- Corresponding Author:
- K. J. Wadher
Department of Pharmaceutical Technology, Smt. Kishoritai Bhoyar College of Pharmacy, Kamptee, Nagpur-441 002
E-mailkamleshwadher@gmail.com.
| Date of Submission | 16 September 2010 |
| Date of Revision | 27 April 2011 |
| Date of Acceptance | 29 April 2011 |
| Indian J Pharm Sci, 2011, 73 (2): 208-215 |
Abstract
Metformin hydrochloride has relatively short plasma half-life, low absolute bioavailability. The need for the administration two to three times a day when larger doses are required can decrease patient compliance. Sustained release formulation that would maintain plasma level for 8-12 h might be sufficient for daily dosing of metformin. Sustained release products are needed for metformin to prolong its duration of action and to improve patient compliances. The overall objective of this study was to develop an oral sustained release metformin hydrochloride tablet by using hydrophilic Eudragit RSPO alone or its combination with hydrophobic natural polymers Gum copal and gum damar as rate controlling factor. The tablets were prepared by wet granulation method. The in vitro dissolution study was carried out using USP 22 apparatus I, paddle method and the data was analysed using zero order, first order, Higuchi, Korsmeyer and Hixson-Crowell equations. The drug release study revealed that Eudragit RSPO alone was unable to sustain the drug release. Combining Eudragit with gum Copal and gum Damar sustained the drug release for more than 12 h. Kinetic modeling of in vitro dissolution profiles revealed the drug release mechanism ranges from diffusion controlled or Fickian transport to anomalous type or non-Fickian transport. Fitting the in vitro drug release data to Korsmeyer equation indicated that diffusion along with erosion could be the mechanism of drug release.
Keywords
Eudragit RSPO, gum copal, gum damar, matrix tablets, release kinetics
Metformin hydrochloride is an orally administered biguanide, which is widely used in the management of type-II diabetes, a common disease that combines defects of both insulin secretion and insulin action [1]. Unlike other antidiabetic drugs metformin HCl does not induce hypoglycemia at any reasonable dose, and hence it is called as an antihyperglycaemic rather than a hypoglycemic drug [2]. It is a hydrophilic drug and is slowly and incompletely absorbed from the gastrointestinal tract, and the absolute bioavailability is reported to be of 50-60 % [3,4]. An obstacle to more successful use of metformin therapy is the high incidence of concomitant gastrointestinal symptoms, such as abdominal discomfort, nausea, and diarrhea that especially occurs during the initial period of treatment. The compound has relatively short plasma half-life of 1.5-4.5 h and the low absolute bioavailability of 50-60 % [5]. Side effects, short half lives, low bioavailability and the need for the administration two to three times a day when larger doses are required can decrease patient compliance. Sustained release products are needed for metformin to prolong its duration of action and to improve patient compliances. Matrix systems are widely used in oral controlled drug delivery because of their flexibility, cost effectiveness, low influence of the physiological variables on its release behavior and broad regulatory acceptance [6,7]. Many researchers investigated various natural, semi-synthetic and synthetic polymeric materials. Cellulose ethers such as hydroxypropylmethylcellulose, sodium carboxymethylcellulose, Eudragit (polymethacrylate) polymer [8,9], ethyl cellulose [10] and some natural gums like guar gum and xanthan gum are widely used hydrophilic polymers as release retardants [11].
Methacrylic resins (Eudragit) appear particularly attractive due to their high chemical stability and compactility properties, and many literatures substantiate use in the development of control release matrix tablet [12,13]. The hydrophilic polymer selected for the present study was Eudragit RSPO, which provide pH-independent drug release to oral dosage forms that can be used for formulating the sustained-release dosage forms [14]. However, the use of hydrophilic matrix alone for extending drug release for highly water soluble drugs is restricted due to rapid diffusion of the dissolved drug through the hydrophilic gel network. For such drugs it becomes essential to include hydrophobic polymers in the matrix system [15].
The natural materials have been extensively used in the field of drug delivery because they are readily available, cost-effective, eco-friendly, capable of multitude of chemical modifications, potentially degradable and compatible due to their natural origin [16]. Gum copal (GC) and gum damar (GD) are natural resinous materials of plant Bursera bipinnata family Burseraceae and Shorea wiesneri family Dipterocarpaceae, respectively. The wide applications of GC and GD propose their strong hydrophobic nature, substantial binding property, compatibility with the physiologic environment [17] and their sustaining property [18]. The objective of this work was to prepare sustained release metformin HCl matrix tablets using synthetic hydrophilic polymer eudragit RSPO alone or in combination with hydrophobic natural polymer, GC and GD to evaluate the in vitro release characteristic and to predict and correlate the release behavior of metformin HCl from the matrices. The influence of the polymer concentration in the tablets was also investigated. The in vitro drug release profiles of the matrices are evaluated, and its release mechanism was studied.
