- *Corresponding Author:
- S. B. Shirsand
Department of Pharmaceutics, H.K.E. Society’s College of Pharmacy, Sedam Road, Gulbarga - 585 104, India
E-mail: shirsand@rediffmail.com
| Date of Submission | 16 March 2009 |
| Date of Revision | 20 April 2010 |
| Date of Acceptance | 20 July 2010 |
| Indian J Pharm Sci, 2010, 72 (4): 431-436 |
Abstract
Fast disintegrating tablets of lorazepam were prepared by effervescent method with a view to enhance patient compliance. A 3΂ full factorial design was applied to investigate the combined effect of two formulation variables: amount of crospovidone and mixture of sodium bicarbonate, citric acid and tartaric acid (effervescent material) on in vitro dispersion time. Crospovidone (2-8% w/w) was used as superdisintegrant and mixture of sodium bicarbonate, citric acid and tartaric acid (6-18% w/w) was used as effervescent material, along with directly compressible mannitol to enhance mouth feel. The tablets were evaluated for hardness, friability, thickness, drug content uniformity and in vitro dispersion time. Based on in vitro dispersion time (approximately 13 s); the formulation containing 8% w/w crospovidone and 18% w/w mixture of sodium bicarbonate, citric acid and tartaric acid was found to be promising and tested for in vitro drug release pattern (in pH 6.8 phosphate buffer), short-term stability and drug-excipient interaction. Surface response plots are presented to graphically represent the effect of independent variables (concentrations of crospovidone and effervescent material) on the in vitro dispersion time. The validity of the generated mathematical model was tested by preparing two extra-design check point formulations. The optimized tablet formulation was compared with conventional marketed tablet for drug release profiles. This formulation showed nearly eleven-fold faster drug release (t 50% 2.8 min) compared to the conventional commercial tablet formulation (t 50% >30 min). Short-term stability studies on the formulation indicated that there were no significant changes in drug content and in vitro dispersion time (P<0.05).
Keywords
32 full factorial design, citric acid and tartaric acid, crospovidone, fast disintegrating tablets, lorazepam, sodium bicarbonate
The major problem faced by many patients with conventional tablet dosage form is difficulty in swallowing. This problem is more apparent when drinking water is not easily available to the patient taking medicine. Hence, patients may not comply with prescription, which results in high incidence of ineffective therapy [1]. The fast dissolving drug delivery system is rapidly gaining acceptance as an important novel drug delivery system. This delivery system emerged from the desire to provide patient with more convenient medication, with better patient compliance than with conventional tablet dosage form. Bioavailability of the drug from this delivery system is significantly greater than from conventional tablets [2-4]. Lorazepam is a benzodiazepine derivative with marked antiepileptic properties. It may be used in the treatment of all types of epilepsy and seizures [5]. Epileptic patients have to strictly adhere to the dosage schedule and any non-compliance of dosage administration may lead to sub-therapeutic levels of drug in the systemic circulation, and hence recurrence of seizures. Since a fast dissolving tablet (FDT) of the drug rapidly disintegrates in the mouth without need of water, such a dosage form will definitely enhance the patient compliance, especially, during travelling or in situations where water is not easily available. Hence, there is an obvious need for the development of fast dissolving tablets to overcome patient non-compliance. Further effervescent tablets have several advantages; (a) they are outstanding due to ease of administration and improved absorption of the active drug through previous dissolution in a buffered medium, (b) effervescent systems can buffer the aqueous solution of drug, so that the stomach pH increases leading to prevention of degradation or inactivation of the active ingredient. This buffering effect (via carbonation) induces the stomach to empty quickly, usually within 20 min into small intestine and results in maximum absorption of active ingredient, (c) effervescent tablets are advantageous since the drug product is already in solution at the time it is consumed, making the absorption faster and more complete when compared to conventional tablet, (d) they dissolve fully in a buffered solution. Reduced localized contact in upper GIT leads to less irritation and greater tolerability. Buffering also prevents gastric acids from interacting with drugs themselves, which can be a major cause of stomach and esophageal upsets, (e) they retain their palatability after lengthy storage; moreover, they produce fizzy tablets, which may have better consumer appeal than the traditional dosage forms, (f) excellent stability that is inherent to effervescent formulations, particularly surpassing liquid forms, (g) drugs delivered using effervescent technology have predictable and reproducible pharmacokinetic profiles that are more consistent than tablets or capsules, (h) effervescent components aid in improving the therapeutic profiles of active ingredients. They also help in solubilization of poorly soluble drugs, (i) effervescence induces penetration enhancement of broad range of compounds ranging in size, structure and other physiological properties. Effervescent blend can be used to obtain programmed drug delivery, (j) in remote areas, especially where parenteral forms are not available due to prohibitive cost, lack of qualified medical staff, effervescent tablets could become an alternative, e.g., the use of chloroquine phosphate effervescent tablets for treating malaria and viral fever and (k) to solve the problems of physicochemical stability and high cost of transporting syrups, effervescent tablets provide a realistic solution. Aim of the present study was to develop and optimize such a FDT for lorazepam using simple and cost-effective methodology.
