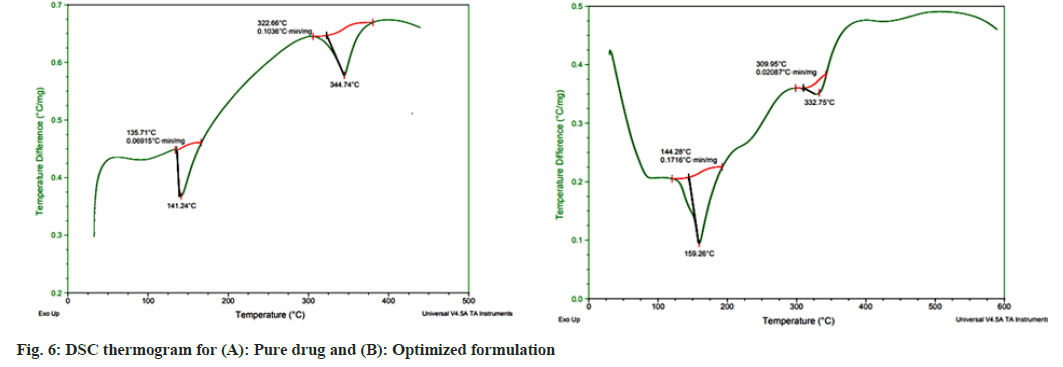
The thermograms of the pure drug showed a sharp endothermic peak at 141° at its melting point as seen in fig. 6, the drug peak was absent in the thermograms of the optimized peak, indicating the amorphous dispersion of the drug in the niosomes, hence confirmed the high confinement of drug in niosomal core.
Both the gels containing optimized LRTD niosomes were appeared white, translucent, and devoid of gritty feelings. The surface pH was found to be adequate for dermal application. The viscosity and spreadability were found to be satisfactory. The value revealed that the gel could be applied easily and would not run out of the skin after application. The drug content of the formulation was found to be adequate as listed in Table 6.
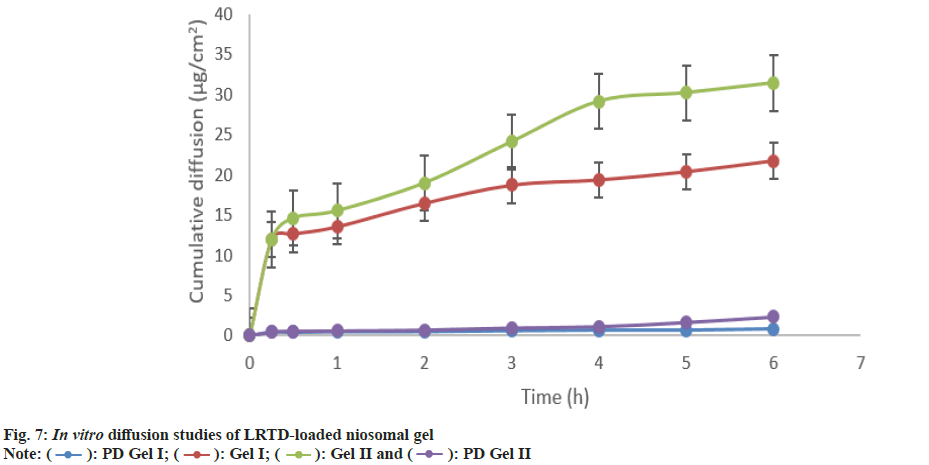
The in vitro diffusion study of the drug from both the nanogels revealed the adequate release of drugs from the gels. The Xanthum gel (Gel II) showed the highest drug diffusion compared to Gel I. This might be related to the swelling efficacy and viscosity of the gelling agents. The diffusion study also confirms the viscosity data obtained for each gel. The in vitro diffusion release of pure drug gels (PD gel I and PD gel II) was much lower compared to the gels loaded with niosomes. The diffusion of the drug from the nanogels is presented in fig. 7.
