- Corresponding Author:
- B. K. Satishbabu
Department of Pharmaceutics, National College of Pharmacy, Balraj Urs Road, Shimoga - 577 201, India
E-mail: bksatishbabu@gmail.com
| Date of Submission | 16 January 2010 |
| Date of Revision | 27 October 2010 |
| Date of Acceptance | 18 November 2010 |
| Indian J Pharm Sci, 2010, 72 (6): 738-744 |
Abstract
A multiple unit oral floating drug delivery system of famotidine was developed to prolong gastric residence time, target stomach mucosa and increase drug bioavailability. Drug and polymer compatibility was studied by subjecting physical mixtures of drug and polymers to differential scanning calorimetry. Cod liver oil entrapped calcium alginate beads containing famotidine, capable of floating in the gastric condition were formulated and evaluated. The gel beads were prepared by emulsion gelation method by employing sodium alginate alone and mixture of sodium alginate and hydrophilic copolymers such as carbopol 934P and hydroxypropylmethylcellulose K15M grade in three different ratios. The effect of selected factors, such as percentage of oil and amount of copolymers on floating properties was investigated. The beads were evaluated for percent drug loading, drug entrapment efficiency, buoyancy and in vitro drug release. The in vitro drug release study of the beads was carried out in simulated gastric media employing a modified Rosette-Rice test apparatus. Wherein, the apparatus was further modified by incorporating a water jacket to the apparatus to circulate hot water to maintain 37±2° for throughout the release study. All the oil entrapped calcium alginate beads floated if a sufficient amount of oil was used. Beads formulated employing sodium alginate alone could not sustain the drug release up to 8 h, whereas beads formulated with mixture of sodium alginate and copolymers demonstrated sustained release of famotidine up to 8 h. The results suggested that cod liver oil entrapped calcium alginate beads were promising as a carrier for intragastric floating drug delivery of famotidine.
Keywords
Calcium alginate beads, famotidine, floating drug delivery systems, gastric residence time, modified rosette rice test apparatus
Introduction
Oral route is the most preferred route of drug delivery due to ease of administration and greater patient compliance [1], although studies revealed that this route is subject to two physiological influences, a short gastric residence time (GRT) and variable gastric emptying time (GET), which may lead to unpredictable bioavailability and times to achieve peak plasma levels. Furthermore, the brief GET in humans, which normally averages 2-3 h through the major absorption zone (stomach and upper part of the intestine), can result in incomplete drug release from the drug delivery system leading to diminished efficacy of the administered dose. Thus, control of placement of a drug delivery system in a specific region of the gastro intestine (GI) tract offers numerous advantages like improved bioavailability and therapeutic efficacy, local delivery of drug and possible reduction of dose size. All these considerations have led to the development of oral controlled release (CR) dosage forms possessing gastric retention capabilities.
Gastroretentive systems can remain in the gastric region for several hours and significantly prolong the gastric residence of the drugs. Prolonged gastric retention improves bioavailability, reduces drug waste, improve solubility of drugs that are less soluble in a high pH environment. It has application also for local drug delivery to the stomach and proximal small intestine [2-4].
Famotidine is a histamine H2-receptor antagonist. It is widely prescribed in gastric ulcers, duodenal ulcers, Zollinger-Ellison syndrome and gastroesophageal reflux disease. In the management of benign gastric and duodenal ulceration the dose is 40 mg daily by oral route at bedtime, for 4 to 8 weeks. In gastroesophageal reflux disease the recommended dose is 20 mg by oral route twice daily for 6 to 12 weeks. Famotidine is incompletely absorbed from GI tract, the low bioavailability (40-45%) and short biological half-life (2.5-3.5 h) of famotidine following oral administration favors development of a sustained release formulation [5,6].
