- *Corresponding Author:
- Anna Pratima G Nikalje
Maulana Azad College of Arts and Science, Dr. Babasaheb Ambedkar Marathwada University, University Campus, Aurangabad, Maharashtra 431004, India
E-mail: annapratimanikalje@gmail.com
| Date of Received | 27 September 2021 |
| Date of Revision | 01 August 2022 |
| Date of Acceptance | 24 March 2023 |
| Indian J Pharm Sci 2023;85(2):426-434 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The current work involves preparation and evaluation of self-micro emulsifying drug delivery system of carvedilol, an nonselective betablocker and alpha-1 blocker. Oral self micro-emulsifying drug delivery system of Carvedilol were prepared by studying the solubility in different oils, surfactants and co-surfactants and formulations were prepared using mixtures of oils, surfactants, and cosurfactants in various proportions. Based on the solubility study, the optimised self micro-emulsifying drug delivery system of carvedilol was prepared using Capryol 90 as oil phase and tween 20 and transcutol P as surfactant and co-surfactant, respectively. The formulations were evaluated for stability, globule size and in vitro performance. To estimate the drug content from various excipients, final microemulsion formulations and in vitro performance study, a stability-indicating validated high-performance liquid chromatography method was developed using Inertsil ODS-3V (150 mm×4.6 mm, 5 μm) high-performance liquid chromatography column. The method was validated for various parametes like accuracy, linearity, precision (intraday and interday) and robustness. All the validation parameters were in acceptable range. The developed method was found to be specific accurate and robust for estimation of carvedilol from its microemulsion formulations.
Keywords
Carvedilol, self-micro emulsifying drug delivery system, high-performance liquid chromatography, content estimation, validation
Amongst the available various dosage forms, oral delivery systems are preferred for chronic treatment. The potent lipophilic molecules which are used in the chronic oral treatment, exhibits low bioavailability owing to their poor aqueous solubility. Nearly 40 % of new drug candidates exhibit low solubility in water due to the lipophilic nature, which leads to poor oral bioavailability, high intra and inter-subject variability and lack of dose proportionality. The dissolution of these molecules in the gastrointestinal fluids is the rate limiting step for the absorption[1].
Self-Emulsifying Drug Delivery System (SEDDS) gained great importance in last two decades as promising lipid-based drug delivery approach for poorly water soluble and low bioavailable drugs, as an alternative strategy to the conventional drug delivery system[2-4]. SEDDS are isotropic systems comprising of oil, surfactant, co-surfatant/co-solvent. Upon oral administrations of SEDDS, the digestive motility of the stomach and intestine provide the agitation necessary for self-emulsification into a fine oil-in-water emulsion which are thermodynamically stable and homogenous systems[4]. This leads to in situ solubilization of drug that can subsequently be absorbed by lymphatic pathways, bypassing the hepatic first-pass effect resulting in increasing the bioavailability.
Carvedilol (CVD), a non-selective beta blocker with selective alpha-1 blocking activity, is Biopharmaceutical Classification System Class II drug which is widely use for the treatment of high blood pressure, Congestive Heart Failure (CHF), and left ventricular dysfunction in people who are otherwise stable[5]. After oral administration CVD is absorbed rapidly from the gastrointestinal tract but due extensive first-pass metabolism by Cytochrome P450 it exerts low bioavailability (about 25 %) with a short elimination half-life of about 2-6 h[6,7]. The CVD shows a pH dependent aqueous solubility in physiological pH range of the gastro-intestine from 1.2 to 7.8. The solubility in water is only about 30 μg/ml, whereas the high solubility (500 to 2500 µg/ml) is observed in pH age of 1.2 to 5.0 and this solubility decreased with increasing the pH which explain its low availability at the absorptive site[8,9]. Thus, the aim of this investigation is to enhance the oral bioavailability of CVD by preparation and characterization of a SEDDS formulation of CVD which will increase the solubility and avoid the extensive hepatic first-pass metabolism by lymphatic absorption, thus resulting in increased bioavailability.
