- *Corresponding Author:
- P. Panzade
Shri Bhagwan College of Pharmacy, Department of Pharmaceutics, Aurangabad-431 003, India
E-mail: prabhakarpanzade@gmail.com
| Date of Submission | 03 October 2013 |
| Date of Revision | 24 December 2014 |
| Date of Acceptance | 20 May 2015 |
| Indian J Pharm Sci 2015;77(3):267-273 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
The objective of present study was to formulate directly compressible orodispersible tablets of quetiapine fumarate by sublimation method with a view to enhance patient compliance. A full 3 2 factorial design was used to investigate the effect of two variables viz., concentration of Indion 414 and camphor. Indion 414 (3-5 % w/w) was used as superdisintegrant and camphor (5-15 % w/w) as subliming agent. The tablets were evaluated for thickness, weight variation, hardness, friability, content uniformity, wetting time, porosity, in vitro disintegration time and in vitro drug release. The formulation containing 5% w/w of Indion 414 and 5% w/w camphor was emerged as promising based on evaluation parameters. The disintegration time for optimized formulation was 18.66 s. The tablet surface was evaluated for presence of pores by scanning electron microscopy before and after sublimation. Differential scanning colorimetric study did not indicate any drug excipient incompatibility, either during mixing or after compression. The effect of independent variables on disintegration time, % drug release and friability is presented graphically by surface response plots. Short-term stability studies on the optimized formulation indicated no significant changes in drug content and in vitro disintegration time. The directly compressible orodispersible tablets of quetiapine fumarate with lower friability, greater drug release and shorter disintegration times were obtained using Indion 414 and camphor at optimum concentrations.
Keywords
Orodispersible tablet, factorial design, Indion 414, sublimation, quetiapine fumarate
Solid oral dosage forms offers noncompliance to patient owing to problems such as dysphagia, risk of choking, and hand tremors. Solid dosage forms also present substantial difficulties in patients such as children, mentally challenged, uncooperative and patient on reduced fluid diet [1]. Moreover, pediatric patients may have ingestion problems owing to underdeveloped muscular and nervous control [2,3]. Also utility of orally administered conventional tablets is limited in conditions of water unavailability [4].
Several innovative drug delivery systems have been documented to conquer the problem of conventional tablets. Novel solid oral dosage form, which disintegrates and dissolves rapidly in saliva without need for drinking water is orodispersible tablet. These tablets usually dissolve within 15 s to 2 min. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach and produce rapid onset of action. Hence, bioavailability of such drug is significantly greater than conventional tablet dosage forms due to avoidance of presystemic metabolism. An orally disintegrating tablet (ODT) is defined as a solid dosage form that dissolves or disintegrates quickly in the oral cavity without the need of water for administration [5]. US-FDA defines ODT as a solid dosage form which, contains a medicinal substance or active ingredient which, disintegrates rapidly within a matter of seconds when placed upon a tongue [6]. The presence of a highly porous surface in the tablet matrix is the key factor for rapid disintegration of ODT. The major advantage from industrial prospect would be extended product life cycle by product differentiation and patent life extension.
Quetiapine fumarate is atypical antipsychotic drug, used in the treatment of schizophrenia and bipolar disorders [7]. However drug is extensively metabolised by the liver limiting its oral bioavailability and making it suitable candidate. The prevalence of psychotic diseases has been increased substantially. Furthermore, approximately 2.4 and 5.7 million American adults or about 1.1 and 2.6 percent of the population age 18 and older get affected by schizophrenia and bipolar disorder, respectively [8-10]. The patients would be benefitted by proposed drug delivery with sudden episode of psychotic attack and need to calm down quickly.
The present study was undertaken to develop orodispersible tablets of quetiapine fumarate with shorter disintegration time, greater drug release and lesser friability with a prospect of assisting various patients who have difficulty in swallowing conventional dosage forms.
Materials and Methods
Quetiapine fumarate was obtained as gift sample from Alkem Pvt. Ltd, Mumbai, India. Pearlitol SD-200, Sucralose, camphor, magnesium stearate, and talc were procured as gift samples from Research Lab Fine Chem Industries, Mumbai. Indion 414 was received from Ion Exchange India Ltd, Gujarat. All other chemicals were of analytical reagent grade.
