- *Corresponding Author:
- H. Kathpalia
Department of Pharmaceutics, Vivekanand Education Society’s College of Pharmacy, Chembur, Mumbai-400 074, India
E-mail: hkathpalia2007@rediffmail.com
| Date of Submission | 25 July 2015 |
| Date of Revision | 07 December 2016 |
| Date of Acceptance | 27 February 2017 |
| Indian J Pharm Sci 2017;79(2):204-211 |
Abstract
The present investigation was undertaken with the objective of formulating taste-masked orally-disintegrating films of the bitter levocetirizine dihydrochloride to enhance the convenience and compliance by the elderly or paediatric or bedridden and non-cooperative patients due to its ease of administration. Scope of this study was to explore the film forming properties of various film formers like modified starch, pullulan, hydroxypropyl methylcellulose and polyvinyl alcohol-polyethylene glycol based polymers. Plasticizers like glycerin, propylene glycol, sorbitol and polyethylene glycol 400 were evaluated by studying their effect on folding endurance, peelablity and in vitro disintegration time. Films were prepared by solvent casting method. Drug loaded films, which composed of the selected polymers with the suitable plasticizer showed excellent film forming capacity along with good folding endurance, in vitro disintegration time within 22 s and >95% drug release in 10 min as compared to marketed immediate release tablets, which showed on 30.4% at the end of 10 min The films prepared were 30-40 mg in weight with pleasant taste with bitter active being successfully taste masked by a mixture of sucralose and monoammonium glycerrhizinate producing an extended sweetness profile. Furthermore, the orally disintegrating films were stable for at least 3 mon when stored at 40° and 75% relative humidity. The formulation developed is simple, easy to prepare and economical with great applicability during the emergency cases such as allergic reactions, whenever immediate onset of action is desired.
Keywords
Orally disintegrating, solvent-casting method, folding endurance, peel ability, Lycoat RS 720, pullulan, HPMC E5, kollicoat IR, monoammonium glycerrhizinate
For the past one decade, there has been an enhanced demand for more patient-friendly and compliant dosage forms. As a result, the demand for developing new technologies has been increasing annually. Orally disintegrating films (ODF) have carved a niche amongst the oral drug delivery systems due to their high patient compliance [1-3]. United States Food and Drug Administration (USFDA) defined the fast dissolving oral thin films as “a thin, flexible, non-friable polymeric film strip containing one or more dispersed/dissolved active pharmaceutical ingredients, which is intended to be placed on the tongue for rapid in vitro disintegration or dissolution in the saliva prior to swallowing for delivery into the gastrointestinal tract” [4].
Levocetirizine dihydrochloride, a third-generation non-sedative antihistamine developed as the active enantiomer of the second-generation antihistamine cetirizine. Levocetirizine is rapidly and extensively absorbed following oral administration with a plasma elimination half-life ranging from 6-10 h [5]. In allergic cold and cough due to sore throat conditions, especially the elderly and paediatric, bedridden and non-cooperative patients experience difficulty in swallowing a tablet type of dosage form [6].
The objective of the present research work was to develop taste masked ODF of levocetirizine dihydrochloride disintegrating within 30 s to enhance the convenience of administration to the patients to improve compliance. Various polymers like modified starch (Lycoat RS 720) [7,8], pullulan [9,10], hydroxypropyl methylcellulose (HPMC E-5) [11] and polyvinyl alcoholpolyethylene glycol (PEG) based polymer (Kollicoat IR) [12] were evaluated for film forming capacity. Plasticizers like glycerin, propylene glycol (PG), sorbitol and PEG 400 [13] were evaluated by studying their effect on folding endurance, peelablity and in vitro disintegration time of the polymer films. Pleasant tasting ODF of levocetirizine dihydrochloride were prepared in which bitter active was successfully taste masked by a mixture of sucralose and monoammonium glycerrhizinate producing an extended sweetness profile [7,14]. The formulation developed was simple, easy to prepare and economical with great applicability and also giving faster In vitro drug dissolution rate as compared to the commercially available immediate release tablets.