Although Eudragit RSPO has been widely used as sustained release material; to our knowledge the property of its combination with GC and Gd has not been evaluated. Hence, in the present work, an attempt has been made to formulate the extendedrelease matrix tablets of metformin HCl using hydrophilic polymer Eudragit RSPO alone or in combination with hydrophobic natural polymer, GC and GD to evaluate the in vitro release characteristics and to predict the release behavior.
Materials and Methods
Metformin HCl was obtained from Universal Medicament Nagpur, India. Microcrystalline cellulose (MCC, Avicel pH 101) and ethyl cellulose were purchased from S. D. Fine Chem. Labs. (Mumbai, India), Eudragit RSPO (ammonium meth acrylic copolymer type A NF) was obtained as gift samples from Degussa India Ltd. (Mumbai, India), and gum copal and gum damar were received as a gift sample from Imex Inc. (Chennai, India). All other ingredients used throughout the study were of analytical grade and were used as received.
Study of physical interaction between drug and polymer
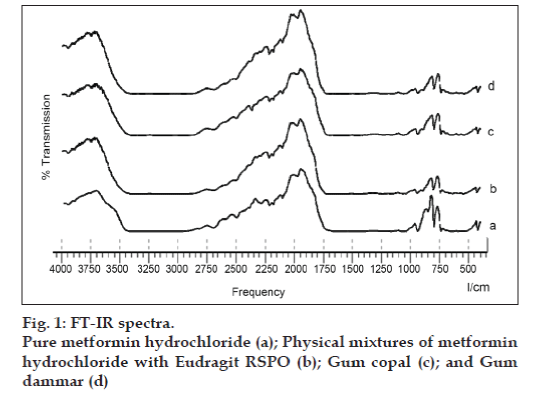
Infrared spectrum was taken by scanning the samples of pure drug and the polymers individually over a wave number range of 4000 to 400 cm–1 using Fourier transform infrared spectrophotometer (FT-IR, Shimadzu 8400S, Shimadzu, Japan). The change in spectra of the drug in the presence of polymer was investigated which indicates the physical interaction of drug molecule with the polymer.
Preparation of Metformin hydrochloride matrix tablets
Matrix tablets, each containing 500 mg metformin HCl were prepared by a conventional non-aqueous wet granulation technique. The composition of various formulations of the tablets with their codes is listed in Table 1. The composition with respect to polymer combination was selected on the basis of trial preparation of tablets. In each formulation, the amount of the active ingredient is 500 mg and the total weight of a tablet is 1000 mg. A batch of 30 tablets was prepared with each formula. The ingredients were passed through a 60-mesh sieve. A blend of all ingredients except glidant and lubricant was mixed, a particular attention had been given to ensure thorough mixing and phase homogenization. Granulation was done manually with a solution of isopropyl alcohol. The wet masses were passed through a 12 mesh sieve and the wet granules produced were first air dried for 10 min and finally at 45-50º in a tray drier for 2 h. The dried granules were sized by a 16-mesh sieve and after lubrication with magnesium stearate. Compression was carried out using 14 mm flat faced circular punches into tablets on an eight station rotary press tablet compression machine (Rimek Minipress I Ahmadabad, India) at a constant compression force. Just before compression, the surfaces of the die and punches were lubricated with magnesium stearate. All the tablets were stored in airtight containers for further study. Prior to compression, granules were evaluated for their flow and compressibility characteristics.
| Formulation | Ingredients | ||||||
|---|---|---|---|---|---|---|---|
| code | Metformin HCl | Eudragit RSPO | Gum copal | Gum damar | MCC | Mg stearate | Total |
| FI | 500 | 200 | - | - | 290 | 10 | 1000 |
| FII | 500 | 300 | - | - | 190 | 10 | 1000 |
| FIII | 500 | 400 | - | - | 90 | 10 | 1000 |
| FIV | 500 | - | 200 | - | 290 | 10 | 1000 |
| FV | 500 | - | 300 | - | 190 | 10 | 1000 |
| FVI | 500 | - | 400 | - | 90 | 10 | 1000 |
| FVII | 500 | - | - | 200 | 290 | 10 | 1000 |
| VIII | 500 | - | - | 300 | 190 | 10 | 1000 |
| FIX | 500 | - | - | 400 | 90 | 10 | 1000 |
| FX | 500 | 200 | 100 | - | 190 | 10 | 1000 |
| FXI | 500 | 150 | 150 | - | 190 | 10 | 1000 |
| FXII | 500 | 100 | 200 | - | 190 | 10 | 1000 |
| FXIII | 500 | 200 | - | 100 | 190 | 10 | 1000 |
| FXIV | 500 | 150 | - | 150 | 190 | 10 | 1000 |
| FXV | 500 | 100 | - | 200 | 190 | 10 | 1000 |
Table 1 Composition of various trial formulations prepared.