Materials and Methods
CVD and its impurities were obtained as gift sample from Ipca Laboratories Ltd., Mumbai, India. Capryol 90, Labrasol, Lauroglycol 90, Lauroglycol FCC, Transcutol P Labrafac (Gattefosse), Capmul MCM (Abitec Corp), Myvacet 9-45K (Kerry Inc.), Miglyol 812 (IOI Oleo GmbH), Tween 20 and 80, Polyethylene glycol 400 were procured from S.D. Fine Chem Ltd. Acetonitrile (High-Performance Liquid Chromatography (HPLC) grade), methanol (HPLC grade), triethylamine (for Chromatography) and orthophosphoric acid 88 % (Emparta® ACS) was purchased from Merck Life Sciences Pvt. Ltd., Mumbai, India. Milli Q water, obtained from Millipore (Massachusetts, USA), was used in preparation of solutions. Reference product (Cardivas, Sun Pharmaceutical Industries Ltd.) was procured from the local market. Buffers and all other chemicals were of analytical grade.
The Waters Alliance chromatographic system was used for estimation of drug content in various oils and surfactant during the screening, from final formulation and for dissolution studies. Cyclomixer (Remi, Mumbai, India) and Sonicator (PCI Analytics, Mumbai, India) were used for preparation and analysis of SMEDDS.
Stability-indicating HPLC method:
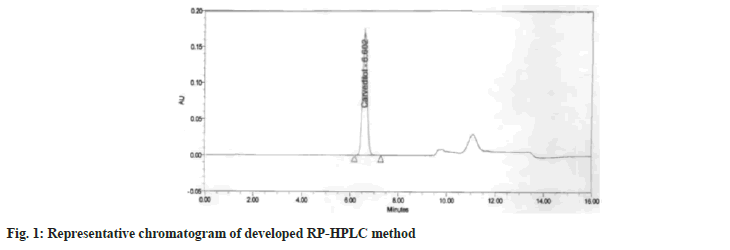
An in-house reverse-phase HPLC method was developed and validated for estimation of CVD from oils, surfactant/cosurfactant (S/CoS) and final developed SMEDDS. The HPLC apparatus consisted of Waters Alliance series sample manager HPLC with quaternary pump, equipped with Waters 2996 Photodiode array detector (Waters, USA), Empower 3 integrator software and Inertsil ODS-3V (150 mm×4.6 mm, 5 µm) HPLC column. The column temperature was kept at 50°. A gradient program was used for the elution of CVD from various excipients and final SMEDDS formulations. The Mobile Phase A (MPA) consisted of 0.02 M potassium dihydrogen phosphate pH 2.8 buffer:acetonitrile (990:10) and Mobile Phase B (MPB) consist of methanol:acetonitrile:buffer (500:400:150) with a gradient program as 0 to 8 min MPA:MPB (55:45); 8.1 to 12 min (0:100); 12.1 to 16 min (55:45) at a flow rate of 1.5 ml/min that led to a retention time of 6.6±0.2 min when detection was carried out at 240 nm. The assay was linear (r2=0.999) in the concentration range 15-270 µg/ml. The method was validated with respect to accuracy and inter and intraday precision as per International Council for Harmonisation guidelines and the relative standard deviation was less than 2 % in both the cases[10]. The method was evaluated for robustness with deliberate changes in column temperature, pH of mobile phase and sample preparation sonication time.
Solubility studies:
Solubility of CVD in various selected excipients (oils, surfactants, and co-surfactants) was determined by shake flask method. An excess amount of CVD was added to eppendorf tube containing selected excipients (each 0.4 g) and mixed using cyclomixer. The eppendorf tubes were incubated in reciprocating water bath shaker (Boekel Scientific, Pennsylvania, USA) at 37° and shaken for 24 h. Samples were centrifuged at 5000 rpm for 15 min and supernatant was taken and subsequently diluted in a different organic solvent depending upon miscibility of oils[11]. The diluted samples were analyzed using developed and validated HPLC method.
Screening of surfactants and co-surfactants based on emulsifying ability
The emulsifying ability of surfactants and co-surfactants was evaluated by their emulsifying potency as reported previously[12]. Briefly, oil, surfactant and co-surfactant were mixed at 1:2:1 ratio and the mixtures were cyclomixed for 5 min and heated at 40° for homogeneous mixing. The obtained mixtures were weighed (50 mg) accurately and diluted with double distilled water to 50 ml to yield a fine emulsion. The resulting emulsions were observed visually for physical appearance, optical clarity and separation of dispersed phase over a period of 12 h and their percentage transmittance was taken at 638.2 nm by Ultraviolet spectrophotometer.