Preparation of orodispersible tablets by sublimation method
All of the formulation components other than lubricant and glidant were accurately weighed, passed through 60-mesh sieve and mixed in vertical blendor for 30 min. Talc and magnesium stearate were passed through 80-mesh sieve, mixed with above blend for 10 min and resultant blend was directly compressed into tablets. The amount of all tablet components other than superdisintegrants and Perlitol SD 200 were kept constant. Round concave tablets of 100 mg in weight and 7 mm diameter were prepared using Cadmach 13 station single sided rotary tablet press. Table 1 outlines the compositions of various ODT formulations studied. Compressed tablets were subjected to the sublimation process in hot air oven at 50° for 6 h [11].
Evaluation of precompression parameters of orodispersible tablets
Prior to compression, powder blends were evaluated for flow and compressibility parameters. Flow properties of powder were determined by angle of repose and compressibility by Carr’s index and Hausner ratio [12,13].
Evaluation of postcompression parameters, thickness and weight variation
The thickness of the tablets was measured using a digital Vernier caliper. Five tablets of each formulation were picked randomly and thickness of each of these tablets was measured. The results are expressed mean±standard deviation (SD) [14]. Twenty tablets were selected at random and average weight was determined using an electronic balance (Shimadzu). Tablets were weighed individually and compared with average weight [15].
Hardness and friability
Five tablets were randomly selected from each batch and hardness of tablets was determined by using Monsanto hardness tester. The mean values and standard deviation for each batch were calculated. The friability of tablets was measured using USP type Roche friabilator. Preweighed tablets (equivalent to 6.5 g) were placed in plastic chambered friabilator attached to motor revolving at a speed of 25 rpm for 4 min. The tablets were then dedusted, reweighed, and percent weight loss was calculated using the formula, % friability=((initial weight–final weight)/ initial weight)×100.
Drug content
Twenty tablets were weighed and powdered. Powder equivalent to a single dose of quetiapine was weighed and assayed for drug content at 248 nm using a UV/Vis double beam spectrophotometer (Jasco V-530). The UV method was validated according to ICH guidelines (Q2 R1).
In vitro disintegration time
The digital tablet disintegration test apparatus (Veego) was used to determine in vitro disintegration time (DT) using distilled water at 37±2°. The time (s) taken by tablet for complete disintegration with no residue remaining in apparatus was recorded as mean±SD [16].
In vitro drug release study
The drug release studies were performed using the USP dissolution test apparatus (VDA-6DR USP Stds.,Veego) employing paddle method. The dissolution test was performed using 900 ml of 0.1 N hydrochloric acid at 37±0.5° and paddle speed of 50 rpm. Samples (5 ml) were collected at predetermined time intervals (1 min) and replaced with equal volume of fresh medium. The study was continued for 10 min, samples were then filtered through 0.45 μm membrane filter [17] and analyzed at 248 nm using UV/Vis spectrophotometer.
Wetting time
Six circular tissue papers of 10 cm diameter were placed in a Petri dish and 10 ml of water containing amaranth dye was added to it to identify complete wetting of tablet surface. A tablet was carefully placed on the surface of tissue paper in Petri dish at ambient temperature. The time taken by water to reach upper surface of the tablet and to completely wet the tablet was noted as wetting time. The study was performed in triplicate and time was recorded using stopwatch.
Measurement of tablet porosity
Porosity of the tablets was calculated from the weight of the tablet (W), tablet volume (V), and true density of powder (ρ) using following Eqn [18], %porosity=((1−weight of Tablet, W)/ volume V)×density (ρ). The true density of powder was determined by a pycnometer.
Drug-excipient compatibility study
Preliminary compatibility studies were performed in closed vial using hot air oven and autoclave. The physical mixture (quetiapine+Indion 414, quetiapine+camphor) were prepared in 1:1 ratio by triturating in mortar and pestle for 10 min. These samples were kept at 40º for 1 mo in hot air oven and at 121º, 15 lb pressure for 15 min in autoclave. Furthermore, samples were observed for their appearance and drug content was determined by assay.
Differential scanning calorimetry
DSC was used to characterize thermal properties of quetiapine, physical mixtures of quetiapine with Indion 414 and camphor, and compressed ODT tablet. The DSC thermograms were recorded using TA-60 thermal analyzer (Shimadzu). The samples were hermetically sealed in aluminium pans and heated at a constant rate of 20°/min over temperature range of 50 to 200°. Inert atmosphere was maintained by purging nitrogen gas at flow rate of 50 ml/min.