In the present study, a multiple unit FDDS of famotidine was designed keeping in view of the all or nothing response of a single unit system [7]. For this purpose cod liver oil entrapped calcium alginate beads of famotidine were formulated which can remain buoyant on the surface of the gastric medium due to the lower density of the entrapped cod liver oil (0.918-0.927 g/ml) than that of gastric fluid which is almost equal to 1.0 g/ml [8,9]. Sodium alginate (SA) was employed to formulate the alginate beads and to sustain the release of the famotidine due its ability to form a stable and bioadhesive gel with calcium ions [10,11]. Hydroxypropylmethylcellulose K15M (HPMC K15) and carbopol 934P (CP) have been incorporated in polymeric matrix to enhance the sustained release properties of the alginate by providing a denser inner matrix [12].
To carry out the in vitro release studies of the beads, a Modified Rosette Rice test apparatus was employed by further modifying it with an outer glass water jacket. Literature reports indicate that the modified dissolution apparatus can more closely simulate most of the in vivo conditions [13,14].
Materials and Methods
Famotidine was received as a gift sample from Dr. Reddy’s Lab Ltd, Hyderabad, India, sodium alginate was purchased from Sigma Aldrich, U.S.A. Hydroxypropylmethylcellulose K15M was a gift from Colorcon Asia Pvt Ltd, Mumbai, India and carbopol 934P was purchased from S.D. Fine Chem Ltd, Mumbai, India. All other reagents and chemicals used were of analytical grade.
Compatibility study
Pure drug, polymers and physical mixture of drug and polymers were subjected to differential scanning calorimetry (DSC) to study the compatibility between drug and polymers (DSC823E, Mettler Toledo system, aluminium pans were employed to place the samples. The heating rate was kept at 10º rise per min up to 200º to better integrate the information. Argon gas was used for purging.
Preparation of floating beads of famotidine
Famotidine (20% w/w of dry polymer weight) was dissolved in water in acidic condition. SA (3% w/v) alone and polymer mixtures (3% w/v) containing SA and CP, and SA and HPMC K15 in 3 different ratios were dissolved in water to get clear polymeric dispersions, then a famotidine solution was added and mixed homogeneously. To the above mixture cod liver oil (20% w/w) was added and stirred to form a homogeneous emulsion. The drug-loaded emulsion was extruded through a 26-G syringe needle into calcium chloride solution (1% w/v) maintained under gentle agitation. The beads were allowed to remain in the same solution for 30 min to improve their mechanical strength. The formed beads were separated, washed with water and allowed to dry at room temperature overnight. Table 1 lists the formulation variables for different formulations of famotidine loaded floating beads. Blank beads without famotidine were also prepared using the same technique.
| Formulation code | SA: CP (3% w/v) |
SA: HK (3% w/v) |
Oil (% w/w) |
|---|---|---|---|
| CF blank | 10:0 | 10:0 | 20 |
| CF1 | 09:01 | - | 20 |
| CF2 | 08:02 | - | 20 |
| CF3 | 07:03 | - | 20 |
| CF4 | - | 09:01 | 20 |
| CF5 | - | 08:02 | 20 |
| CF6 | - | 07:03 | 20 |
SA, sodium alginate; CP, carbopol 934P; HK, hydroxypropylmethylcellulose K15; -, components not included in formulations.
Table 1: Formulation variables of various Famotidine bead Formulations.
Evaluation of floating beads
The prepared beads were evaluated for drug loading efficiency (LE) and drug entrapment efficiency (EE). An accurately weighed sample of beads (100 mg) was crushed in a mortar and added to 100 ml of phosphate buffer pH 7.4. This mixture was kept over night under stirring to elute complete drug from the polymer matrix. The mixture was filtered and analyzed spectrophotometrically at a wavelength of 265 nm (UV Spectrophotometer, 1601, Shimadzu, Japan) against blank bead mixture, which was treated similarly. The percent drug loading was calculated according to the following Eqn, percent LE = (drug load- drug loss)/drug load × 100 and the percent entrapment efficiency (%EE) was calculated according to the following Eqn, EE (%) = (actual drug content/ theoretical drug content)×100. The mean surface diameter of the beads was determined in dry state using a dial thickness meter.
Floating properties
The time between the introduction of the FDDS into the medium and its buoyancy to the upper one third of the dissolution vessel (floating lag time) and the time for which the formulation constantly floated on the surface of the medium (floating duration) were measured simultaneously as a part of dissolution studies.