Construction of pseudo ternary phase diagrams:
Ternary phase diagram of oil, surfactant:co-surfactant and water was plotted in which each of the component represented apex of the triangle. Ternary mixtures with varying compositions of surfactant, co-surfactant and oil were prepared. Twenty five experiments were conducted with varying concentrations of oil, surfactant and cosurfactant. The oil, surfactant and cosurfactant concentration was varied from 10 % to 90 %. Drug, 25 mg was added to mixture of oily phase, surfactant and cosurfactant followed by stirring using cyclomixer (Remi, Mumbai, India). Compositions were evaluated for microemulsion formation by diluting 240 mg each of the 25 mixtures from each phase diagram to 25 ml with 0.45 µm filtered distilled water. The mixtures were equilibrated for 12 h to examine for any signs of phase separation. Microemulsions which showed precipitation of the drug, phase separation or cracking were rejected. Microemulsions having globule size 200 nm or below were considered desirable. The area of microemulsion formation was identified for respective systems and phase diagram was plotted. The mean globule size of most stable microemulsion was recorded by photon correlation spectroscopy (Beckmann N5 coulter, Miami, USA)[11,13].
Preparation of SMEDDS:
The required quantity of oil, surfactant and cosurfactant were mixed thoroughly by stirring on cyclomixer (Remi, Mumbai, India). The requisite quantity of CVD was weighed accurately and dissolved in the mixture by stirring. The mixtures were equilibrated for 12 h to observe for any signs of phase separation. Accurately weighed 240 mg of the SMEDDS were filled in size “3” hard gelatin capsules until further evaluation.
Evaluation of SMEDDS:
Appearance: The SMEDDS formulations before dilution were evaluated visually for color, appearance and consistency and for homogeneity of oil-surfactant mixture and absence of precipitation of drug.
Globule size analysis: The average globule size and polydispersity index of microemulsions were determined by the photon correlation spectroscopy (Beckmann N5 coulter, Miami, USA). Microemulsions were diluted with double distilled water to ensure that the light scattering intensity was within the instruments sensitivity range. Measurements were made in triplicate for all the microemulsions[14].
Drug content: SMEDDS equivalent to 25 mg CVD were weighed, suitably diluted with methanol and estimated for drug content by developed and validated HPLC method. The analysis was performed in Triplicate[14].
Effect of pH on globule size: To study the effect of pH, 240 mg of the system was diluted to 10 ml using buffer solutions. The effect of 0.7 % HCl (pH 1.45), pH 4.5 (Acetate buffer), pH 6.8 (Phosphate Buffer) was determined using photon correlation spectroscopy.
Effect of dilution and pH of dilution on SMEDDS: Robustness of the selected formulations for dilution was assessed by exposing them to 100-, 400- and 1000-fold dilution with water, 0.7 % HCl (pH 1.45), pH 4.5 (Acetate buffer), pH 6.8 (Phosphate Buffer). The diluted microemulsions were stored for 6 h and monitored for any physical changes (such as precipitation or phase separation)[15].
Effect of CVD loading: Drug was incorporated at dose levels of 6.25, 12.5, 25, 37.5 and 50 mg in blank formulations F2 and F5. SMEDDS equivalent to 25 mg drug was dispersed in 10 ml double distilled water and evaluated for globule size, drug precipitation and phase separation, if any.
Thermodynamic stability: The objective of thermodynamic stability is to evaluate the phase separation and effect of temperature variation on SMEDDS formulations. Microemulsions were subjected to centrifugation at 15 000 rpm for 15 min and observed visually for phase separation. Optimized SMEDDS formulations were subjected to the freeze-thaw cycles by storing them at -20° for 24 h and then at 40° for another 24 h.
In vitro dispersion test: F2 and F5 SMEDDS were filled in size ‘3’ hard gelatin capsules and evaluated for dispersion using United States Pharmacopeia (USP) type II apparatus. Dissolution was performed in 900 ml, 0.7 % HCl pH adjusted to 1.45 with NaOH buffer at 50 rpm as indicated in USP monograph. The samples were withdrawn at predetermined time intervals and analyzed using HPLC λmax of 240.0 nm. The analysis was conducted on six replicates and the average drug release±S.D. was calculated[11,15].
Results and Discussion
The SEDDS of CVD was prepared using the different combinations of oils and surfactant. An HPLC method was developed for estimation of CVD from various oils and it formulation. Representative chromatogram of developed method is given as fig. 1. The method precision showed percentage RSD of 0.2 % and the intermediate precision also showed the percentage RSD of 0.4 % which was very well within the acceptance criteria of 2.0 %. The accuracy study was performed by recovery at 50 %, 100 % and 150 % level in triplicate. The mean recovery value of 100.4 % was observed against the acceptance criteria of 80 %-120 %. The robust ness studies performed by deliberate changes in the column temperature by ±2° showed RSD of 0.8%, change in pH of buffer by ±0.2 units showed RSD of 1.1 %, whereas change in sample preparation time i.e. sonication time by ±5 min showed RSD of 0.4 %. The above validation results indicate that the developed method is accurate, precise and robust in nature for its intended use.