Materials and Methods
Lorazepam and crospovidone (CP) were gift samples from Centaur Chemicals Pvt. Ltd., Chickboli, India, and Wockhardt Research Centre, Aurangabad, India, respectively. Directly compressible mannitol (Pearlitol SD200), sodium stearyl fumarate (SSF) were generous gifts from Strides Arco Labs, Bangalore, Glenmark Ltd., Nashik and Alkem Labs Pvt Ltd, Mumbai, India. All other chemicals used were of analytical reagent grade.
Preparation of fast disintegrating tablets of lorazepam
Fast disintegrating tablets of lorazepam were prepared by effervescent method [6] according to the formulae given in Table 1. Lorazepam, mannitol, pineapple flavour, aspartame and cros-povidone were accurately weighed and sifted through sieve No. 44. Sodium bicarbonate and anhydrous citric acid and tartaric acid were pre-heated at a temperature of 80º to remove absorbed/ residual moisture and were thoroughly mixed in a mortar to get a uniform powder and then added to other ingredients. The ingredients after sifting through sieve No. 44 were thoroughly mixed in geometrical order. The blend thus obtained was directly compressed at 8 mm size to get a tablet of 150 mg weight.
| Ingredients (mg/Tablet) | Formulation code | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EF1 | EF2 | EF3 | EF4 | EF5 | EF6 | EF7 | EF8 | EF9 | C1 | C2 | |
| Lorazepam | 1.0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Crospovidone | 3.0 | 3.00 | 3.00 | 7.50 | 7.50 | 7.50 | 12.00 | 12.00 | 12.00 | 5.25 | 9.75 |
| Sodium bicarbonate | 9.0 | 18.00 | 27.00 | 9.00 | 18.00 | 27.00 | 9.00 | 18.00 | 27.00 | 13.50 | 22.50 |
| Citric acid+ tartaric acid | 9.0 | 18.00 | 27.00 | 9.00 | 18.00 | 27.00 | 9.00 | 18.00 | 27.00 | 13.50 | 22.50 |
| Aspartame | 1.5 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Flavour | 1.5 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Talc | 3.0 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Sodium stearylfumarate | 1.5 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Mannitol SD200 | 120.5 | 102.5 | 84.5 | 116.0 | 98.0 | 80.0 | 111.5 | 93.5 | 75.5 | 109.2 | 86.75 |
| Total | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 | 150.0 |
EF=Formulations prepared by effervescent method using cropsovidone as super-disintegrant at three different levels and mixture of sodium bicarbonate, citric acid and tartaric acid as effervescent material. C1 and C2 are extra design check-point formulations
Table 1: Factorial Design Formulations Of Lorazepam Prepared By Effervescent Method
Evaluation of fast dissolving tablets
To determine weight variation, twenty tablets were selected randomly from each formulation and were weighed individually using a Shimadzu digital balance (BL-220H). The individual weights were compared with the average weight for obtaining the weight variation [7]. Ten tablets from each formulation were selected randomly and their thickness was measured with a screw gauge for calculating thickness variation. Hardness of the tablets was measured using a Monsanto Hardness Tester (Pharmalab, Ahmedabad, India) and friability of a sample of twenty fast dissolving tablets was measured using a USP type-II Roche friabilator (Pharmalab, Ahmedabad, India). Pre-weighed tablets were placed in a plastic chambered friabilator attached to a motor revolving at a speed of 25 rpm for 4 min. The tablets were then dusted, reweighed and percentage weight loss (friability) was calculated (Table 2 and 3).