Materials and Methods
Levocetirizine dihydrochloride was gifted by Cipla Ltd. and sucralose was obtained from Ipca Labs. Polymers like pullulan were obtained from Gangwal Chemicals, Lycoat RS 720 from Roquette Pharma, HPMC E-5 from Colorcon Ltd., Kollicoat IR from BASF. monoammonium glycerrhizinate (Magnasweet) was obtained from Unicorn Natural Products Pvt. Ltd. All other chemicals and reagents were of analytical grade and were purchased from Loba Chemie, Mumbai.
Screening of polymers for ODF:
Lycoat RS 720 (25%), pullulan (2-5%), HPMC E-5 (5- 15%) and Kollicoat IR (2-15%) were evaluated as film forming agents and glycerin, PG, sorbitol and PEG 400 as plasticizers for their suitability in ODFs. Plasticizer was dissolved in small quantity of purified water and then the polymer was dissolved in it followed by deaeration by sonication. Films were cast on plastic petri plate and completely dried in oven at 60°.
The films were evaluated for film forming ability, folding endurance [15], ease of peel ability, tackiness, uniformity and in vitro disintegration. The concentration of polymer, which formed smooth, homogeneous, nontacky, flexible, fast disintegrating and easily peelable film was selected for formulation of drug-loaded film.
Determination of threshold value of bitterness of levocetirizine dihydrochloride:
A panel comprising of six healthy volunteers (age 22- 25 y) were selected for this study. Prior consent has been taken from the participants as the study involved only holding the drug solution in the mouth for 30 s but not to swallow it. Then the solution was spitted out followed by rinsing the mouth with water. A series of solutions of levocetirizine dihydrochloride of concentrations 10, 20, 30, 40 and 50 μg/ml was prepared in phosphate buffer of pH 6.8. The volunteers were asked to hold 10 ml of each solution in oral cavity for 30 s and rate the taste on a scale from 0 to 4 (0: no bitterness, 1: threshold bitterness, 2: bitter, 3: moderate bitterness and 4: strongly bitter). The mouth was rinsed with distilled water and a gap of 30 min was allowed between successive tests. Based on opinion of volunteers, threshold bitterness concentration of levocetirizine dihydrochloride was determined. Various trials were taken by physical mixture of the drug with sucralose, with and without peppermint powder, with and without Magnasweet to mask the bitter taste of the drug and the ratings obtained have been shown in Table 1.
| Attributes | Rating scale | |||
|---|---|---|---|---|
| After taste | 1- no | 2- slight | 3- extreme | - |
| Flavour intensity | 1- not strong | 2- moderate | 3- strong | 4- very strong |
| Bitterness | 1- no bitterness | 2- bitterness at the end | 3- bitter | 4- extremely bitter |
Table 1: Taste attributes rating scale
Preparation of drug-loaded films:
Drug-loaded films were prepared by solvent casting method [16] for the optimized film compositions of each polymer. Levocetirizine dihydrochloride was incorporated at a concentration of 5 mg per 4 cm2 film area. Amount of drug added into the film forming solution was calculated by considering total amount of solution to be poured in order to obtain films with desired thickness when casted on the calculated total surface area of the petri plate. Weighed quantities of the polymer and plasticizer were added to 3/4th the quantity of water, stirred until the polymer was dissolved and kept aside to remove the entrapped air. Levocetirizine dihydrochloride along with water soluble sweetener sucralose and monoammonium glycerrhizinate was dissolved in the remaining quantity of water and peppermint powder was then added to it under stirring. Both solutions were then mixed till a homogenous solution was obtained and then deaerated by sonication. Film was casted on plastic petri plate and completely dried at 60°. Dried films were peeled off and then stored in airtight container under dehumidified conditions until taken up for further evaluation.