Swelling study
The swelling behavior of the calcium alginate beads was studied in 0.1N HCl (pH 1.2) and phosphate buffer pH 7.4. Previously weighed (W1) beads were immersed in respective media. The weight (W2) of the beads was determined for 8 h: Every 30 min for the first 2 h and then every h after that. The swelling index (SI) of each batch was calculated using the following Eqn, percent SI = (W2–W1)/W1×100.
In vitro drug release study
In vitro release characteristics of famotidine floating gel beads were evaluated employing 0.1N HCl (pH 1.2). Dissolution of floating beads was carried out in a modified Rosette Rice test apparatus. A glass beaker of 100 ml was modified at the base by adding a tapped glass tube outlet so that the glass beaker can hold 70 ml of dissolution medium. This apparatus was housed inside an outer glass jacket through which hot water at 37±2º was circulated continuously. The medium was stirred at 75 rpm on a magnetic stirrer. A burette connected to a reservoir was mounted above the beaker to deliver fresh dissolution medium at a flow rate of 10 ml/ 30 min. Sampling was done at every 30 min till 8 h. The sample aliquots were analyzed spectrophotometrically at a wavelength of 265nm (UV Spectrophotometer, 1601, Shimadzu, Japan).
Stability study
The formulation CF4 was subjected for three month stability study according to ICH guidelines by exposing the beads in a suitable packing mode to the temperature 40±2° and relative humidity 75±5% in programmable environmental test chamber (CHM-10S, Remi Instruments Ltd., Mumbai, India). At the end of every month the beads were analyzed for the drug content, floating behavior and in vitro drug release.
Results and Discussion
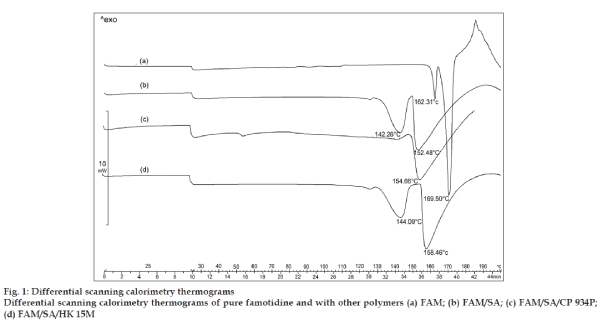
The DSC thermograph of pure famotidine showed two endothermic peaks (fig. 1a), a first small endothermic peak at 162.31° and the second sharp endothermic peak at 169.50°. The DSC thermograms of physical mixture of famotidine and the polymers showed no characteristic peaks of the polymers and famotidine peaks were still present but slightly shifted from their original positions (fig. 1b to 1d). The findings indicate that the drug and the polymers are compatible with each other.
The percent drug loading of various famotidine bead formulations ranged between 39 and 53.5 (the active drug content varied between 0.848 and 1.561 mg in a 100 mg sample). The entrapment efficiency for various famotidine floating bead formulations was found to vary between 29.3 and 55.2% and given in Table 2. The prepared beads were almost spherical and translucent. The mean surface diameter of all the formulations was given in Table 2. The lies between 1.734±0.006 (SD) and 1.751±0.032 mm (SD).
| Formulation Code | Mean diameter ±SD (mm) |
Drug content* (mg) |
% LE | % EE | Floating behavior | |
|---|---|---|---|---|---|---|
| FLT (min) | Floating duration (h) | |||||
| CF blank | 1.714±0.05 | - | - | - | 0 | 24 |
| CF | 1.751±0.03 | 0.849±0.03 | 39.01 | 29.34 | 0 | 24 |
| CF1 | 1.734±0.00 | 1.160±0.06 | 49.3 | 43.92 | 0 | 24 |
| CF2 | 1.735±0.00 | 1.561±0.02 | 49.9 | 55.15 | 0 | 24 |
| CF3 | 1.738±0.00 | 1.273±0.01 | 39.98 | 44.66 | 0 | 24 |
| CF4 | 1.740±0.02 | 0.966±0.03 | 43.98 | 44.13 | 0 | 24 |
| CF5 | 1.742±0.01 | 0.963±0.06 | 53.5 | 44.51 | 0 | 24 |
| CF6 | 1.741±0.03 | 1.076±0.08 | 52.9 | 38.55 | 0 | 24 |
SD; Standard deviation; %LE, percentage loading efficiency; %EE, percentage entrapment efficiency; *average drug content per 100 mg of beads±SD (n = 3).