Evaluation of granules
The granules were evaluated for angle of repose, loose bulk density (LBD), tapped bulk density (TBD), compressibility index and drug content. Angle of repose was determined by funnel method. Bulk density and tapped density were determined by cylinder method, and Carr’s index (CI) was calculated using the following equation. Carr’s index=(TBD-LBD)×100/TBD. Hausner's ratio was related to interparticle friction and could be used to predict powder flow properties. Hausner's values of the prepared granules ranged from 1.12 to 1.25 was thought to indicate good flow properties [19].
Evaluation of tablets
The prepared matrix tablets were evaluated for hardness, weight variation, thickness, friability and drug content [19]. Hardness of the tablets was tested using a Strong-Cobb hardness tester (Tab-machine, Mumbai, India). Friability of the tablets was determined in a Roche friabilator (Campbell Electronics, Mumbai, India). The thickness of the tablets was measured by vernier caliper. Weight variation test was performed according to the official method [20]. Drug content was analyzed by measuring the absorbance of standard and samples at λ=233 nm using UV/Vis spectrophotometer (Shimadzu 1601, Kyoto, Japan).
in vitro drug release studies
Drug release studies were conducted using USP-22 dissolution apparatus-2, paddle type (Electrolab, Mumbai, India) at a rotational speed of 50 rpm at 37±0.5º. The dissolution media used were 900 ml of 0.1 mol/l HCl for first 2 h followed by pH 6.8 phosphate buffer solution for 12 h. Sink condition was maintained for the whole experiment. Samples (10 ml) were withdrawn at regular intervals and the same volume of pre-warmed (37±0.5º) fresh dissolution medium was replaced to maintain the volume constant. The samples withdrawn were filtered through a 0.45 μ membrane filter (Nunc, New Delhi, India) and the drug content in each sample was analyzed after suitable dilution with a UV spectrophotometer (Shimadzu UV-1700) at 233 nm [21]. The dissolution test was performed in triplicate. Drug dissolved at specified time periods was plotted as cumulative percent release versus time (h) curve.
Kinetic Analysis of release data
The release data obtained were treated according to zero-order (R=k1t), first-order (R=k1t), Higuchi (R=k3√t) [22], Korsmeyer-Peppas (log R=log k4+n log t) equation, Hixson–Crowell equations ((UR) 1/3= k5 t) [23] to find the equation with the best fit. Where R and UR are the released and unreleased percentages, respectively, at time (t); k1, k2, k3, k4, and k5 are the rate constants of zero-order, first-order, Higuchi matrix, Peppas-Korsmeyer, and Hixon-Crowell model, respectively. In order to compare the release profile of different formulas with possible difference in release mechanisms (n values), a mean dissolution time (MDT) [14] was calculated using Eq. MDT=(n/ n+1).K-1/n, Where n = release exponent and K= release rate constant.
Statistical Analysis
The data was subjected to two ways ANOVA followed by Bonferroni post test for analyzing the statistical difference using the software GraphPad Prism (San Diego, CA) and in all the cases P < 0.001 was considered as significant.
Results and Discussion
FTIR studies revealed that metformin HCl showed two typical bands at 3369 and 3283 cm–1 due to N-H primary stretching vibration and a band at 3170 cm–1 due to N-H secondary stretching and characteristics bands at 1623 and 1560 cm–1 assigned to C=N stretching. No significant change in the appearance of characteristic peaks of pure drug spectra was observed (fig. 1). This indicates that the drug is compatible with the polymers used in the investigation.
The granules of proposed formulations were evaluated for LBD, TBD, Compressibility index, angle of repose and Hausner’s ratio (Table 2). An angle of repose of less than 30 degrees indicates good flow properties. This was further supported by the lower compressibility index. Granules with Carr’s index values around 21% and below are considered to have fair and excellent flow properties [19].
Table 3 gives the physical parameters such as hardness, thickness, friability and weight uniformity of all the fabricated tablets. All the tablets of different formulations showed acceptable results with respect to weight variation, drug content uniformity, friability. All formulations showed less than 1% (w/w) friability, which was within the prescribed limits [24]. According to the Pharmacopoeial recommendation for tablets weighing more than 324 mg, ±5% deviation from the mean weight is acceptable [20]. As the results show, the average weight deviation percentage of 20 tablets taken from each formulation was less than ±0.5%, and all the formulations met the requirement. The manufactured tablets showed low weight variations and a high degree of drug content uniformity among different batches of the tablets, and drug content was more than 95%.