Evaluation of drug-loaded ODF:
The drug-loaded films obtained were evaluated for organoleptic and palatability characteristics. The thickness of each film was measured at five different locations (centre and four corners) using a vernier calliper. Data were represented as a mean±SD of triplicate films. Films were cut into 2×2 cm square strips. The moisture uptake by the films was determined by exposing them to 75% relative humidity (RH) at room temperature (25±2°) for one week [17]. The uptake of moisture by the films was measured and calculated as percent increase in weight. Tack is the tenacity with which the film adheres to an accessory that has been pressed into contact with the film. Tackiness evaluation [18] was carried out by gently pressing the film between fingertips and results were noted in qualitative terms as tacky or non-tacky. Film softening upon storage was checked by storing the films in desiccators at room temperature for 48 h. Films were then evaluated for softening and integrity.
The folding endurance of the film was determined by repeatedly folding one film at the same place till it broke. The number of times that film could be folded at the same place without breaking was noted; which gave the value of the folding endurance. In vitro disintegration time was determined by placing (2×2 cm2) film in a glass Petri dish containing 6 ml of distilled water. The time required for the film to break was noted as In vitro disintegration time. Typical disintegration time for ODFs is 5-30 s [19]. Test was conducted in triplicates. Drug content determination was done by dissolving three samples of 1 cm2 surface area of film in 0.1 N hydrochloric and then analysing the amount of drug by UV spectrophotometer at 231 nm. The In vitro dissolution test was performed for each type of film using the USP dissolution apparatus II at 37±0.5° with stirring speed of 50 rpm in 900 ml 0.1 N hydrochloric acid and this was compared to the dissolution profile obtained with marketed immediate release tablets, Xyzal 5 mg under similar dissolution conditions. Film size required for dose delivery (2×2 cm2) was used. Five ml aliquot of dissolution medium was collected at time intervals of 1, 2, 5, 10, 15 and 30 min and replaced with equal volumes of 0.1 N hydrochloric acid. The collected samples were filtered and the concentration of the dissolved levocetirizine dihydrochloride was determined using UV spectrophotometer at 231 nm. The results were presented as an average of three such concentrations.
Stability studies:
Stability studies of optimized batch of ODFs were performed under accelerated stability conditions (40±2°/75±5% RH) kept in stability testing chamber for three months according to International Conference on Harmonisation (ICH) guidelines. The samples were evaluated for their physical characteristics, in vitro drug dissolution and assay initially and at the end of 1 and 3 mon of storage period.
Results and Discussion
The present study was undertaken to investigate polymers like Lycoat RS 720, pullulan, HPMC E-5 and Kollicoat IR for their film forming capacity. Preliminary trials (plain films) were undertaken for designing the orodispersible films where the effect of various concentrations of the different film-forming agents and plasticizers on the characteristics of the films was assessed. Initially investigation was focused on development of polymer films with uniform appearance, good peelability from substrate, nontackiness, optimum flexibility, in vitro disintegration time of less than 30 s. Although no requirements for in vitro disintegration time have been specified for ODFs, a limit of 30 s was decided as a selection criterion for screening studies.
As per results of evaluation tests given in Table 2 and 3, Lycoat RS 720 at concentration of 25% w/w formed films with good peelability, while at lower concentration of 10% w/w, no film formation was observed. To impart flexibility and improve wetting property of Lycoat films, PG and polysorbate 80 were added. At 2% w/w concentration of PG, film was found to be brittle with low folding endurance. Hence PG concentration was increased to 4% w/w, which resulted in flexible film formation with good folding endurance. For both the trials, polysorbate 80 concentration was fixed i.e. 2% w/w. But Lycoat films were nondisintegrating at both the concentrations of PG; hence PG was replaced with sorbitol. Sorbitol was tried at 2% and 4% w/w concentration. It was observed that at 4% w/w concentration of sorbitol, film flexibility was maintained without affecting the stability. It was later found that in combination with soya lecithin, polysorbate was required in smaller concentration. 0.1% w/w polysorbate 80 and 0.8% w/w soya lecithin was incorporated in the films to aid in wetting and dissolution of film. No film softening upon storage was observed. Thus the polymer film of batch number LF09 had acceptable film properties and hence it was selected for drug loading.