Selection of suitable oils and surfactants is an important step in the design of SMEDDS containing poorly water-soluble drugs. The volume of the formulation is critical and should be kept minimal to deliver the therapeutic dose of the drug in an encapsulated form. Therefore the study was performed to identify oils and surfactants that possess good solubilizing capacity for CVD. The pre-concentrate mixture should be clear, monophasic liquid at ambient temperature and should have good solvent properties to allow presentation of the drug in solution[11,16].
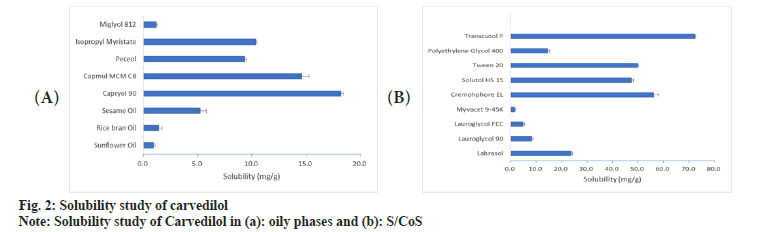
Oils represent one of the most important excipients in the SMEDDS not only because it can solubilize marked amount of hydrophobic drugs but can also determine the self-microemulsification efficiency and absorption of drugs from the Gastrointestinal (GI) tract. It is well established that modified or hydrolyzed vegetable oils have good solubilization[17,18] and emulsifying properties[19]. In the present investigation, modified oils like capryol 90, capmul MCM C8 and isopropylmyristate were found to solubilize more amount of CVD than other naturally available oils (fig. 2a). Isopropylmyristate is not used for oral systems hence was not considered for further evaluation. Literature cites that active pharmaceutical ingredients are more soluble in medium-chain triglycerides than long-chain triglycerides because they possess higher ester content per g than long-chain triglycerides[20].
Fig. 2b shows the solubility of CVD in various surfactants and co-surfactants. Cremophor EL exhibited maximum solubility of 56.3 mg/g followed by Tween 20 (50.1 mg/g), and solutol HS-15 (47.426 mg/g). In addition to their ability to solublize poorly soluble drugs, nonionic surfactants are also known to be less irritant and cytotoxic than their anionic and cationic counterparts; and are less affected by pH and changes in ionic strength that are likely to be encountered in gastrointestinal environment[19]. The surfactant and cosurfactant was selected on the basis of their ability to solubilize the drug and their emulsification efficiency. The selection of surfactant would be governed by their emulsification efficiency towards the oily phase. Amongst the cosurfactants, Transcutol P (72.3 mg/g) showed the highest solubility of CVD followed by Labrasol (23.9 mg/g) and Polyethylene Glycol (PEG) 400 (14.6 mg/g).
The turbidimetric method was performed to screen the various surfactants and co-surfactants to emulsify the selected oily phase. The intensity of light passing through such dispersions is attributed to the scattering of light which occurs due to absence of optical homogeneities in the medium[12]. The percentge transmittance could directly be used to correlate the relative droplet size of the emulsion and are depicted in Table 1 and Table 2. The emulsification evaluation distinctly indicates the capability of various combinations of surfactant and co-surfactant to emulsify oils. Amongst the various surfactant evaluated tween 20, solutol HS15 and cremophor EL showed good emulsifying ability towards capryol™ 90 than capmul® MCM (Table 1 and Table 2).
| Surfactants | Percentage transmittance values with Co-surfactants for Capryol 90 | |||
|---|---|---|---|---|
| Transcutol P | Capmul C8 | Labrasol | PEG 400 | |
| Solutol HS15 | 95.1±3.24 % | 70.10±5.01 % | 68.10±4.31 % | 88.15±3.01 % |
| Tween 20 | 90.25±2.01 % | 81.05±2.98 % | 75.20±1.89 | 92.6±1.58 % |
| Cremophor EL | 98.35±2.58 % | 25.9±1.27 % | 70.35±2.47 | 95.15±1.22 % |
Table 1: Emulsification studies for Capryol 90 as oil phase.
| Surfactants | Percentage transmittance values with Co-surfactants for Capmul MCM | |||
|---|---|---|---|---|
| Transcutol P | Capmul C8 | Labrasol | PEG 400 | |
| Solutol HS15 | 89.1±2.14 % | 68.80±3.14 % | 86.12±2.22 % | 78.25±2.11 % |
| Tween 20 | 89.50±4.31 % | 71.85±2.18 % | 70.80±9.81 | 82.0±1.78 % |
| Cremophor EL | 88.37±2.08 % | 35.1±1.97 % | 60.3782.41 | 85.1341.42 % |
Table 2: Emulsification studies for Capmul MCM as oil phase.