| Formulation | LBD | TBD | Angle of repose | Carr’s index | Hausner’s ratio |
|---|---|---|---|---|---|
| FI | 0.232 | 0.273 | 26.31±0.57 | 15.52 | 1.18 |
| FII | 0.377 | 0.453 | 26.82±0.23 | 13.2 | 1.15 |
| FIII | 0.307 | 0.35 | 26.69±0.59 | 12.3 | 1.14 |
| FIV | 0.33 | 0.38 | 25.31±0.79 | 13.15 | 1.15 |
| FV | 0.312 | 0.358 | 26.21±0.32 | 12.84 | 1.15 |
| FVI | 0.318 | 0.368 | 26.10±0.53 | 13.58 | 1.16 |
| FVII | 0.393 | 0.453 | 25.81±0.61 | 13.24 | 1.15 |
| FVIII | 0.323 | 0.376 | 26.28±0.33 | 14.09 | 1.16 |
| FIX | 0.346 | 0.404 | 26.021±0.93 | 14.35 | 1.16 |
| FX | 0.267 | 0.307 | 26.02±0.61 | 13.33 | 1.15 |
| FXI | 0.384 | 0.434 | 25.94±0.56 | 17.85 | 1.22 |
| FXII | 0.307 | 0.444 | 26.05±0.65 | 13.46 | 1.16 |
| FXIII | 0.307 | 0.357 | 25.94±0.56 | 13.84 | 1.15 |
| FXIV | 0.266 | 0.312 | 26.32±0.87 | 14.66 | 1.16 |
| FX | 0.384 | 0.441 | 25.98±0.40 | 13.46 | 1.15 |
Table 2Physical properties of the granules
| Formulation | Hardness† | Friability† (%) | Weight | Drug content*(%) | Thickness† (mm) |
|---|---|---|---|---|---|
| code | (kg/cm2) | variation* | |||
| FI | 8.25±0.52 | 0.292±0.145 | 1002.28±9.13 | 98.13 | 4.45±0.07 |
| FII | 8.58±0.38 | 0.298±0.243 | 1001.58±5.13 | 99.34 | 4.44±0.03 |
| FIII | 8.37±0.25 | 0.298±0.123 | 1002.24±9.46 | 92.73 | 4.42±0.07 |
| FIV | 7.89±0.53 | 0.195±0.101 | 998.23±11.13 | 99.19 | 4.99±0.08 |
| FV | 7.91±0.71 | 0.289±0.117 | 1003.28±5.13 | 99.34 | 4.96±0.07 |
| FVI | 7.76±0.56 | 0.220±0.098 | 1001.28±6.13 | 96.34 | 4.98±0.02 |
| FVII | 8.01±0.90 | 0.301±0.003 | 1001.38±9.13 | 98.34 | 5.08±0.02 |
| FVIII | 8.15±0.68 | 0.276±0.125 | 1003.08±3.13 | 99.74 | 5.22±0.02 |
| FIX | 7.82±0.51 | 0.213±0.118 | 999.28±9.13 | 95.44 | 5.11±0.04 |
| FX | 8.17±0.52 | 0.198±0.008 | 1002.88±2.34 | 94.64 | 4.47±0.06 |
| FXI | 8.25±0.69 | 0.218±0.101 | 1001.48±5.65 | 93.56 | 4.46±0.02 |
| FXII | 8.50±0.32 | 0.248±0.071 | 1004.03+3.13 | 95.39 | 4.42±0.07 |
| FXIII | 8.08±0.38 | 0.236±0.016 | 1001.28+5.53 | 99.17 | 4.45±0.05 |
| FXIV | 8.28±0.52 | 0.217±0.120 | 1002.28+10.33 | 99.29 | 4.45±0.04 |
| FX | 8.34±0.75 | 0.204±0.080 | 1001.48+11.53 | 95.37 | 4.49±0.03 |
Table 3 Physical properties of the matrix tablets
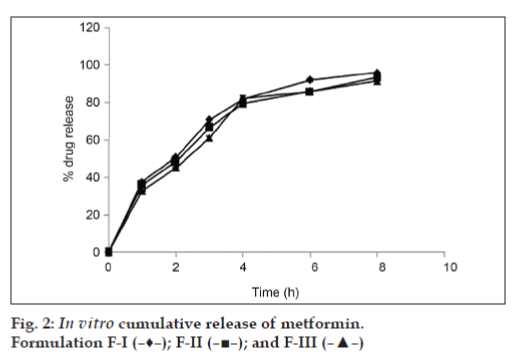
The results of dissolution studies of formulations F-I, F-II, and F-III, composed of eudragit RSPO (20, 30 and 40%) are shown in fig. 2. Tablets F-I, F-II and F-III released 43.88, 47.37 and 48.64% of metformin HCl at the end of 2 h; and 92.35, 92.73, and 93.69% of drug at the end of 8 h., respectively. No significant difference (P<0.001) in release rate was observed between tablets containing either 30 or 40% of Eudragit RSPO (92.35, 92.73% at 8 h). Further increase in concentration of Eudragit did not significantly (P<0.001) affect the release rate. On this basis, 30% of Eudragit RSPO was selected for further studies.