| Formulation | LF01 | LF02 | LF03 | LF04 | LF05 |
|---|---|---|---|---|---|
| Film forming capacity | Poor | Poor | Very good | Good | Good |
| Peelability | Non-peelable | Non-peelable | Peelable | Brittle during peeling | Non-peelable |
| Appearance | Transparent | Transparent | Transparent | Transparent | Transparent |
| Tackiness | Non-tacky | Non-tacky | Non-tacky | Tacky | Non-tacky |
| Folding endurance | - | 4 | 29 | 9 | 11 |
| In vitrodisintegration time (s) | Non-disintegrating | Non-disintegrating | Non-disintegrating | 38 | 31 |
| Film softening at25°, 60% RH | Softening | Softening | Softening | No film softening | No film softening |
| Inference | No film formation | Brittle films Low folding endurance | Non disintegrating film | Brittle films | Non-peelable |
| Acceptability | No | No | No | No | No |
Table 2: Physico-Mechanical properties of lycoat Rs 720 polymer films
| Formulation | LF06 | LF07 | LF08 | LF09 |
|---|---|---|---|---|
| Film forming capacity | Good | Good | Good | Very good |
| Peelability | Peelable | Peelable | Peelable | Peelable |
| Appearance | Transparent | Transparent | Transparent | Transparent |
| Tackiness | Tacky | Non-tacky | Slight tacky | Non-tacky |
| Folding endurance | 28 | 20 | 33 | 31 |
| In vitrodisintegration time (s) | 36 | 29 | 21 | 18 |
| Film softening at 25°, 60% RH | No film softening | No film softening | No film softening | No film softening |
| Inference | Tacky | Folding endurance need to be improved | Slight tacky | Optimum |
| Acceptability | No | No | No | Yes |
Table 3: Physico-Mechanical properties of lycoat Rs 720 polymer films (continued)
As per the results of evaluation tests given in Table 4; pullulan formed good peelable film at 2% w/w and 5% w/w concentration with in vitro disintegration time of less than 30 s. Hence lower concentration of pullulan was selected for further trials. Effect of different plasticizers was studied. Sorbitol was selected, which showed good folding endurance and no film softening. Thus 5% w/w pullulan film of batch number PF05 containing 0.5% sorbitol had acceptable film properties hence was selected for drug loading.
| Formulation | PF01 | PF02 | PF03 | PF04 | PF05 |
|---|---|---|---|---|---|
| Film forming capacity | Good | Good | Good | Good | Very good |
| Peelability | Non-peelable | Peelable | Peelable | Brittle during peeling | Easily peelable |
| Appearance | Transparent | Transparent | Transparent | Opaque | Transparent |
| Tackiness | Non-tacky | Non-tacky | Tacky | Non-tacky | Non-tacky |
| Folding endurance | 20 | 35 | 29 | 30 | 40 |
| In vitro disintegration time (s) | 28 | 16 | 20 | 18 | 15 |
| Film softening at 25°, 60% RH | Softening | No film softening | Softening | Softening | No film softening |
| Inference | Low folding endurance | Good folding endurance | Brittle and tacky | Brittle films | Optimum |
| Acceptability | No | No | No | No | Yes |
Table 4: Physico-Mechanical properties of pullulan polymer films
As per results of evaluation tests given in Table 5, HPMC E-5 formed good peelable film at 5% w/w. Effect of different plasticizers was studied at 0.7% concentration with 0.1% polysorbate. PG was selected as it gave the least D.T. Further trial was carried out with increasing concentration of HPMC from 5 to 7% to increase the thickness of the film along with 1% PG. In vitro disintegration time in 6 ml water was 10 s; hence HF05 was the optimized batch, which was selected for drug loading.