| Formulation code | Variable levels in coded form* | In vitro dispersion time | |
|---|---|---|---|
| X1 | X2 | ||
| EF1 | -1 | -1 | 57.19 |
| EF2 | -1 | 0 | 53.04 |
| EF3 | -1 | +1 | 43.64 |
| EF4 | 0 | -1 | 44.51 |
| EF5 | 0 | 0 | 32.71 |
| EF6 | 0 | +1 | 22.35 |
| EF7 | +1 | -1 | 25.09 |
| EF8 | +1 | 0 | 13.56 |
| EF9 | +1 | +1 | 13.18 |
| C1 | -0.5 | -0.5 | 42.24 |
| C2 | +0.5 | +0.5 | 31.13 |
Where-1=3.0 mg, 0=7.5 and +1=12 mg (X1 is the amount of crospovidone). Where -1=9.0 mg of NaHCO3 and 9.0 mg citric acid and tartaric acid, 0=18.0 mg of NaHCO3 and 18.0 mg citric acid and tartaric acid +1=12 mg of NaHCO3 and 12 mg citric acid and tartaric acid (X2 is the amount of effervescent materials). -0.5= 5.25 mg (X1 is the amount of crospovidone and 13.5 mg of NaHCO3 and 13.5 mg of citric acid + tartaric acid (X2 is the amount of effervescent material) +0.5=9.75 mg (X1 is the amount of crospovidone) and 22.5 mg of NaHCO3 and 22.5 mg citric acid and tartaric acid (X2 is the amount of effervescent material)
Table 2: Formulation And Evaluation Of 32 Full Factorial Design
| Parameters | Formulation code | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EF0 | EF1 | EF2 | EF3 | EF4 | EF5 | EF6 | EF7 | EF8 | EF9 | C1 | C2 | |
| Hardness* | 3.60± | 3.16± | 3.33± | 3.30± | 3.43± | 3.50± | 3.36± | 3.50± | 3.36± | 3.33± | 3.00± | 3.36± |
| (kg/cm2)± SD | 0.28 | 0.57 | 0.66 | 0.26 | 0.05 | 0.10 | 0.32 | 0.10 | 0.32 | 0.32 | 0.03 | 0.032 |
| Thickness (mm) | 2.4 | 2.74 | 2.85 | 2.80 | 2.78 | 2.90 | 2.90 | 2.56 | 2.54 | 2.70 | 2.90 | 2.85 |
| Friability (%) | 0.52 | 0.50 | 0.41 | 0.42 | 0.51 | 0.42 | 0.43 | 0.51 | 0.43 | 0.40 | 0.43 | 0.42 |
| In vitro | 180.27± | 57.19± | 53.04± | 43.64± | 44.51± | 32.71± | 22.35± | 25.09± | 13.56± | 13.18± | 42.24± | 31.13± |
| dispersion time* | 0.14 | 0.87 | 1.23 | 1.29 | 3.40 | 1.32 | 1.46 | 1.62 | 0.65 | 1.03 | 0.74 | 2.72 |
| (sec) ±SD | ||||||||||||
| Drug content* | 101.74± | 104.23± | 103.59± | 104.28± | 102.74± 103.36± 102.74± 103.25± 103.36± | 104.28± | 103.54± | 105.37± | ||||
| (%)±SD | 1.25 | 1.87 | 0.60 | 0.66 | 2.15 | 1.21 | 2.15 | 1.12 | 1.21 | 0.66 | 0.61 | 0.67 |
| Weight variation | 146-152 mg (within the IP limits of ±7.5%) | |||||||||||
*Average of three determinations. Formulation EF9 was selected as the best and used in further studies
Table 3: Evaluation Of Factorial Design Fdt Formulations
Drug content uniformity was determined by weighing ten tablets, pulverizing to a fine powder. A quantity of powder equivalent to 1 mg of lorazepam was extracted into methanol and the solution was filtered through a 0.22 μm membrane filter disc, (Millipore Corporation). The lorazepam content was determined by measuring the absorbance at 231 nm (UV/Vis spectrophotometer, Shimadzu 1700) after appropriate dilution with methanol. The drug content was determined using standard calibration curve. The mean percent drug content was calculated as an average of three determinations [8].
In vitro dispersion time was determined by placing one tablet in a beaker containing 10 ml of pH 6.8 phosphate buffer at 37±0.5º and the time required for complete dispersion was determined [9]. Wetting time was determined by carefully placing a tablet on to a twice folded circular tissue paper placed in a Petri-dish having internal diameter of 5cm containing 6 ml of water. The time required for water to reach the upper surface of the tablet and to completely wet it was noted as the wetting time. The weight of the tablet prior to placing in the Petri-dish was noted (wb) using a Shimadzu balance. The wetted tablet was removed and reweighed (wa). Water absorption ratio (R) was then determined according to the following equation; R=100×(wa– wb)/wb, where, wb and wa were tablet weights before and after water absorption, respectively.