Table 2: Evaluation Parameters of Various Famotidine Bead Formulations
The floating ability of prepared beads was evaluated along with dissolution studies. The beads without oil sank immediately in 0.1N HCl (pH 1.2), while beads containing 20% w/w amount of cod liver oil (CF to CF6) demonstrated instantaneous and excellent floating ability. Floating behavior of prepared beads is given in Table 2.
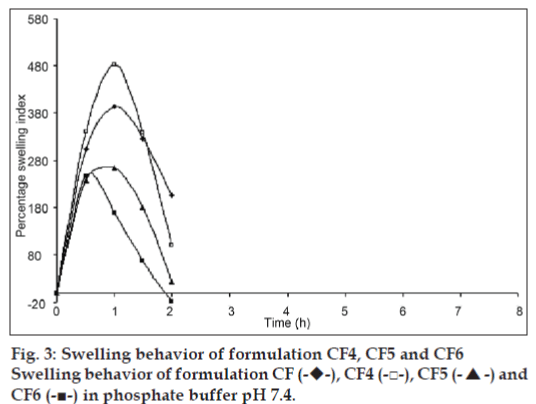
The swelling behavior study of the beads was performed in 0.1N HCl and phosphate buffer pH 7.4. The swelling ratio of the beads in 0.1N HCl was not significantly changed. The beads showed significant swelling in phosphate buffer pH 7.4 and the results of swelling indices of formulations CF to CF6 are shown in figs. 2 and 3.
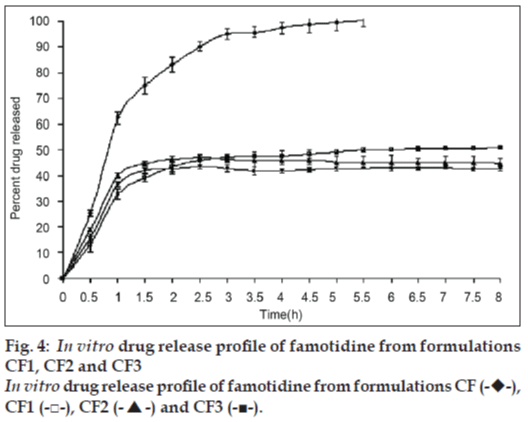
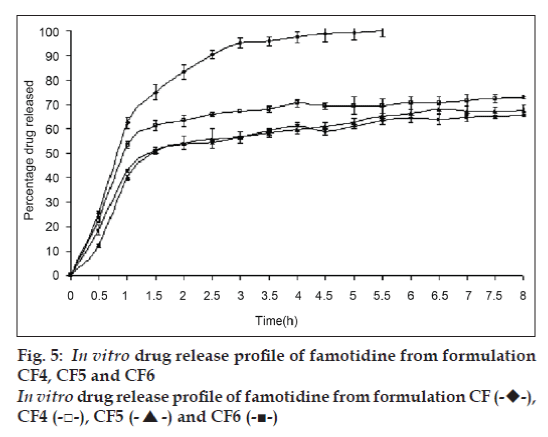
In vitro drug release study of famotidine floating beads was carried in 0.1N HCl (pH 1.2), for a period of 8 h. In the 0.1N HCl, the beads exhibited a biphasic release profile as an initial rapid drug release phase (burst effect) was followed by a sustained, gradually increasing drug release phase after 1 h extending up to 8 h. Formulation CF contained only SA could not sustain the famotidine release up to 8 h. It released complete drug at the end of 5 h 30 min. whereas formulations contained CP; CF1, CF2 and CF3 released 50.94, 44.92 and 42.81% of drug respectively at the end 8 h and the release profile is shown in fig. 4 .The formulations contained HPMC K15; CF4, CF5 and CF6 released 72.79, 67.70 and 65.57% of the drug at the end of 8 h respectively. The release profile from these beads is shown in fig. 5.