These studies indicated that cremophor EL was relatively superior surfactants in emulsifying oils and along with transcutol P and PEG 400 as a co-surfactants. Hence, pseudoternary phase diagrams were constructed using capryol 90 with various combinations of surfactants and co-surfactants.
Upon gentle agitation self-micro emulsifying systems form very fine oil-water emulsions, when they are mixed with aqueous media. Surfactant and co surfactant are predominantly adsorbed at the interface of oil and water, which reduces the interfacial energy. Thus reduction in the free energy required for the emulsification improves the thermodynamic stability of the microemulsion formulations. Therefore, the selection of oil and surfactant, and the mixing ratio of oil to S/CoS, play an important role in the formation of the microemulsion[21].
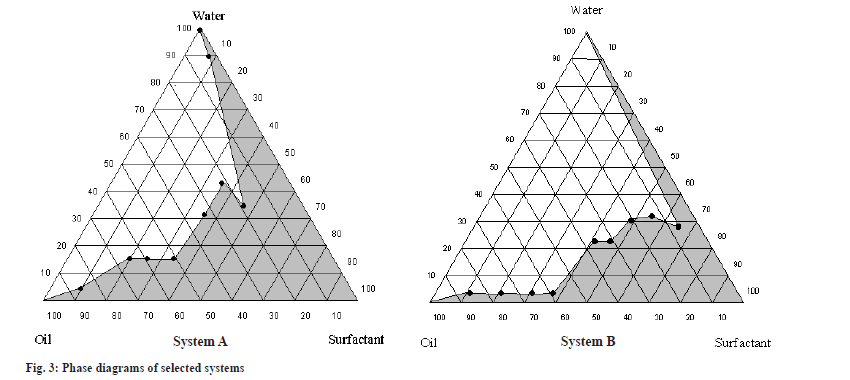
In the present study, capryol 90 was tested for phase behavior studies with different combinations of cremophore EL and solutol HS-15 with transcutol P, labrasol and PEG as the S/CoS mixture. As seen from the ternary plots, capryol 90 gave a wider microemulsion region at all combinations of cremophore EL/transcutol P as S/CoS ratios and solutol HS-15/PEG 400 as S/CoS ratios. The microemulsion area increased as the S/Cos ratios increased. However, it was observed that increasing the surfactant ratio resulted in formation of viscous solutions. Thus, an S/CoS ratio 1:1 for cremophore EL and transcutol P system and 2:1 for solutol HS-15 and PEG 400 system was selected during the formulation study. The phase diagram constructed with the selected system is given in fig. 3.
System A comprises of crapryol 90 as oil phase and cremophore EL:transcutol P (1:1) as S/CoS, System B comprises of crapryol 90 as oil phase and solutol HS-15:PEG 400 (2:1) as S/CoS. The composition of SMEDDS formulation trial is depicted in Table 3. The particle size and its distribution have a significant influence on the physical stability and in vivo fate of colloidal drug carriers such as microemulsions[22,23]. Therefore, it was essential to determine the effect of formulation variables on the particle size of the resulting microemulsions. The results of globule size analysis are indicated in Table 4. Formulations F3, F7 and F8 exhibited highest globule size (>100 nm) in comparison to other formulations. F1 and F2 were found to give globule size of less than 50 nm with nearly similar globule size distribution. Hence, taking into consideration the mean globule sizes of the formulations it was decided to evaluate F2 and F5 for further studies. Both the formulations can be classified as Type IIIB formation per Pouton, resulting in very fine globule size due to high content of hydrophilic co-solvents[17].