The ex vivo permeation study of the formulations confirmed the in vitro diffusion study and showed a good permeation of the drug through the animal skin. Xanthum gum gel (Gel II) showed better permeation among the other gel bases, as shown in fig. 8. The permeation parameters were calculated for the ex vivo permeation study and listed in Table 7. The drug flux and permeation constant of the xanthum gel base were found to be significantly higher than the other gel bases. Hence among the three bases, the xanthum gum was found to be the best one in terms of permeation and drug diffusion and was selected for skin irritation test.
| Permeation parameters | GEL II | GEL I | PD GEL I | PD GEL II |
|---|---|---|---|---|
| Drug flux (Jss) | 1.7 | 0.7 | 0.084 | 0.106 |
| Permeation constant (Kp) | 9.6 | 6.2 | 0.4 | 0.8 |
Table 7: Permeation Parameters
The skin irritation study was performed with Gel II and found that there was no irritation on the application of the gel to the animal skin. Hence it can be concluded that the LRTD loaded niosomal gel of xanthum gum was found to be safe on administration.
In conclusion, the research was focused on the formulation and characterization of LRTD encapsulated niosomal gel for topical application for the treatment of skin allergy. The objective of the research was to overcome the low oral bioavailability and side effects associated with conventional delivery of LRTD. The study was experimented to formulate a suitable niosomal gel of LRTD, which can increase the permeability of the drug through the skin on application. A statistical design was integrated into the development of niosomes of LRTD to achieve high drug entrapment and a nanosized vesicle suitable for skin penetration. The optimized formulation thus obtained from the model validation of Box Behnken design was incorporated into three types of gels. Among them, the niosomal gel of xanthum gum was found to be the best for its mechanical and rheological properties, and in vitro and ex vivo testing of the drug. Hence it can be concluded that the niosomal gel of LRTD can be a promising alternative approach to control the allergic conditions of the skin and can overcome the difficulties associated with conventional delivery of the drug.
Acknowledgements:
The authors are grateful to the management and principal of Krupanidhi College of Pharmacy, Bengaluru for providing the resource to complete the proposed work. We thank hereby Stride Arco Bangalore, for their generous support for the gift sample of the drug. We acknowledge MNCF, IISC for their support to conduct AFM study.
Conflict of interests:
Authors declared there is no conflict of interest.
References
- Identifying bug bites and stings, and how to treat them. Health line; 2023.
- Loratadine. AHFS® Patient Medication InformationTM; 2023.
- Elkomy MH, El Menshawe SF, Abou-Taleb HA, Elkarmalawy MH. Loratadine bioavailability via buccal transferosomal gel: Formulation, statistical optimization, in vitro-in vivo characterization, and pharmacokinetics in human volunteers. Drug Deliv 2017;24(1):781-91.
[Crossref] [Google Scholar] [PubMed]
- Loratadine (Claritin): Side effects, interactions, uses, dosage, warnings. Everyday health; 2020.
- PubChem compound Summary for CID 3957, Loratadine: C22H23ClN2O2. National Center for Biotechnology Information; 2023.
- Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: Novel sustained release nonionic stable vesicular systems—an overview. Adv Colloid Interface Sci 2012;183:46-54.
[Crossref] [Google Scholar] [PubMed]
- Tran YT, Tran GN, Hoang AL, Vu GT. Niosomes loaded with diclofenac for transdermal administration: Physico-chemical characterization, ex vivo and in vivo skin permeation studies. J Appl Pharm Sci 2020;10(12):53-61.
- Singh P, Ansari H, Dabre S. Niosomes-a novel tool for anti-ageing cosmeceuticals. J Pharm Res 2016;6(10):6691-703.
- Chandu VP, Arunachalam A, Jeganath S, Yamini K, Tharangini K, Chaitanya G. Niosomes: A novel drug delivery system. Int J Novel Trends Pharm Sci 2012;2(1):25-31.
- Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J Control Rel 2014;185(1):22-36.
[Crossref] [Google Scholar] [PubMed]
- Khan R, Irchhaiya R. Niosomes: A potential tool for novel drug delivery. J Pharm Investig 2016;46:195-204.