IR spectroscopy
FTIR spectra were recorded for quetiapine, physical mixture, and compressed tablet using IR-spectrophotometer (Bruker alpha T, India). The samples were prepared in KBr dish and scanned over 400 to 4000 cm-1.
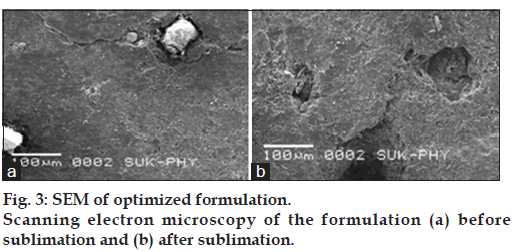
Scanning electron microscopy
The surface morphology of optimized formulation before and after sublimation of camphor was studied using (JSM-6360, Jeol Ltd., Japan). The tablet surface was sputter coated for 10 min with gold by using fine coat ion sputter and examined under SEM.
Stability study
The optimized formulation was subjected to stability study according to ICH guidelines, at room temperature, 30±2°/60%RH±5% and 40±2°/75% RH±5% condition in stability chamber (HMG, India) for three mo. Tablets were assayed for drug content for 90 d at the interval of one mo.
Experimental design
Preliminary trial formulations of quetiapine fumarate were designed by sublimation method using two superdisintegrant (Indion 414 and Kyron T-314) in different concentration (0.5-5% w/w) along with camphor (5-15% w/w) as subliming agent. From preliminary studies, Indion 414 was found superior hence selected for further investigation. The 32 factorial design was used for the optimization of variables (Design Expert 8.0.7.1). The two independent factors, concentration of Indion-414 (X1) and concentration of camphor (X2), were set to three different levels and experimental trials were performed for all nine possible combinations. The dependent responses measured, were DT (Y1), friability (Y2), and percent drug release (Y3). The experimental design is outlined in Table 1.
| Ingredient (mg) | Formulation code | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | |
| QuetiapineFumarate | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 |
| Indion?414 | 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | 5 |
| Camphor | 5 | 5 | 5 | 10 | 10 | 10 | 15 | 15 | 15 |
| Sucralose | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Magnesium Stearate | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Talc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Pearlitol SD?200 | 63 | 62 | 61 | 58 | 57 | 56 | 53 | 52 | 51 |
| Total weight | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 1: 32 Factorial Design Formulations
Results and Discussion
The present study was focused on development of ODTs of quetiapine fumarate that disintegrate within few seconds. Preliminary studies were performed to optimize the concentrations of superdisintgrant, subliming agent and other excipients in the formulation. The ODTs were prepared by sublimation method employing Indion 414 as superdisintegrants and camphor as subliming agent. Indion 414 is effective at low concentration, nontoxic in human and can be used in direct compression hence was selected as superdisintegrant. Directly compressible mannitol (Pearlitol SD 200) was used as a diluent owing to its water solubility, disintegrant property and pleasant mouth feel. Moreover, it produces cooling sensation due to its negative heat of solution. Sucralose was employed as sweetening agent as unpleasant taste could be masked. The factorial formulations were evaluated for flow properties and all the formulations exhibited good flow properties. The various flow parameters are presented in Table 2.
| Formulation code | Bulk density (g/cm3) | Tapped density (g/cm3) | Carr’s index (%) | Hausner ratio | Angle of repose (θ) | Flow |
|---|---|---|---|---|---|---|
| F1 | 0.795 | 0.84 | 18.36 | 1.19 | 29.24 | Good |
| F2 | 0.816 | 0.85 | 18.51 | 1.15 | 29.68 | Good |
| F3 | 0.743 | 0.91 | 17.36 | 1.31 | 26.56 | Good |
| F4 | 0.82 | 0.86 | 15.86 | 1.21 | 27.92 | Good |
| F5 | 0.73 | 0.96 | 13.20 | 1.18 | 29.24 | Good |
| F6 | 0.71 | 0.88 | 11.85 | 1.22 | 26.54 | Good |
| F7 | 0.72 | 0.84 | 12 | 1.10 | 27.92 | Good |
| F8 | 0.752 | 0.90 | 11.62 | 1.19 | 29.68 | Good |
| F9 | 0.86 | 0.95 | 13.40 | 1.14 | 27.02 | Good |
Table 2: Flow Properties Of Designed Formulations
The result of post compression parameters indicated that, all the formulated tablets were of uniform weight with acceptable weight variation and thickness. Hardness of all formulations was maintained at 3-3.5 kg/cm2 and friability loss was between 0.33 to 1.14%. The hardness and friability studies revealed that the tablets possessed good mechanical resistance. The sublimating agent increased the friability of tablets that may be attributed to increased porosity. The ODTs showed Drug content in the range of 98-101%, which, was within acceptable limits. The results are depicted in Table 3.