| Ingredients (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|
| CVD | 3.125 | 3.125 | 3.125 | 3.125 | 3.125 | 3.125 | 3.125 | 3.125 |
| Capryol 90 | 10.0 | 8.6 | 11.3 | 8.6 | 7.5 | 6.0 | 7.5 | 6.0 |
| Cremophore EL | 10.0 | 12.9 | 11.3 | 12.9 | - | - | - | - |
| Solutol HS-15 | - | - | - | - | 15.0 | 18.0 | 15.0 | 18.0 |
| Transcutol P | 10.0 | 12.9 | - | - | - | - | 7.5 | 6.0 |
| Labrasol | - | - | 7.5 | 12.9 | - | - | - | - |
| PEG 400 | - | - | - | - | 7.5 | 6.0 | - | - |
Table 3: Composition of various SMEDDS formulations.
| Formulations | Globule size (nm) |
|---|---|
| F1 | 38.95±0.58 |
| F2 | 34.35±0.51 |
| F3 | 107.6±1.23 |
| F4 | 97.6±0.35 |
| F5 | 55.3±1.21 |
| F6 | 54.1±6.22 |
| F7 | 107.3±1.31 |
| F8 | 105.8±1.95 |
Note: Each value is a mean±SD of two determinations
Table 4: Globule size analysis of SMEDDS formulations.
Both formulations appeared clear, transparent, homogenous liquids at room temperature. F2 and F5 SMEDDS were diluted in 1:1 ratio and observed for birefringence by placing between polarizing plates. Formulations appeared completely dark when observed between polarizing plates in crossed position because of inability of light to pass. This indicates the microemulsions produced from the SMEDDS were optically isotropic. Also no precipitation was observed for both formulations after 24 h.
The formulation was characterized for various parameters and the results are discussed below. The drug content of various self-microemulsifying formulations was found to be greater than 98 % of the theoretical value, which was considered quite satisfactory (Table 5). The effect of pH on globule size was studied to evaluate the effect of GI tract pH on the mean globule size and stability of emulsion. SMEDDS were diluted with different buffer systems and its effect on the mean globule size is given in Table 6. The formulations showed mean globule size in the range of 32-83 nm. However, an increase in the globule size was observed when pH 6.8 Phosphate buffer was used as dilution medium but it was still below 100 nm.
| Formulation | F2 | F5 |
|---|---|---|
| Drug content (%) | 100.01±0.98 | 99.42±0.87 |
Note: Data is expressed as mean±SD, (n=3)
Table 5: Drug content of optimized formulations.
| Formulation | F2 | F5 |
|---|---|---|
| 0.7 % HCl pH 1.45 | 32.06±0.29 | 58.39±1.97 |
| pH 4.5 (Acetate buffer) | 36.84±0.61 | 76.74±2.32 |
| pH 6.8 (Phosphate Buffer) | 38.16±3.44 | 83.97±4.21 |
Note: Data is expressed as mean±SD, (n=3)
Table 6: Effect of pH on particle size of selected SMEDDS.
The ability of a microemulsion to be diluted without any drug precipitation is essential for its use as a drug delivery vehicle since, after administration, it will almost certainly be diluted by body fluids. These studies were performed to study the robustness of the formulations to pH simulating GI tract conditions. SMEDDS were diluted with aqueous phase and allowed to stand for 6 h to determine their short-term physical stability. The resulting microemulsions were robust to all dilutions and did not show any separation or drug precipitation.
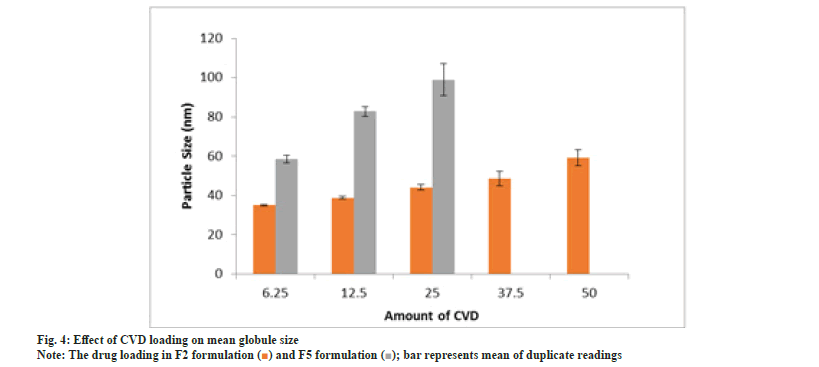
Effect of CVD loading on mean globule size is shown in fig. 4. Drug incorporation can have significant influence on mean globule size and needs to be investigated[24-26]. Formulation F5 upon dilution resulted in drug precipitation. This suggests F5 could not incorporate more than 25 mg CVD. An increase in globule size was observed with an increase in drug load in case of F2 and F5.