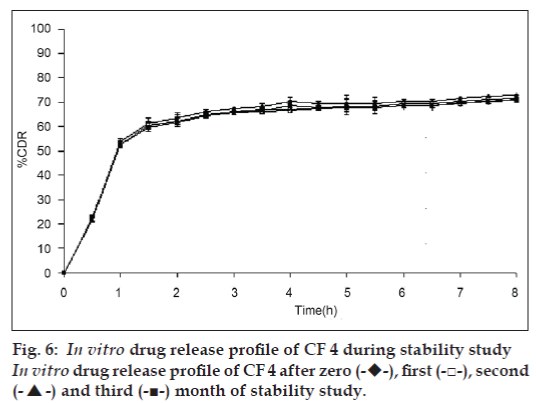
In view of potential utility of the formulation, stability study was carried out on formulation CF4 for three months according to ICH guidelines. At the end or each month, the formulation was subjected to drug assay, floating behavior and in vitro release studies. The results were shown in Table 3 and fig. 6.
| Formulation code | Drug content ±SD (mg) |
Floating behavior | Drug release at the end of 8h | |
|---|---|---|---|---|
| FLT (min) | Floating duration | |||
| Zero month | 0.966±0.035 | 0 | 24 | 72.79±0.318 |
| First month | 0.973±0.138 | 0 | 24 | 71.54±0.321 |
| Second month | 0.962±0.140 | 0 | 24 | 70.92±0.297 |
| Third month | 0.970±0.133 | 0 | 24 | 71.04±0.685 |
Table 3: Stability Studies of Formulation Cf4
The thermograms obtained by subjecting pure famotidine and physical mixture of famotidine and polymers (fig. 1) showed no possible drug polymer incompatibility. The thermograph of pure famotidine showed a sharp peak at 169.50°, whereas the famotidine peaks obtained in the physical mixtures of famotidine and polymers were broader and slightly shifted from their original positions which could be possibly due to the presence of other functional groups of polymers which may be neglected.
Alginate can form gel by ionotropic gelation with divalent calcium ions. When an emulsion of cod liver oil containing alginate was dropped into calcium chloride solutions, spherical beads were then formed instantaneously in which intermolecular cross-links were formed between the divalent calcium ions and the negatively charged alginate molecules. The gel beads were easily formed without any sophisticated equipment. Homogenization of the emulsion is a must, as without homogenization, the oil separated from the alginate solution despite being mixed by stirrer. Alginate might have helped to emulsify the mixture of water and oil phase during the homogenization process. However the emulsifying property was limited when the oil concentration was increased to more than 30%. At this concentration and above the oil started leaking from the beads. The oil-entrapped beads were spherical, translucent and slightly yellowish. It was found that a minimum of 20% w/w cod liver oil was necessary to impart satisfactory buoyancy to the beads.
The percentage drug loading efficiency (%LE) of the famotidine loaded beads are shown in Table 2. The %LE was obtained in the range of 39% to 53%. Formulation CF 5 gave the highest %LE and the formulation CF gave the lowest %LE. Similarly the percentage entrapment efficiency of the beads Table 2 was obtained in the range of 29% to 55%. Formulation CF2 showed the highest drug entrapment and formulation CF showed the lowest entrapment of drug. The low drug loading and the low entrapment efficiency of the CF formulation was may be due to the highly porous nature of the alginate matrix, due to which the drug may diffuse back into the cross linking solution from the bead matrix during cross linking period. Incorporation of the copolymers such CP and HPMC K15 which have higher viscosity might have increased the viscosity of the polymer matrix forming a rigid barrier for the drug to diffuse back into the cross linking solution. Thus, formulations contained these copolymers showed better drug loading and drug entrapment efficiency compared to CF formulation. The effect of copolymers in increasing the drug loading in the beads is also evident from the results that, increase in the copolymer concentration in the bead formulation further enhances the drug loading and the drug entrapment efficiency of the beads.