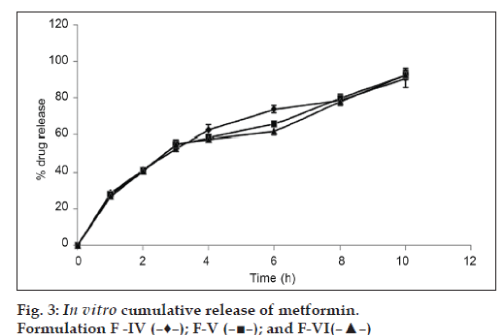
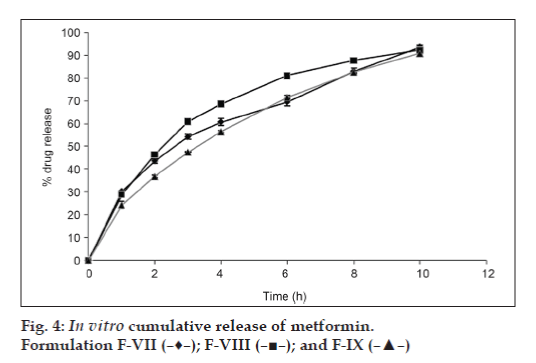
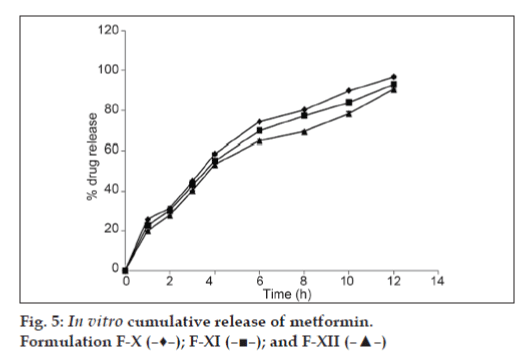
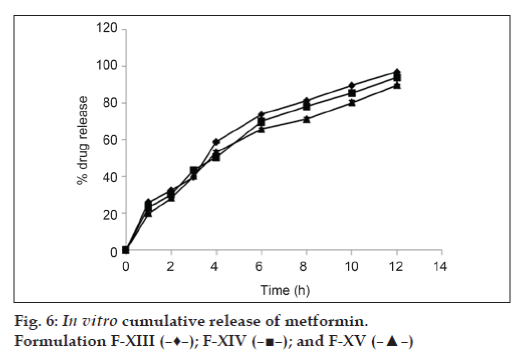
The results of dissolution studies of formulations F-IV, F-V, and F-VI, composed of GC (20, 30 and 40%) are shown in fig. 3. Tablets F-IV, F-V, and F-VI, released 41.19, 40.11 and 40.47% of metformin HCl at the end of 2 h; and 90.72, 88.56 and 83.12% of drug at the end of 10 h, respectively. Formulations F-VII, F-VIII, and F-IX, composed of GD (20, 30 and 40%) are shown in fig. 4. Tablets F-VII, F-VIII, and F-IX, released 43.61, 46.36 and 36.73% metformin HCl at the end of 2 h; and 93.53, 92.22 and 90.77% of drug at the end of 10 h, respectively. The results of dissolution studies of formulations F-X, F-XI, and F-XII, composed of combination of Eudragit RSPO and GC (75:25, 50:50 and 25:75% respectively) are shown in fig. 5. Tablets F-X, F-XI, and F-XII, released 31.62, 29.39 and 28.60% metformin HCl at the end of 2 h; and 96.96, 93.52 and 90.66% of drug at the end of 12 h, respectively. The results of dissolution studies of formulations F-XIII, F-XIV, and F-XV composed of combination of Eudragit RSPO and GD (75:25, 50:50 and 25:75%, respectively) are shown in fig. 6. Tablets - F-XIII, F-XIV, and F-XV, released 31.36, 30.96 and 28.89% of metformin HCl at the end of 2 h; and 95.16, 91.83, and 89.71% of drug at the end of 12 h, respectively. As indicated in fig. 2, tablets containing Eudragit RSPO (20, 30 and 40%) alone showed initial burst release during first hour (31.32, 33.14 and 37.47%, respectively). Eudragit RSPO contains quaternary ammonium groups, and solubilization of these quaternary ammonium groups in acidic pH leads to formation of pores in the matrix, thereby releasing metformin HCl in the acidic pH. This phenomenon may be attributed to surface erosion or initial disaggregation of the matrix tablet prior to gel layer formation around the tablet core [25].