Centrifugation and freeze-thaw cycling are accelerated tests to determine the stability of microemulsions under stress conditions. Centrifugal force is used to determine the behavioral changes occurring in the colloidal particles in the presence of gravitational field. The formulation F2 was found to remain stable after centrifugation process, freeze-thaw cycle and no phase separation or drug precipitation was observed.
The dispersion/release profile of CVD from both optimized SMEDDS is indicated in fig. 5. The formulations were found to release more than 90 % drug in 5 min in all the three dissolution media i.e 0.7 % HCl (pH 1.45), pH 4.5 Acetate Buffer and pH 6.8 Phosphate buffer. The reference drug product showed the pH dependent drug release from the tablet formulation. The SMEDDS formulations results in the instantaneous dispersion of the formulation resulting in faster release as compared to Tablets formulation in all pH ranges. Also, the SMEDDS showed pH independent release indicating the drug is in the solution form and is readily available for absorption thought out the GI tract. Data is expressed as mean±SD, n=3.
A SMEDDS formulation of a poorly water soluble drug, CVD was formulated as direct filled hard gelatin capsules, which are convenient for oral administration. The formulation F2 and F5 were found to be the optimized formulation on the basis of results of pseudoternary phase diagram, in vitro dispersion/release, droplet size and physicochemical stability. The optimized formulation showed rapid self-emulsification in an aqueous media. The developed RP-HPLC method was validated and successfully applied for estimation of CVD from developed SMEDDS formulation of CVD. The results from the study show the effectiveness of SMEDDS formulation to enhance solubility and dissolution characteristics of sparingly soluble compounds like CVD.
Conflict of interest:
The authors declared no conflict of interest.
References
- Vipul PP, Tushar RD, Pankaj PK, Samir A, Rajesh AK. Self emulsifying drug delivery system: A conventional and alternative approach to improve oral bioavailability of lipophilic drugs. Int J Drug Develop Res 2010;2:859-70.
- Shukla JB, Patel SJ. Formulation and evaluation of self micro emulsifying system of candesartan cilexetil. Int J Pharm Pharm Sci 2010;2:143-6.
- Kim DS, Cho JH, Park JH, Kim JS, Song ES, Kwon J, et al. Self-microemulsifying drug delivery system (SMEDDS) for improved oral delivery and photostability of methotrexate. Int J Nanomed 2019;14:4949-60.
[Crossref] [Google Scholar] [PubMed]
- Dokania S, Joshi AK. Self-microemulsifying drug delivery system (SMEDDS)-challenges and road ahead. Drug Deliv 2015;22(6):675-90.
[Crossref] [Google Scholar] [PubMed]
- Stoschitzky K, Koshucharova G, Zweiker R, Maier R, Watzinger N, Fruhwald FM, et al. Differing beta‐blocking effects of carvedilol and metoprolol. Eur J Heart Fail 2001;3(3):343-9.
[Crossref] [Google Scholar] [PubMed]
- Tanwar Y, Chauhan C, Sharma A. Development and evaluation of carvedilol transdermal patches. Acta Pharm 2007;57(2):151-9.
[Crossref] [Google Scholar] [PubMed]
- Ubaidulla U, Reddy MV, Ruckmani K, Ahmad FJ, Khar RK. Transdermal therapeutic system of carvedilol: Effect of hydrophilic and hydrophobic matrix on in vitro and in vivo characteristics. AAPS Pharmscitech 2007;8(1):E13-20.
[Crossref] [Google Scholar] [PubMed]
- El-Say KM, Hosny KM. Optimization of carvedilol solid lipid nanoparticles: An approach to control the release and enhance the oral bioavailability on rabbits. PLoS ONE 2018;13(8):e0203405.
[Crossref] [Google Scholar] [PubMed]
- Hamed R, Awadallah A, Sunoqrot S, Tarawneh O, Nazzal S, AlBaraghthi T, et al. pH-dependent solubility and dissolution behavior of carvedilol-case example of a weakly basic BCS class II drug. AAPS Pharmscitech 2016;17:418-26.
[Crossref] [Google Scholar] [PubMed]
- International Conference on Harmonization. Validation of analytical procedures: Text and methodology Q2 (R1). In : International Conference on Harmonization 2005 Geneva: IFPMA.
- Desai NS, Nagarsenker MS. Design and evaluation of self-nanoemulsifying pellets of repaglinide. AAPS PharmSciTech 2013;14:994-1003.