- Mahale NB, Thakkar PD, Mali RG, Walunj DR, Chaudhari SR. Niosomes: Novel sustained release nonionic stable vesicular systems-an overview. Adv Colloid Interface Sci 2012;183:46-54.
[Crossref] [Google Scholar] [PubMed]
- Ferreira SC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GC, et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal Chim Acta 2007;597(2):179-86.
[Crossref] [Google Scholar] [PubMed]
- Sailaja AK, Shreya M. Preparation and characterization of naproxen loaded niosomes by ether injection method. Nano Biomed Eng 2018;10(2):174-80.
- Mishra N, Srivastava V, Kaushik A, Chauhan V, Srivastava G. Formulation and in vitro evaluation of niosomes of Aceclofenac. JSIR 2014;3(3):337-41.
- Bhattacharyya S, Sogali BS. Application of statistical design to assess the critical process parameters of ethanol injection method for the preparation of liposomes. Dhaka Univ J Pharm Sci 2019;18(1):103-11.
- Patel D, Dasgupta S, Dey S, Roja Ramani Y, Ray S, Mazumder B. Nanostructured lipid carriers (NLC)-based gel for the topical delivery of aceclofenac: Preparation, characterization, and in vivo evaluation. Sci Pharm 2012 Sep;80(3):749-64.
[Crossref] [Google Scholar] [PubMed]
- Shilakari Asthana G, Sharma PK, Asthana A. In vitro and in vivo evaluation of niosomal formulation for controlled delivery of clarithromycin. Scientifica 2016;2016:6492953.
[Crossref] [Google Scholar] [PubMed]
- Niu L, Panyam J. Freeze concentration-induced PLGA and polystyrene nanoparticle aggregation: Imaging and rational design of lyoprotection. J Control Rel 2017;248:125-32.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee B, Patra B, Layek B, Mukherjee A. Sustained release of acyclovir from nano-liposomes and nano-niosomes: An in vitro study. Int J Nanomed 2007;2(2):213-25.
[Google Scholar] [PubMed]
- Pilch E, Musiał W. Selected physicochemical properties of lyophilized hydrogel with liposomal fraction of calcium dobesilate. Materials 2018;11(11):2143.
[Crossref] [Google Scholar] [PubMed]
- Hv RR, Bhattacharyya S. In vitro evaluation of mucoadhesive in situ nanogel of celecoxib for buccal delivery. Ann Pharm Fr 2021;79(4):418-30.
[Crossref] [Google Scholar] [PubMed]
- Akbari J, Saeedi M, Enayatifard R, Morteza-Semnani K, Hashemi SM, Babaei A, et al. Curcumin Niosomes (curcusomes) as an alternative to conventional vehicles: A potential for efficient dermal delivery. J Drug Deliv Sci Technol 2020;60:102035.
- Akhtar N, Arkvanshi S, Bhattacharya SS, Verma A, Pathak K. Preparation and evaluation of a buflomedil hydrochloride niosomal patch for transdermal delivery. J Liposome Res 2015;25(3):191-201.
[Crossref] [Google Scholar] [PubMed]
- Ning M, Guo Y, Pan H, Chen X, Gu Z. Preparation, in vitro and in vivo evaluation of liposomal/niosomal gel delivery systems for clotrimazole. Drug Dev Indu Pharm 2005;31(4-5):375-83.
[Crossref] [Google Scholar] [PubMed]
- Shinde UA, Kanojiya SS. Serratiopeptidase niosomal gel with potential in topical delivery. J Pharm 2014;2014:382959.
[Crossref] [Google Scholar] [PubMed]
- Vyas J, Vyas P, Raval D, Paghdar P. Development of topical niosomal gel of benzoyl peroxide. Int Scholar Res Notice 2011;2011:1-6.
- Budhiraja A, Dhingra G. Development and characterization of a novel antiacne niosomal gel of rosmarinic acid. Drug Deliv 2015;22(6):723-30.
[Crossref] [Google Scholar] [PubMed]
- Uprit S, Sahu RK, Roy A, Pare A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm J 2013;21(4):379-85.