| Formulation | zero order | First order | Higuchi | Hixon-Crowell | Korsmeyer-Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r2 | k | r2 | k | r2 | k | r2 | K | N | r2 | K | |
| FI | 0.86 | 14.22 | 0.9939 | -0.3213 | 0.9905 | 34.059 | 0.9778 | -0.078 | 0.5470 | 0.986 | 31.780 |
| FII | 0.821 | 14.639 | 0.9953 | -0.3407 | 0.989 | 35.276 | 0.9679 | -0.081 | 0.5180 | 0.982 | 34.566 |
| FIII | 0.781 | 15.115 | 0.9834 | -0.3731 | 0.9815 | 36.591 | 0.9494 | -0.087 | 0.4770 | 0.982 | 34.566 |
| FIV | 0.826 | 10.871 | 0.9702 | -0.2305 | 0.9942 | 29.235 | 0.9598 | -0.057 | 0.4970 | 0.992 | 29.567 |
| FV | 0.848 | 10.727 | 0.9684 | -0.2293 | 0.9948 | 28.750 | 0.9707 | -0.056 | 0.4960 | 0.990 | 28.925 |
| FVI | 0.85 | 10.535 | 0.9542 | -0.2236 | 0.9907 | 28.209 | 0.9628 | -0.055 | 0.4965 | 0.984 | 28.286 |
| FVII | 0.837 | 11.037 | 0.9778 | -0.245 | 0.9976 | 29.640 | 0.9768 | -0.059 | 0.5806 | 0.996 | 30.954 |
| FVIII | 0.782 | 11.680 | 0.9939 | -0.2668 | 0.9891 | 31.620 | 0.9594 | -0.064 | 0.5008 | 0.982 | 31.935 |
| FIX | 0.906 | 10.706 | 0.9956 | -0.2251 | 0.9967 | 28.435 | 0.9934 | -0.056 | 0.5806 | 0.998 | 24.709 |
| FX | 0.887 | 9.639 | 0.9722 | -0.2447 | 0.9907 | 28.132 | 0.9898 | -0.055 | 0.5772 | 0.987 | 24.227 |
| FXI | 0.896 | 9.247 | 0.9878 | -0.2066 | 0.9903 | 26.942 | 0.9885 | -0.050 | 0.5970 | 0.987 | 22.329 |
| FXII | 0.906 | 8.586 | 0.9825 | -0.1713 | 0.9928 | 24.974 | 0.9845 | -0.043 | 0.5879 | 0.994 | 21.044 |
| FXIII | 0.876 | 9.596 | 0.9899 | -0.2315 | 0.9927 | 28.069 | 0.9903 | -0.054 | 0.5614 | 0.986 | 24.945 |
| FXIV | 0.883 | 9.229 | 0.9960 | -0.2008 | 0.9927 | 26.965 | 0.9865 | -0.049 | 0.5821 | 0.991 | 23.073 |
| FX | 0.913 | 8.590 | 0.9869 | -0.1713 | 0.9927 | 24.956 | 0.9879 | -0.043 | 0.6103 | 0.994 | 20.176 |
Table 4 in vitro release kinetics parameters
Metformin HCl release profile of GC and GD matrix tablets is shown in fig. 2 and fig. 3, respectively. As regards the effect of gum concentration, decrease in drug release rate was observed when GC and GD content in the matrix were increased. This may be due to the reason that the gums in higher concentrations in the tablets might have produced dense matrix around the drug particles, providing more barriers for them to escape and dissolve. Further, such dense matrix, specifically when it is hydrophobic in nature, may be expected to favor less penetration of the dissolution medium in the tablets. This may also be the auxiliary reason for obtaining slow drug release profiles through GC and GD matrix tablets. In low concentrations (10% w/w), GC showed significant sustained drug delivery compared to GD. Tablets with 20% w/w GC and GD showed 85.56%, and 90.83% total drug release at the end of 10 h respectively. This may be due to the low solubility of GC compared to GD at pH 1.2 and pH 6.8. Both the gums in 30% w/w concentration retarded metformin HCl release beyond 10 h. Drug release from GC and GD matrix followed zero order and Higuchi square root kinetics respectively.
In formulations containing combinations of hydrophilic and hydrophobic polymers, FX, FXI, FXII, (fig. 5) and F-XIII, F-XIV, F-XV (fig 6), showed a significant difference (P<0.001) of drug release as compared with 10 and 20% of either of the Eudragit preparation. Hydrophilic eudragit when combined with hydrophobic GC and GD (FX-FXV) no burst release was observed, which may be due to the tendency to mask these quaternary ammonium groups to some extent, thereby modifying release of the drug. It is reported in the literature that more than 30% release of drug in the first hour of dissolution indicates the chance of dose dumping. The results showed probability of dose dumping from matrix tablets prepared without GC and GD.
To describe the kinetics of drug release from matrix tablets, release data was analyzed according to different kinetic equations. The data were analyzed by the regression coefficient method and regression coefficient values (r2) of all batches were shown in Table 4. On analyzing regression coefficient values of all batches, it was found that Batch F-I, II, III tablet exhibited almost zero-order kinetics. Batch F-IV, F-V, F-VI, F-VII and F-VIII tablet followed Higuchi model, whereas Batches F-IX, F-X, F-XI, and F-XII tablet followed first order kinetics.