| Formulation | HF01 | HF02 | HF03 | HF04 | HF05 | HFO6 |
|---|---|---|---|---|---|---|
| Film forming capacity | Good | Good | Very good | Good | Very good | Poor |
| Peelability | Peelable | Peelable | Easily peelable | Peelable | Easily peelable | Non-peelable |
| Appearance | Transparent | Opaque | Transparent | Transparent | Transparent | Transparent |
| Tackiness | Non-tacky | Non-tacky | Non-tacky | Non-tacky | Non-tacky | Tacky |
| Folding endurance | 20 | 22 | 35 | 25 | 40 | - |
| In vitrodisintegration time (s) | 20 | 16 | 10 | 15 | 10 | - |
| Film Softening at 25°, 60% RH | Softening | Softening | No film softening | Softening | No film softening | Softening |
| Inference | Low folding endurance | Film formed was not uniform | Less D.T compare to others | Brittle films | Optimum | Film was not formed |
| Acceptability | No | No | Yes | No | Yes | No |
Table 5: Physico-Mechanical properties of hpmc E-5 polymer films
The present study revealed that Kollicoat IR did not form a film at 2% concentration but formed good peelable film at 5 and 10% w/w concentrations. Film softening was seen with 5% concentration of Kollicoat IR; hence 10% concentration was finalized. Polysorbate 80 (0.1%) was added as wetting agent. From the results as shown in Table 6, batch KF03 had acceptable film properties; hence it was selected for drug loading. Solvent casting being most preferred offered great uniformity and films had fine gloss and better physical properties. The films were easy to prepare without the use of organic solvents.
| Formulation | KF01 | KF02 | KF03 | KF04 |
|---|---|---|---|---|
| Film forming capacity | Poor | Good | Very good | Poor |
| Peelability | Non-peelable | Peelable | Easily peelable | Non-peelable |
| Appearance | - | Transparent | Transparent | - |
| Tackiness | - | Non-tacky | Slight tacky | - |
| Folding endurance | - | 30 | 37 | - |
| In vitro disintegration time (s) | - | 29 | 21 | - |
| Film Softening at25°, 60% RH | softening | softening | No film softening | softening |
| Inference | Film was not formed | Non-uniform film | Optimum | Film was not formed |
| Acceptability | No | No | Yes | No |
Table 6: Physico-Mechanical properties of kollicoat ir polymer films
Most volunteers reported that 20 μg/ml as the threshold value of bitterness of levocetirizine dihydrochloride. This shows that the drug is extremely bitter and requires efficient taste masking method before formulating as orally disintegrating dosage forms. In order to ensure patient compliance and to allow the mouth disintegrating films to become a viable delivery system, bitterness masking becomes essential. Taste masking of levocetirizine dihydrochloride, which is extremely bitter was successfully achieved using a combination of sucralose as the sweetener, peppermint as flavour and monoammonium glycerrhizinate as the flavour enhancer and bitter taste masking agent. Sucralose as a sweetener has an initial burst of sweetness, which dissipates rapidly but in combination with monoammonium glycerrhizinate, which is a flavour enhancer produced a sweetness profile that extended itself over the time of the product being experienced in the mouth. From the scores given by volunteers for the taste masking trials (Table 7); the batch F03 with sucralose 2 mg, peppermint powder 2 mg and monoammonium glycerrhizinate 0.2 mg was selected as effectively taste masked batch.
| Batch No | After taste | Flavour intensity | Bitterness |
|---|---|---|---|
| F01 | 3 | 1 | 3 |
| F02 | 2 | 1 | 3 |
| F03 | 1 | 2 | 1 |
Table 7: Taste scoring given by volunteers
Drug loading in the optimized formula for each polymer (Table 8) was successful without any change in film characteristics. Results of evaluation tests performed for the characterization of levocetirizine dihydrochloride ODFs are given in Table 9. Films of formula LODF01 were non-tacky, smooth and transparent with thickness of 0.2 mm. Also in vitro dissolution time of this film was within 30 s; hence it was observed that drug loading did not affect the properties of Lycoat polymer films. Drug-loaded pullulan film was non-tacky, transparent and disintegrated in 15 s. HPMC E-5 film containing levocetirizine dihydrochloride disintegrated within 10 s. Film obtained was non-tacky, transparent and folding endurance was 40. Drug-loaded Kollicoat IR film was non-tacky, flexible, and transparent and disintegrated within 21 s. Kollicoat IR consists of 75% polyvinyl alcohol and 25% of PG hence there was no need for additional plasticizer.