In vitro drug release study
In vitro dissolution studies of the optimized fast dissolving tablets of lorazepam and commercial conventional tablet was performed according to USP XXIII Type-II dissolution apparatus (Electrolab, model TDT-06N) employing a paddle stirrer at 50 rpm using 900 ml of pH 6.8 phosphate buffer at 37±0.5º as dissolution medium [10]. One tablet was used in each test. Aliquotes of the dissolution medium (5 ml) were withdrawn at specific time intervals (2, 4, 6, 8, 10, 15 and 30 min) and replaced with the equal volume of fresh medium. The samples were filtered through 0.22 μm membrane filter disc and analyzed for drug content by measuring the absorbance at 233 nm. Drug concentration was calculated from the standard calibration curve and expressed as cumulative percent drug dissolved. The release studies were performed in replicates of six.
Stability testing
Accelerated stability studies on formulation EF9 were carried out by storing 15 tablets in an amber coloured rubber stoppered vials at 40o/75% RH over a period of 3 mo. At intervals of one month, the tablets were visually examined for any physical changes and changes in drug content. At the end of 3 mo period, the formulation was also subjected to dissolution studies.
Optimization of the formulation using 32 full factorial design
Based on the results of the preliminary trial formulations of 13 batches of three different combinations of effervescent materials, a 32 full factorial design was used for simultaneous evaluation of two formulation variables and their interaction, each at three levels and trials were performed at all nine possible combinations [11]. The best combination (blend of sodium bicarbonate-citric acid and tartaric acid) [12] of effervescent material was used in the optimization of the formulations. The effect of the independent variables, viz., crospovidone (X1) and blend of sodium bicarbonate-citric acid and tartaric acid (X2) on the dependent variable in-vitro dispersion time (Y1) was evaluated.
Results and Discussion
Lorazepam FDT were prepared by effervescent method; formulation optimization was done using a 32 full factorial design employing crospovidone as super-disintegrant and blend of sodium bicarbonate-citric acid and tartaric acid as an effervescent material, along with directly compressible mannitol (Pearlitol SD 200), which was used to enhance the mouth feel, based on the results of preliminary trial formulations. A total of nine formulations, a control formulation (EF0 without super disintegrant) and two extra-design check point formulations (C1 and C2 to check validity of the developed polynomial equation), were designed.
Powder blends were evaluated for the flow parameters such as angle of repose, tapped density, bulk density and Carr’s index. As the material was free flowing (angle of repose values were found to be <30o and Carr’s index <15%).
The tablets were evaluated for weight variation, uniformity of drug content, hardness, friability, in vitro dispersion time, in vitro dissolution studies, tablets obtained were of uniform weight (due to uniform die fill), with acceptable variation as per IP specifications (±7.5%). Drug content was found to be in the range of 102-105%, which is within acceptable limits. Hardness of the tablets was found to be 3.0 to 3.5 kg/cm2. Friability below 1% was an indication of good mechanical resistance of the tablets. Formulation EF9 was found to be promising and displayed an in vitro dispersion time of 13 s, which facilitates faster dispersion in the mouth.
Based on the results of preliminary trail formulations of 13 batches, optimization of the FDT formulation has been done using a 32 full factorial design (formulations EF1 to EF9). PCP Disso 2000 V3 software was used to develop a polynomial equation for the dependent variable in vitro dispersion time. Formulation EF9 containing 8% w/w crospovidone, 18% w/w mixture of sodium bicarbonate-citric acid and tartaric acid was found to be promising with an in vitro dispersion time of 13 s against 180 s displayed by control formulation (EF0), which does not contain the super-disintegrant crospovidone.