[Crossref] [Google Scholar] [PubMed]
- Aiyalu R, Govindarjan A, Ramasamy A. Formulation and evaluation of topical herbal gel for the treatment of arthritis in animal model. Brazil J Pharm Sci 2016;52:493-507.
- Goyal G, Garg T, Malik B, Chauhan G, Rath G, Goyal AK. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv 2015;22(8):1027-42.
[Crossref] [Google Scholar] [PubMed]
- Qumbar M, Imam SS, Ali J, Ahmad J, Ali A. Formulation and optimization of lacidipine loaded niosomal gel for transdermal delivery: In vitro characterization and in vivo activity. Biomed Pharmacother 2017;93:255-66.
[Crossref] [Google Scholar] [PubMed]
- Kumar L, Verma R. In vitro evaluation of topical gel prepared using natural polymer. Int J Drug Deliv 2010;2(1):58-63.
- Pillai O, Panchagnula R. Transdermal delivery of insulin from poloxamer gel: Ex vivo and in vivo skin permeation studies in rat using iontophoresis and chemical enhancers. J Control Release 2003;89(1):127-40.
[Crossref] [Google Scholar] [PubMed]
- Abu Hashim II, Abo El-Magd NF, El-Sheakh AR, Hamed MF, Abd El-Gawad AE. Pivotal role of Acitretin nanovesicular gel for effective treatment of psoriasis: Ex vivo–in vivo evaluation study. Int J Nanomed 2018;13:1059-79.
[Crossref] [Google Scholar] [PubMed]
- Hussain A, Samad A, Singh SK, Ahsan MN, Haque MW, Faruk A, et al. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv 2016;23(2):642-57.
[Crossref] [Google Scholar] [PubMed]
- Patel KK, Kumar P, Thakkar HP. Formulation of niosomal gel for enhanced transdermal lopinavir delivery and its comparative evaluation with ethosomal gel. AAPS PharmSciTech 2012;13(4):1502-10.
[Crossref] [Google Scholar] [PubMed]
- Soliman SM, Malak NSA, Gazayerly O El, Rahim AAA. Delivery of celecoxib : In vitro permeation, anti-inflammatory. Drug Discov Ther 2010;4(6):459–71.
- Bhattacharyya SA. A Niosomal gel of cefoperazone sodium for topical application. ACTA Pharm Sci 2021;59(3):2.
- Nahar S, Islam S, Khandaker SI, Nasreen W, Hoque O, Dewan I. Formulation and evaluation of metoprolol tartrate loaded niosomes using 23 factorial design. J Pharm Res Int 2018;22(6):1-7.
 ): PD Gel I; (
): PD Gel I; ( ): Gel I; (
): Gel I; ( ): Gel II and (
): Gel II and ( ): PD Gel II
): PD Gel II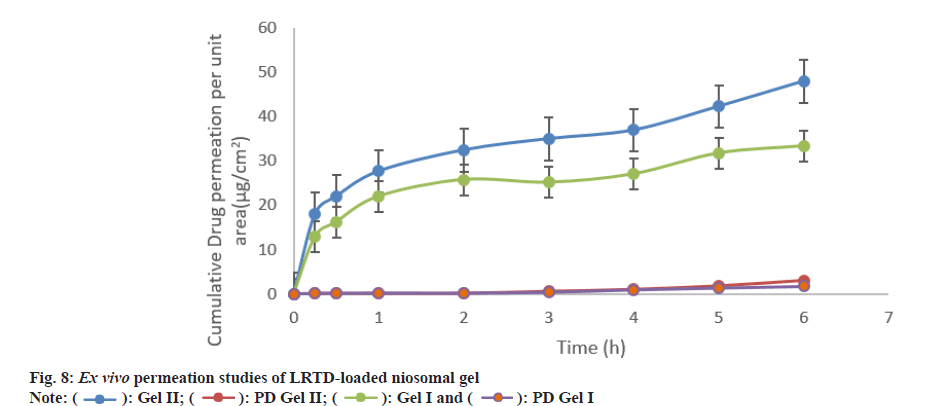
 ): PD Gel I
): PD Gel I