| Ingredients | Concentration (%w/w) | |||
|---|---|---|---|---|
| LODF01 (Lycoat RS 720) |
LODFO2 (Pullulan) |
LODF03 (HPMC E-5) |
LODF04 (Kollicoat IR) |
|
| Lycoat RS 720 | 25 | - | - | - |
| Pullulan | - | 5 | - | - |
| HPMC E-5 | - | - | 7 | - |
| Kollicoat IR | - | - | - | 10 |
| Polysorbate 80 | 0.1 | 0.1 | 0.1 | 0.1 |
| Soy lecithin | 0.8 | - | - | - |
| Sorbitol | 4 | 0.5 | - | - |
| Propylene glycol | - | - | 1 | - |
| Sucralose | 1 | 1 | 1 | 1 |
| Monoammonium glycerrhizinate |
0.1 | 0.1 | 0.1 | 0.1 |
| Peppermint powder | 0.8 | 0.8 | 0.8 | 0.8 |
| Purified water | q.s. | q.s. | q.s. | q.s. |
Table 8: Optimized composition of levocetirizine dihydrochloride odf
| PARAMETERS | LODF01 LYCOAT |
LODF02 PULLULAN |
LODF03 HPMC E-5 |
LODF04 KOLLICOAT IR |
|---|---|---|---|---|
| Appearance | Smooth surface and transparent | Smooth surface and transparent | Smooth surface and transparent | Smooth surface and transparent |
| Taste | Sweet and minty smooth taste | Sweet and minty smooth taste | Sweet and minty smooth taste | Sweet and minty smooth taste |
| Tack test | Non-tacky | Non-tacky | Non-tacky | Non-tacky |
| %Moisture uptake | 0.46 | 0.6 | 0.82 | 0.62 |
| Thickness (mm) | 0.2 | 0.2 | 0.2 | 0.2 |
| In vitrodisintegration time (s) | 18 | 15 | 10 | 21 |
| Folding endurance | 31 | 40 | 40 | 37 |
| Film softening upon storage | No film softening | No film softening | No film softening | No film softening |
| %Drug content | 98.29 | 101.36 | 99.2 | 102 |
| Inference | Acceptable | Acceptable | Acceptable | Acceptable |
Table 9: Physico-Chemical properties of levocetirizine dihydrochloride odf
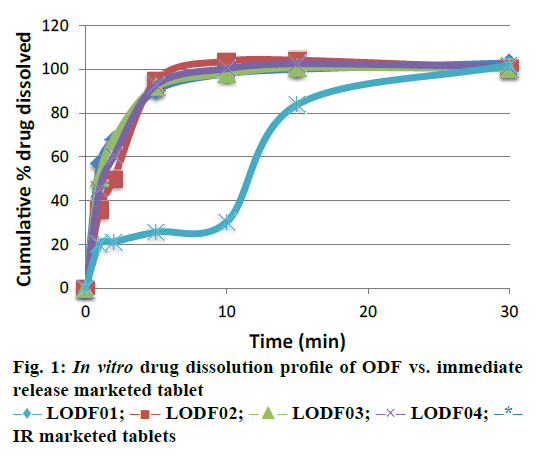
In vitro dissolution study of all the batches showed >95 % drug dissolution in 10 min, whereas the marketed immediate release tablets of levocetirizine dihydrochloride, Xyzal 5 mg tablets shows only 30.4% dissolution in 10 min and 101.9% dissolution in 30 min (Figure. 1). It was found that the optimized formulations were stable for at least three months at accelerated condition (40±2°/75±5% RH) as no significant change in appearance, thickness, folding endurance, In vitro disintegration time, drug content and In vitro dissolution time and extent was observed (Table 10 and 11).