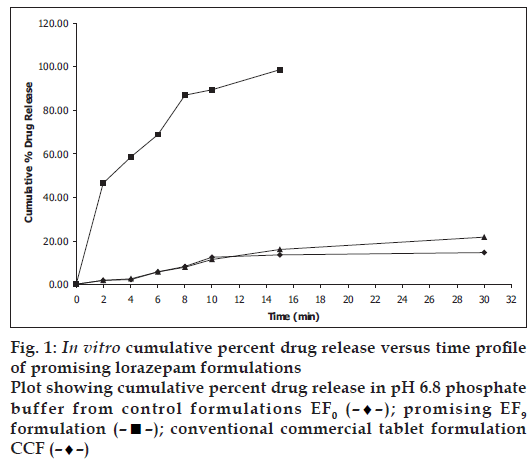
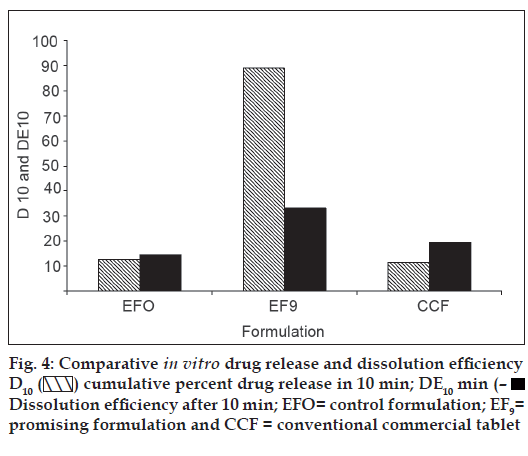
In vitro dissolution parameters, including dissolution efficiency [13] of the promising formulation EF9, the control (EF0) and the commercial conventional tablet formulation (CCF) are shown in Table 4 and the dissolution profiles, and dissolution parameters depicted in figs. 1 and 4 respectively. This data reveals that overall, the formulation EF9 has shown nearly eleven-fold faster drug release (t50% 2.8 min) when compared to CCF (t50% > 30 min).
| Formulation code | D5 (%) | D10 (%) | DE5min (%) | DE10min (%) | t50% (min) | t70% (min) | t90% (min) |
|---|---|---|---|---|---|---|---|
| EF0 | 5.00 | 12.59 | 6.00 | 14.43 | >30 | >30 | >30 |
| EF9 | 62.00 | 89.00 | 13.93 | 33.02 | 2.80 | 6.10 | 9.0 |
| CCF | 4.00 | 11.38 | 10.00 | 19.45 | >30 | >30 | >30 |
EF0 is control formulation, EF9 is promising fast disintegrating tablet formulation, CCF is conventional commercial tablet formulation, D5 is percent drug released in 5 min, D10 is percent drug release in 10 min, D15 is percent drug release in 15 min, DE10min is dissolution efficiency at 10 min, t50% is time for 50% drug dissolution, t70% is time for 70% drug dissolution, t90% is time for 90% drug dissolution
Table 4: In Vitro Dissolution Parameters In Ph 6.8 Phosphate Buffer
IR spectroscopic studies indicated that the drug is compatible with all the excipients. The IR spectrum of EF9 showed all the characteristic peaks of lorazepam, thus confirming that no interaction of drug occurred with the components of the formulation. Short-term stability studies of the above formulation indicated that there are no significant changes in drug content and in vitro dispersion time at the end of 3 mo period (P<0.05).
Polynomial equation for 32 full factorial design with two independent variables i.e., proportion of crospovidone (X1) and proportion of sodium bicarbonate, citric acid and tartaric acid as effervescent material (X2), at three levels is [14] Y = b0 + b1X1 + b2X2+ b12X1X2 + b11X12 + b22X22, where Y is dependent variable, b0 arithmetic mean response of nine batches, and b1 estimated coefficient for factor X1. The main effects (X1 and X2) represent the average results of changing one factor at a time from its low to high value. The interaction term (X1X2) shows how the response changes when two factors are simultaneously changed. The polynomial terms X12 and X22 are included to investigate nonlinearity.