[Crossref] [Google Scholar] [PubMed]
- Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm 2007;329(1-2):166-72.
[Crossref] [Google Scholar] [PubMed]
- Patel P, Solanki S, Mahajan A, Mehta F, Shah K. Preparation and characterization of novel self nano emulsifying drug delivery system of allopurinol. Res J Pharm Technol 2021;14(4):2108-14.
- Patel K, Padhye S, Nagarsenker M. Duloxetine HCl lipid nanoparticles: Preparation, characterization, and dosage form design. AAPS Pharmscitech 2012;13:125-33.
[Crossref] [Google Scholar] [PubMed]
- Pol AS, Patel PA, Hegde D. Peppermint oil based drug delivery system of aceclofenac with improved anti-inflammatory activity and reduced ulcerogenecity. Int J Pharm Biosci Technol 2013;1(2):89-101.
- Dholakiya A, Dudhat K, Patel J, Mori D. An integrated QbD based approach of SMEDDS and liquisolid compacts to simultaneously improve the solubility and processability of hydrochlorthiazide. J Drug Deliv Sci Technol 2021;61:102162.
- Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’drug delivery systems. Eur J Pharm Sci 2000;11:S93-8.
[Crossref] [Google Scholar] [PubMed]
- Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm 2000;50(1):179-88.
[Crossref] [Google Scholar] [PubMed]
- Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother 2004;58(3):173-82.
[Crossref] [Google Scholar] [PubMed]
- Bhandari V, Avachat A. Formulation and characterization of self emulsifing pellets of carvedilol. Brazil J Pharm Sci 2015;51:663-71.
- Shah M, Agrawal AG. Self-microemulsifying system. In: Colloid Science in Pharmaceutical Nanotechnology; 2019.
- Marti-Mestres G, Nielloud F. Main surfactants used in pharmaceutical field. In: Nielloud F, Marti-Mestres G, editors. Pharmaceutical Emulsions and Suspensions. Drug and Pharmaceutical Sciences. 2000; 105:1-18.
- Kim CK, Cho YJ, Gao ZG. Preparation and evaluation of biphenyl dimethyl dicarboxylate microemulsions for oral delivery. J Control Release 2001;70(1-2):149-55.
[Crossref] [Google Scholar] [PubMed]
- Subramanian N, Ray S, Ghosal SK, Bhadra R, Moulik SP. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol Pharm Bull 2004;27(12):1993-9.
[Crossref] [Google Scholar] [PubMed]
- Li P, Ghosh A, Wagner RF, Krill S, Joshi YM, Serajuddin AT. Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int J Pharm 2005;288(1):27-34.
[Crossref] [Google Scholar] [PubMed]
- Kang BK, Lee JS, Chon SK, Jeong SY, Yuk SH, Khang G, et al. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm 2004;274(1-2):65-73.
[Crossref] [Google Scholar] [PubMed]
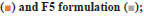 bar represents mean of duplicate readings.
bar represents mean of duplicate readings.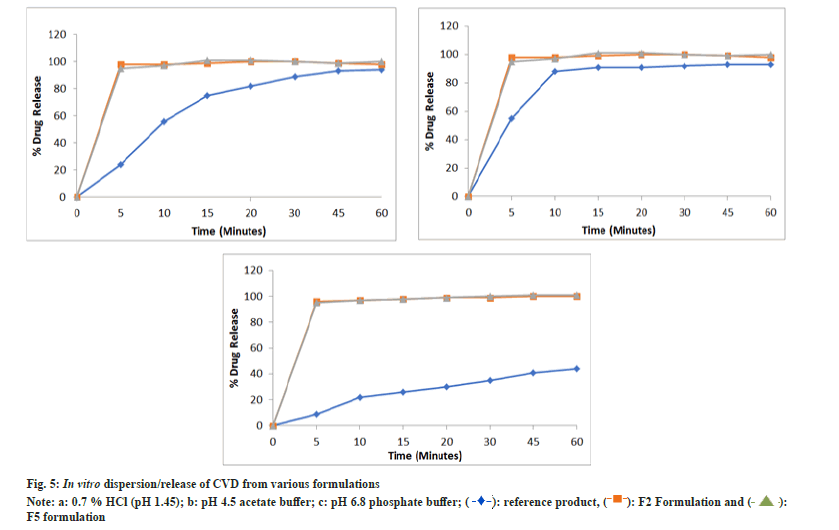
 F5 formulation.
F5 formulation.