| Physical | Conditions | LODF01 (Lycoat RS 720) | LODF02 (Pullulan) | ||||
|---|---|---|---|---|---|---|---|
| parameters | (°/%RH) | Initial | 30th | 90th | Initial | 30th | 90th |
| day | day | day | day | ||||
| Appearance | 40/75 | Transparent | Transparent | Transparent | Transparent | Transparent | Transparent |
| 25/60 | Transparent | Transparent | Transparent | Transparent | Transparent | Transparent | |
| Thickness (mm) | 40/75 | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm |
| 25/60 | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | |
| Folding endurance | 40/75 | 31 | 30 | 32 | 40 | 38 | 36 |
| 25/60 | 32 | 29 | 30 | 35 | 33 | 30 | |
| % Moisture uptake | 40/75 | 0.45 | 0.4 | 0.42 | 0.6 | 0.55 | 0.58 |
| 25/60 | 0.52 | 0.42 | 0.48 | 0.55 | 0.5 | 0.48 | |
| In vitro disintegration time (s) | 40/75 | 18 | 20 | 18 | 20 | 18 | 20 |
| 25/60 | 20 | 22 | 20 | 20 | 22 | 20 | |
| Assay % | 40/75 | 98 | 98.5 | 101.5 | 101 | 102.2 | 102 |
| 25/60 | 99 | 101 | 100.2 | 102 | 101.5 | 100.5 | |
Table 10: Stability data of lycoat and pullulan odf
| Physical | Conditions | LODF03 (HPMC) | LODF04 (Kollicoat) | ||||
|---|---|---|---|---|---|---|---|
| parameters | (°/%RH) | Initial | 30th | 90th | Initial | 30th | 90th |
| day | day | day | day | ||||
| Appearance | 40/75 | Transparent | Transparent | Transparent | Transparent | Transparent | Transparent |
| 25/60 | Transparent | Transparent | Transparent | Transparent | Transparent | Transparent | |
| Thickness (mm) | 40/75 | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm |
| 25/60 | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | 0.2 mm | |
| Folding endurance | 40/75 | 40 | 38 | 36 | 37 | 38 | 36 |
| 25/60 | 35 | 36 | 30 | 32 | 30 | 35 | |
| %Moisture uptake | 40/75 | 0.8 | 0.78 | 0.8 | 0.6 | 0.62 | 0.6 |
| 25/60 | 0.58 | 0.6 | 0.65 | 0.58 | 0.58 | 0.65 | |
| In vitro disintegration time (s) | 40/75 | 20 | 22 | 20 | 20 | 25 | 22 |
| 25/60 | 25 | 22 | 24 | 22 | 23 | 21 | |
| Assay % | 40/75 | 99 | 100 | 101.5 | 101 | 102.2 | 102 |
| 25/60 | 98 | 101.5 | 100.2 | 99.5 | 101.2 | 100.8 | |
Table 11: Stability data of hpmc and kollicoat odf
Lycoat RS 720 (25%), pullulan (5%), HPMC E-5 (7%) and Kollicoat IR (5%) of levocetirizine formed flexible, peelable, transparent fast dissolving films weighing around 50 mg and showing disintegration time within 30 s. The films showed faster drug release and dissolution as compared to commercial immediate release tablets and were attractive in appearance and produced sweet after taste, thus fulfilling the objectives of the present research work. These ODF can yield significantly better or at least comparable pharmacokinetic profile (AUC, Tmax, Cmax) as the conventional immediate release solid dosage forms [20] and hence may prove to be more efficacious. The present research work demonstrated that the bitter taste of water soluble drug levocetrizine dihydrochloride can be completely eliminated by a combination of sucralose, peppermint powder and monoammonium glycerrhizinate, which can also give extended sweetness profile. Thus the development of paper thin ODF providing all the homogeneity advantages of a liquid formulation with convenience and stability of a solid dosage form can be a viable option when rapid action is required especially in case of non-cooperative patients.