The in vitro release profiles of drug from all these formulations could be best expressed by Higuchi’s equation as the plots showed highest linearity (r2=0.98 to 0.99) [22]. To confirm the diffusion mechanism, the data were fitted into Korsmeyer-Peppas equation [23]. The formulations showed good linearity (r2=0.97 to 0.98) with slope (n) between 0.477-0.5879, which appears to indicate a coupling of diffusion and erosion mechanisms-so called anomalous diffusion.
The time taken to release 25% (t25), 50% (t50), and 75% (t75) of drug from different formulations was determined (Table 5). Tablets containing combination of Eudragit RSPO with GC (F-X, XI and F-XIII) required 1.6, 0.9, 1.4 h and 6.6, 7.9 and 8.5 h to release 25% and 75% of drug, respectively. While combination of Eudragit RSPO with GD (F-XIV, XV and F-XVI) required 1.4, 1.8, 1.4 h and 7.1, 7.4 and 8.4 h to release 25% and 75% of drug, respectively. These values were significantly higher than those obtained in matrix tablets formulated with either Eudragit RSPO or GC and GD alone, which clearly indicated sustained release nature of the combination of both Eudragits with GC and GD.
Mean dissolution time (MDT) value is used to characterize drug release rate from a dosage form and indicates the drug release retarding efficiency of polymer. Tablets prepared with combination of Eudragit RSPO with GC and GD (F-X, F-XI, F-XII and F-XIII, F-XIV, F-XV) showed higher MDT value (3.98, 4.11, and 4.45 h; 3.97, 4.19 and 4.28, respectively). This finding can be attributed to the hydrophobic nature of GC and GD, which retarded drug release from the matrix.
The synthetic hydrophilic matrix of Eudragit RSPO alone could not sustain the release of the metformin HCl effectively for 12 h. Results of the present study demonstrated that combination of both synthetic hydrophilic (Eudragit RSPO) with natural hydrophobic polymers (GC and CD) could be successfully employed for formulating sustainedrelease matrix tablets. Diffusion coupled with erosion might be the mechanism for the drug release from hydrophilic and hydrophobic polymer based matrix tablets which can be expected to reduce the frequency of administration and decrease the dose-dependent side effects associated with repeated administration of conventional metformin HCl Tablets.
| Formulation | t25% | t50% | t75% | MDT |
|---|---|---|---|---|
| code | (h) | (h) | (h) | |
| F1 | 0.8 ±0.2 | 1.9±0.2 | 3.7±0.2 | 2.21±0.2 |
| F2 | 0.8±0.1 | 2.0±0.2 | 4.1±0.4 | 2.22±0.2 |
| F3 | 0.6±0.1 | 2.2±0.2 | 4.3±0.3 | 2.33±0.3 |
| F4 | 0.7±0.1 | 2.9±0.1 | 6.6±0.2 | 2.98±0.1 |
| F5 | 0.8±0.2 | 3.0±0.2 | 6.8±0.4 | 3.31±0.1 |
| F6 | 0.8±0.2 | 3.1±0.4 | 7.1±0.5 | 3.49±0.2 |
| F7 | 0.7±0.1 | 2.8±0.2 | 6.4±0.3 | 2.43±0.1 |
| F8 | 1.1±0.2 | 2.6±0.3 | 5.2±0.5 | 3.23±0.2 |
| F9 | 1.2±0.3 | 3.4±0.3 | 6.8±0.3 | 3.14±0.2 |
| F10 | 0.8±0.2 | 3.2±0.2 | 7.1±0.4 | 3.98±0.2 |
| F11 | 0.9±0.2 | 3.4±0.3 | 7.7±0.3 | 4.13±0.4 |
| F12 | 1.0±0.1 | 4.0±0.4 | 9.0±0.3 | 4.44±0.1 |
| F13 | 0.8±0.1 | 3.2±0.2 | 7.1±0.2 | 3.92±0.2 |
| F14 | 1.4±0.2 | 3.5±0.3 | 6.9±0.3 | 4.15±0.3 |
| F15 | 1.4±0.1 | 4.4±0.4 | 8.6±0.4 | 4.29±0.2 |
Table 5 in vitro dissolution parameters
metformin HCl effectively for 12 h. Results of the present study demonstrated that combination of both synthetic hydrophilic (Eudragit RSPO) with natural hydrophobic polymers (GC and CD) could be successfully employed for formulating sustainedrelease matrix tablets. Diffusion coupled with erosion might be the mechanism for the drug release from hydrophilic and hydrophobic polymer based matrix tablets which can be expected to reduce the frequency of administration and decrease the dose-dependent side effects associated with repeated administration of conventional metformin HCl Tablets.
Acnowledgements
The authors thank the Universal Medicament, Nagpur, India for providing Metformin HCl as gift sample and S. K. B. College of Pharmacy, Kamptee, Nagpur, India for providing necessary facilities to carry out this work.