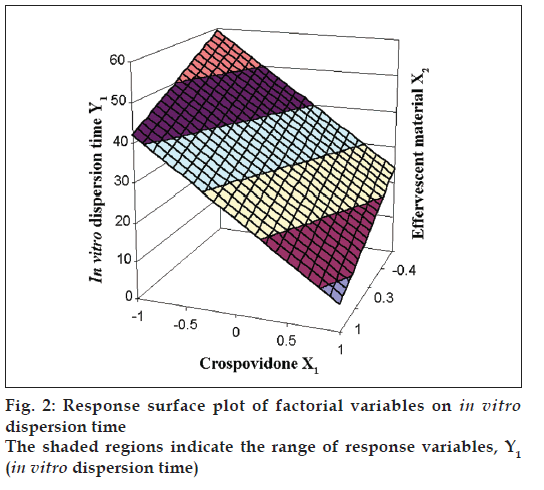
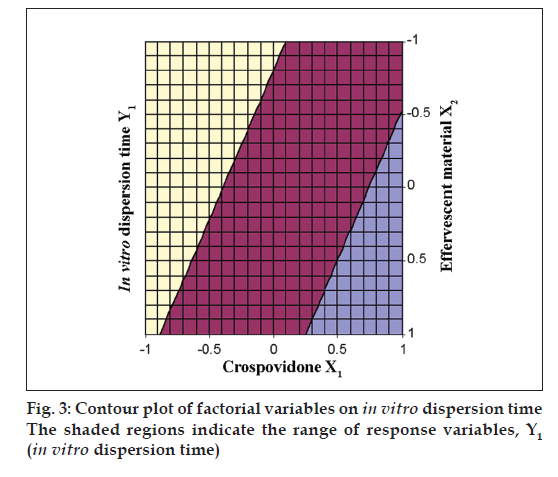
The Eqn. derived for in vitro dispersion time of the factorial formulations is Y1= 33.11–8.83 X1–4.33 X2. The negative sign for coefficients of X1 and X2 indicate that as the concentration of disintegrants increases, in vitro dispersion time decreases. The data clearly indicates that the in vitro dispersion time values are strongly dependent on the selected independent variables. Validity of the above equation was verified by designing two extra design check point formulations (C1 and C2) and determining the in vitro dispersion time. The in vitro dispersion time values predicted from the equation for these formulations are 44.73 and 17.73 s, where those observed from experimental results are 46.27 and 19.95 s, respectively. The closeness of the predicted and observed values for C1 and C2 in the method indicates validity of derived equation for the dependent variable (in vitro dispersion time). The computer generated response surface and contour plots for the dependent variable are shown in fig. 2 and 3, respectively.
The results a 32 full factorial design reveal that the amounts of crospovidone (X1) and effervescent material (X2) significantly affect the dependent variable (Y1), the in vitro dispersion time. It is thus concluded that, by adopting a systematic formulation approach, an optimum point can be reached in the shortest time with minimum efforts. Effervescent technique would be an effective approach compared with the use of more expensive adjuvants in the formulation of fast dissolving tablets with improved drug dissolution, patient compliance, convenience and acceptability.
References
- Seager H. Drug delivry products and Zydis fast dissolving dosage forms. J Pharm Pharmacol 1998;50:375-82.
- Indurwade NH, Rajyaguru TH, Nakhat PD. Novel approach- Fast dissolving tablets. Indian Drugs 2002;39:405-9.
- Kuchekar BS, Badhan AC, Mahajan HS. Mouth dissolving tablet: A novel drug delivery system. Pharm Times 2003;35:7-14.
- Reddy LH, Ghosh BR. Fast dissolving drug delivery systems: A review of the literature. Indian J Pharm Sci 2002;64:331-6.
- Sweetman SC. editor, Martindale: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002. p. 688-9.
- Kaushik D, Dureja H, Saini TR. Formulation and evaluation of olanzapine mouth dissolving tablets by effervescent formulation approach. Indian Drugs 2002;41:410-2.
- Banker GS, Anderson NR. The theory and practice of industrial pharmacy. In: Lachman L, Libermann HA, Kanig JL, editors. 3rd ed. New Delhi: Varghese Publishing House; 1987. p. 293-9.
- Ministry of Health and Family Welfare. Indian Pharmacopoeia: New Delhi: Controller of Publications, Govt. of India; 1996. p. 735.
- Bi YX, Sunada H, Yonezawa Y, Danjo K. Evaluation of rapidly disintegrating tablets by direct compression method. Drug Dev Ind Pharm 1999;25:571-81.
- Bhagwati ST, Hiremath SN, Sreenivas SA. Comparative evaluation of disintegrants by formulating cefixime dispersible tablets. Indian J Pharm Edu Res 2005;39:194-7.
- Bolton S. Pharmaceutical Statistics. 2nd ed. New York: Marcel Decker Inc; 1990. p. 234-6.
- Ansel HC, Allen LV, Popovich NG, editors. Pharmaceutical Dosage Forms and Drug Delivery Systems. 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2000. p. 176.
- Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol 1974;27:48-9.
- Gohel M, Patel M, Amin A, Agarwal A, Dave R, Bariya N. Formulation design and optimization of mouth dissolve tablets of nimesulide using vacuum drying technique. AAPS PharmSciTech 2004;5:e36.
 cumulative percent drug release in 10 min; DE10 min (–■–) Dissolution efficiency after 10 min; EFO= control formulation; EF9=
promising formulation and CCF = conventional commercial tablet
cumulative percent drug release in 10 min; DE10 min (–■–) Dissolution efficiency after 10 min; EFO= control formulation; EF9=
promising formulation and CCF = conventional commercial tablet