Acknowledgements
The authors thank Cipla Ltd. Mumbai, for providing levocetirizine dihydrochloride as gift sample for this work. They also thank Dr. (Mrs.) Supriya Shidhaye, Principal, VES College of Pharmacy, Mumbai for providing the required facilities to carry out this research work.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Aurora J, Pathak V. Oral disintegrating dosage forms, an overview. Drug Del Tech 2005;5:50-54.
- Bala R, Pawar P, Khanna S, Arora S. Orally dissolving strips: a new approach to oral drug delivery system. Int J Pharm Investig 2013;3:67-76.
- Kathpalia H, Gupte A. An introduction to fast dissolving oral thin film drug delivery system: a review. Curr Drug Del 2013;10:667-84.
- http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/022524orig1s000chemr.pdf.
- https://www.drugbank.ca/drugs/DB06282.
- Hirani J, Rathod D, Vadalia K. Orally Disintegrating Tablets: A Review. Trop J of Pharm Res 2009;8:161-72.
- Kathpalia H, Sule B, Gupte A. Development and evaluation of orally disintegrating film of tramadol hydrochloride. Asian J Biomed Pharm Sci 2013;3:27-32.
- Preis M, Pein M, Breitkreutz J. Development of a taste-masked orodispersible film containing dimenhydrinate. Pharmaceutics 2012;4:551-62.
- Foss D, Hoffman A, Shen S, StuckyAM, Turner JL. Edible pullulan films containing flavouring. US Patent Number-US20090011115A1;2009.
- Renuka M, Avani A. Formulation and characterization of rapidly dissolving films of cetirizine hydrochloride using pullulan as a film forming agent. Indian J Pharm Educ Res 2011;45:72-7.
- Daud A, Bonde M, Sapkal N, Gaikwad N. To study the effect of solvent, viscosity and temperature on mouth dissolving film of Withaniasomniferalinn. Asian J Pharm 2012;6:212-7.
- Mahesh A, Shastri N, Sadanandam M. Development of taste masked fast disintegrating films of LevocetrizineDihydrchloride for oral use. Current Drug Del 2010;7:21-7.
- Mishra R, Amin A. Formulation development of taste masked rapidly dissolving films of cetirizine hydrochloride. Pharm Tech 2009;33:48-56.
- Myers GL, Hariharan MS, Davidson K, Sanghvi P. Stabilized amine-containing actives in oral film composition. US Patent Number-US9095577 2015;2015.
- Panda BP, Dev NS, Rao MEB. Development of innovative orally fast disintegrating dosage forms: a review. Int J Pharm Sci Nanotech 2012;5:1666-74.
- Hoffmann EM, Breitenbach A, Breitkreutz J. Advances in orodispersible films for drug delivery. Expert Opin Drug Del 2011;8:299-316.
- Radhakishan UR, Chavan V, Tribhuvan N. Mouth dissolving films and their patents: An overview. Int Res J Pharm 2012;3:39-42.
- Bhupinder B, Sarita J, Mandeep K, Harmanpreet S. Orally fast dissolving films: innovations in formulation and technology. Int J Pharm Sci Rev Res 2011;9:50-4.
- Barnhart S. Thin film oral dosage forms. In: Rathborne MJ, Hadgraft J, Roberts MS, Lane ME, editors. Modified release drug delivery technology: Drugs and the pharmaceutical sciences. 2nd ed. New York: Marcel Dekker; 2008. p. 209-16.
- Zhang H, Chen H, Li XJ, Zhang Q, Sun YF, Liu CJ, et al. Pharmacokinetics and safety profiles of novel diethylstilbestrol orally dissolving film in comparison with diethylstilbestrol capsules in healthy Chinese male subjects. Int J ClinPharmacolTher 2014;52:407-15