The mean particle diameter of the blank oil entrapped alginate beads containing no drug were found to be 1.714±0.052 (SD) mm, but the drug loading to the beads increases the size of the beads of formulation CF to 1.751±0.032 (SD) mm. Incorporation of copolymers to the bead formulation results in decreasing of the bead diameter as in case of formulations CF1 to CF6. As the process parameters were kept constant, the added materials were responsible for the change in the size of the calcium alginate beads.
The floating ability of prepared beads was evaluated along with dissolution studies. The beads without oil sank immediately in 0.1N HCl (pH 1.2), while beads containing sufficient amount of cod liver oil (CF to CF6) demonstrated instantaneous and excellent floating ability. The beads remained afloat through out the study period 8 h and the beads continued float till 24 h. Thus, floating ability was found to be directly related to the amount of oil entrapped in the polymer matrix.
The swelling behavior study of the bead was performed in 0.1N HCl and phosphate buffer pH 7.4 the swelling ratio of the beads in 0.1 N HCl was not significantly changed. The beads were also not significantly swollen and eroded in the dissolution media (0.1N HCl). Thus, from these results, it could be assumed that the drug release was not under the control of the swelling behavior but rather was controlled by the dissolution of the famotidine in the dissolution medium and diffusion of the famotidine through polymer matrix. Whereas, when beads are introduced to phosphate buffer pH 7.4, they swelled significantly and eroded in the mean time. Formulation CF, Swelled to its maximum within 1h of the study, but was completely eroded at the end of 2 h. Formulations contained CP i. e. CF1 and CF2 showed increasing swelling till the 8 h, whereas CF3 showed maximum swelling at the end of 5h. after which, the erosion of the bead matrix predominated (fig. 2). All the formulations containing HPMC K15 i. e. CF4, CF5 and CF6 swelled to their maximum at the end of 1h and were completely eroded at the end of 3 h.
The gel beads in the 0.1 N HCl (pH 1.2), exhibited a biphasic release profile as an initial rapid drug release phase was followed by a slower and sustained, gradually increasing drug release phase after 1h. Formulation CF released 62.37±2.21% of the drug within 1h but could not sustain the drug release over the following 7 h and released 100% drug at the end of 5 h 30 min. formulations CF1, CF2 and CF3, which contain CP along with SA released 50.94, 44.92 and 42.81% of drug respectively at the end 8 h (fig. 4) and formulations CF4, CF5 and CF6, which contain HPMC K15 released 72.79, 67.70 and 65.57% of the drug at the end of 8 h respectively (fig. 5). The results show that incorporation of rate controlling polymers such as CP and HPMC K15 can sustain the drug release from the oil entrapped calcium alginate beads. The results also show that as the concentration of the both copolymers increases in the formulation, the drug release was further decreased and more sustaining of drug release was observed.
Various release kinetic models were applied to the data obtained from in vitro release studies to elucidate the mechanism of drug release from the floating gel beads, such as zero order, first order, and higuchi and korsemeyer peppas models Table 4. It was observed that for the formulation CF, the r2 was higher when fitted to first order equation (r2 = 0.9892), which indicates that a first order release from the formulation CF. whereas all the other formulations, CF1 to CF6 followed Higuchi model. As the n values of the Korsemeyer-peppas model for all the formulations was found to be less than 0.5, it suggested that the drug release from the beads followed fickian diffusion. Result of stability study on the formulation CF4 showed no significant changes in the drug content, Floating behavior and in vitro drug release characteristics of the bead formulations.