| Batchcode | Hardness(kg/cm2) | Thickness(mm) | Drugcontent (%) | In vitroDT (sec) | Wettingtime (sec) | Friability(%) | % Drug releasein 1 min | % Porosity |
|---|---|---|---|---|---|---|---|---|
| F1 | 3?3.5 | 2.60±0.20 | 99.94±0.37 | 29.08±0.98 | 29.16±0.98 | 0.383±0.12 | 51.32±0.1 | 12.76±0.96 |
| F2 | 3?3.5 | 2.66±0.35 | 98.31±0.14 | 24.16±0.98 | 23.33±1.50 | 0.334±0.10 | 62.46±0.1 | 13.48±1.49 |
| F3 | 3?3.5 | 2.63±0.30 | 100.9±0.73 | 18.66±1.03 | 16.66±1.21 | 0.367±0.10 | 72.42±0.3 | 14.64±1.65 |
| F4 | 3?3.5 | 2.66±0.20 | 98.83±0.11 | 26.83±1.47 | 23.83±1.32 | 0.726±0.17 | 55.88±0.6 | 23.71±0.97 |
| F5 | 3?3.5 | 2.66±0.15 | 98.26±0.17 | 23.16±0.75 | 22.5±1.51 | 0.68±0.10 | 68.83±0.1 | 25.36±1.71 |
| F6 | 3?3.5 | 2.63±0.32 | 99.15±0.13 | 19.33±1.03 | 16.8±1.60 | 0.60±0.15 | 74.64±0.1 | 27.69±1.07 |
| F7 | 3?3.5 | 2.63±0.15 | 99.04±0.05 | 24.5±1.048 | 22.66±1.36 | 1.14±0.14 | 55.39±0.1 | 39.69±1.07 |
| F8 | 3?3.5 | 2.60±0.20 | 98.85±0.03 | 22±0.89 | 19.16±1.16 | 1.13±0.16 | 75.13±0.6 | 40.91±0.81 |
| F9 | 3?3.5 | 2.63±0.15 | 99.16±0.05 | 17.66±1.36 | 16.33±1.21 | 1.11±0.14 | 75.38±0.1 | 41.01±1.25 |
Table 3: Evaluation Of Factorial Design ODT Formulations
The most important parameter that needs to be optimized in the development of mouth dissolving tablets and selection of optimized formulation is DT of tablets. DT was decreased with increasing concentration of superdisintegrant. At low concentration of superdisintegrant tablets showed high DT that may be owing to insufficient swelling of tablet. But at higher concentration DT was decreased considerably (Table 3) due to optimum swelling of tablets required for effective disintegration and wicking action of superdisintegrant. Formulation F3 was found promising with lowest DT of 18.66 s.
The wetting time for all formulations was found between 16-30 s (Table 3). This wide variation was observed due to developmental changes in the formulation to attain preliminary objectives. Formulations F3, F6 and F9 exhibited lowest wetting time but difference was not significant. However wetting time was decreased with increasing concentration of camphor except F6 formulation showed slight increase in wetting time but difference was not significant among various formulations. The decrease in wetting time could be attributed to increased number of pores on the tablet surface owing to sublimation of camphor from the tablets. Furthermore, as F3 formulation showed comparable wetting time at low concentration of subliming agent, it was considered for further study (Table 3).
All the formulations exhibited % porosity in the range of 12.76 to 41.01 for camphor concentration in the range of 5 to 15 mg (Table 3). It explicitly indicates that % porosity was increased with increasing concentration of subliming agent. However optimum porosity was observed for F3 formulation using low concentration of subliming agent as evident from lower wetting time. Moreover high % porosity was observed for other formulations at higher concentration of subliming agent but that led to enhanced friability. Hence high porosity led to substantial decrease in wetting time owing to faster water uptake and also facilitated wicking action of superdisintegrant bringing about faster disintegration.