References
- Stith BJ, Goalstone ML, Espinoza R, Mossel C, Roberts D, Wiernsperger N. The antidiabetic drug metformin elevates receptor tyrosine kinase activity and inositol 1, 4, 5-triphosphate mass in Xenopus oocytes. Endocrinology 1996;137:2990-9.
- Wiley A. Insulin and oral hypoglycemic drugs. In: Williams DA, Lemke TL editors,. Foyes Principle of medicinal chemistry. New York: Lippincott Williams and Wilkins; 2002. p. 641-8.
- Martindale SC. The Complete Drug Reference. London: The Pharmaceutical Press; 2002.
- Dunn CJ, Peters DH. Metformin: A review of its pharmacological properties and therapeutic use in non-insulin-dependent diabetes mellitus. Drugs 1995;49:721-49.
- Defang O, Shufang N, Wei L. in vitro and in vivo evaluation of two extended Release preparations of combination metformin and glipizide. Drug Dev Ind Pharm 2005;31:677-85.
- Salsa T, Veiga F, Pina ME. Oral control release dosge form I.Cellulose ether polymer in hydrophilic matrices. Drug Dev Ind Pharm 1997;23:929-38.
- Chien YW. Novel drug delivery systems. New York: Marcel Dekker; 1992. p. 1-43.
- Mehta KA, Kislaloglu MS, Phuapradit W, Malik AW, Shah NH. Release performance of a poorly soluble drug from a novel Eudragit-based multiunite erosion matrix. Int J Pharm Sci 2001;213:7-12.
- Kibbe AH. Handbook of pharmaceutical excipients. Washington DC: American Pharmaceutical Association; 2000.
- Sanchez L, Teresa F, Fernandez A, Alvarez F, Rabasco A, Mura P. Development of sustained release matrix tablets of didanosine containing methacrylic and ethylcellulose polymers. Int J Pharm Sci 2002;234:213-21.
- Reddy KR, Mutalik S, Reddy S. Once-daily sustained-release matrix tablets of Nicorandil: Formulation and in vitro evaluation. AAPS PharmSciTech 2003;61:4.
- Rodriguez L, Caputo O, Cini M, Cavallari C, Grecchi R. in vitro release of theophylline from directly compressed matrices containing methacrylic acid copolymers and/or dicalcium posphate dihydrate. II Farmaco 1993;48:1597-604.
- Rao VM, Engh K, Qiu Y. Design of pH-independent controlled release matrix tablets for acidic drugs. Int J Pharm Sci 2003;252:81-6.
- Gohel MC, Patel TP, Bariya SH. Studies in preparation and evaluation of pH independent sustained-release matrix tablets of verapamil HCl using directly compressible Eudragits. Pharm Dev Technol 2003;8:323-33.
- Liu J, Zhang F, McGinity JW. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. Eur J Pharm Biopharm 2001;52:181-90.
- Bharadwaj TR, Kanwar M, Lal R. Gupta A. Natural gums and modified natural gums as sustained-release carriers. Drug Dev Ind Pharm 2000;26:1025-38.
- Morkhade MM, Fulzele SV, Satturwar PM, Joshi SV. Gum Copal and Gum Damar: Novel Matrix Forming Materials for Sustained Drug Delivery. Indian J Pharm Sci 2006;68:53-8.
- Kidokoro M, Haramiishi Y, Sagasaki S, Shimizu T, Yamamoto Y. Application of Fluidized Hot-Melt Granulation (FHMG) for the Preparation of Granules for Tableting; Properties of Granules and Tablets Prepared by FHMG. Drug Dev Ind Pharm 2000;28:67-76.
- Wells J. Pharmaceutical preformulation: The physiochemical properties of drug substances. In: Aulton ME, editor, Pharmaceutics the science of dosage form design London: Churchill Livingstone; 2002. p. 247.
- Indian Pharmacopoeia, Government of India, Ministry of health and family welfare. 4th ed. New Delhi: The Controller of Publications; 1996.
- Basak SC, Rahman J, Ramlingam M. Design and in vitro testing of a floatable gastroretentive tablet of metformin hydrochloride. Pharmazie2007;62:145-8.
- Higuchi T. Mechanism of sustained-action medication: Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 1963;52:1145-9.
- Korsmeyer RW, Gurny R, Doelker EM, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm 1983;15:25-35.
- Banker GS, Ander LR. Tablets. In: Lachman L, Liberman HA, Kanig JL, editors. The theory and practice of industrial pharmacy Mumbai: Varghese Publishing House; 1987. p. 293-345.
- Ebube NK, Hikal A, Wyandt CM, Beer DC, Miller LG, Jones AB. Sustained release of acetaminophen from heterogeneous matrix tablets, influence of polymer ratio, polymer loading and coactive on drug release. Pharm Dev Technol 1997;2:161-70.