| Formulation code | Zero order (r2) | First order (r2) | Higuchi model (r2) | Korsemeyer model (n) |
|---|---|---|---|---|
| CF | 0.713 | 0.9892 | 0.9025 | 0.4936 |
| CF1 | 0.566 | 0.6331 | 0.7974 | 0.3606 |
| CF2 | 0.3129 | 0.3188 | 0.5622 | 0.1943 |
| CF3 | 0.3618 | 0.3798 | 0.6088 | 0.2275 |
| CF4 | 0.5011 | 0.6237 | 0.7424 | 0.2956 |
| CF5 | 0.6489 | 0.7821 | 0.8501 | 0.4538 |
| CF6 | 0.6005 | 0.7228 | 0.8242 | 0.3402 |
Table 4: Kinetics of In Vitro Famotidine Release from Floating Beads
In the present work, cod liver oil entrapped floating alginate beads containing famotidine were prepared to prolong the residence time of the drug in the stomach for more than 8 h. The drug release from the beads was evaluated under more simulated conditions using a flow through dissolution apparatus. The beads showed excellent floating properties throughout the study period. It was found that SA alone could not sustain the drug release for sufficient period of time whereas incorporation of rate controlling polymers such as carbopol and hydroxypropylmethylcellulose as copolymers can effectively sustain the drug release from the bead formulations.
References
- Garg S, Sharma S. Gastroretentive drug delivery systems. Business Briefing: Pharmatech 2003; 5:160-6.
- Singh BN, Kwon H. Floating drug delivery systems: An approach to oral controlled drug delivery via gastric retention. J Control Release 2000;63:235-59.
- Dave BS, Amin AF, Patel MM. Gastroretentive drug delivery system of ranitidine hydrochloride: Formulation and in vitro evaluation. AAPS PharmSciTech 2004; 5:e34.
- Arora S, Ali J, Ahuja A, Khar RK, Baboota A. Floating drug delivery system: A review. AAPS PharmSciTech 2005; 6:47.
- Available from: www.parpharm.com/pdf/product/0608.pdf. [Last accessed on 2010 Nov 3].
- Jaimini M, Rana AC, Tanwar YS. Formulation and evaluation of famotidine floating tablets. Curr Drug Deliv 2007; 4:51-5.
- Ichikawa M, Watanabe S, Miyake Y. A new multiple-unit oral floating dosage form, I: Preparation and evaluation of floating and sustained release characteristics. J Pharm Sci 1991;80:1062-6.
- British Pharmacopoeia. Vol. 1. London: The Stationery Office on behalf of the Medicines and Healthcare products Regulatory Agency; 2003. p. 297-8.
- Bardonett PL, Faivre V, Pugh WJ, Piffaretti JC, Falson F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J Control Release 2006;111:1-18.
- Murata Y, Sasaki N, Miyamoto E, Kawashima S. Use of floating alginate beads for stomach- specific drug delivery. Eur J Pharm Biopharm 2000;50:221-6.
- Tonnesen HH, Karlsen J. Alginate in drug delivery systems. Drug DevInd Pharm 2002;28:621-30.
- Li S, Lin S, Daggy BP, Mirchandani HL, Chien YW. Effect of HPMC and Carbopol on the release and floating properties of Gastric Floating Drug Delivery System using factorial design. Int J Pharm 2003;253:13-22.
- Gohel MC, Mehta PR, Dave RK, Bariya NH. A more relevant dissolution method for evaluation of floating drug delivery system. J Dissol Tech 2004;11:21-5.
- Karande AD, Yeole PG. Comparative assessment of different dissolution apparatus for floating drug delivery systems. J Dissol Tech 2006; 25:20-3.
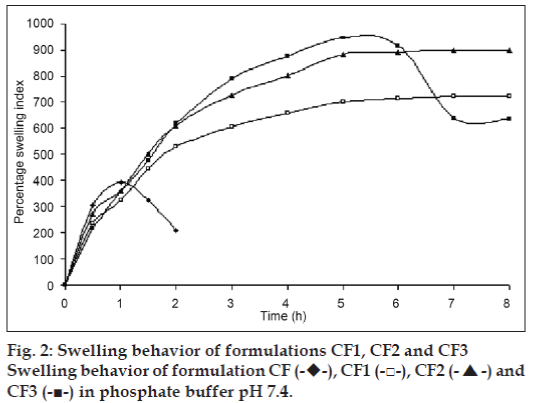
 , CF1 (-□-), CF2 (-▲-) and CF3 (-■-) in phosphate buffer pH 7.4.
, CF1 (-□-), CF2 (-▲-) and CF3 (-■-) in phosphate buffer pH 7.4.