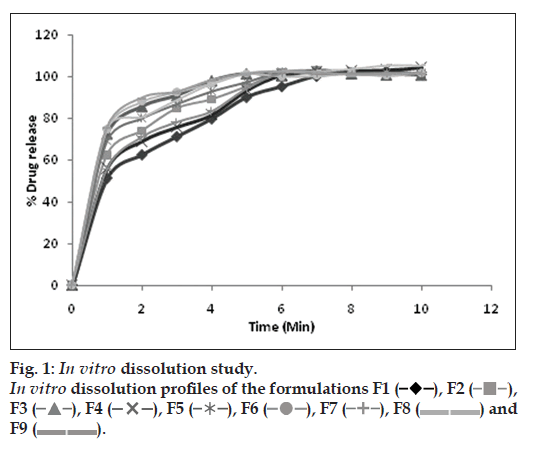
The aim of study was to assess in vitro dissolution behavior of developed formulations. The drug released in 1 min was considered and the results are presented in Table 3 and fig. 1. The results indicate that F9 formulation exhibited maximum % drug release (75.38±0.08). However F3 formulation showed comparable % drug release at low concentration of superdisintegrant and camphor hence selected for further evaluation.
Preliminary compatibility studies of drug and excipients in hot air oven at 40º for 1 mo and at 121º, 15 lb pressure for 15 min in autoclave revealed no change in color, odour and assay. However compatibility was further confirmed by DSC and FTIR studies. The DSC and FTIR studies revealed that drug and excipients are compatible with each other. The substantial changes in IR absorption bands and DSC thermograms were not observed for drug in physical mixture and tablet formulation as shown in fig. 2.
The formation of pores was confirmed by SEM analysis by subjecting tablet before and after sublimation. It was evident from the results presented in fig. 3 that pores were formed on the surface of tablet after sublimation of camphor.
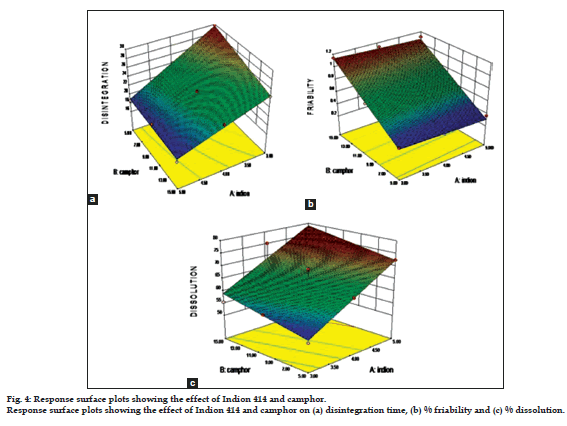
Analysis of variance for disintegration study, % drug release at 60 s (%DR60sec) and friability was performed. The coefficients X1 (Indion 414) and X2 (Camphor) showed significant effect (P<0.05) on selected responses.
The response surface plots for dependent variables %DR60sec, disintegration time and friability were generated and the effect of independent variables, X1 and X2 on the responses was studied (fig. 4).
The effect of formulation variables on disintegration time can be described by the model Eqn, Y1 (disintegration time) = +22.91-4.25X1-1.42X2+1.08X1X2. The negative sign for coefficient X1 and X2 indicated that as the concentration of superdisintegrant and camphor increased, DT decreased (R2=0.9881) indicating good correlation between independent and dependent variables. The term with (P<0.01) was considered significant.
The parameter friability can be described by the model Eqn., Y2 (friability)=+0.72-0.027X1+0.38X2, the negative sign for coefficient X1 suggests increase in concentration of superdisintegrant decreased the friability and positive X2 indicates that as concentration of camphor increasesd friability also increased. The effect of independent variables on friability was significant (P<0.01) and indicates good correlation (R2=0.9836).
The model Eqn for % drug reléase was Y3 (% drug release)=+65.72+9.98X1+3.28X2, the positive sign for X1 and X2 indicated that as the concentration of superdisintegrant and camphor increased, percent drug release also increased. R2 value of 0.8972 for percent drug release indicating good correlation between independent and dependent variable. The term with (P<0.01) was considered significant.
Stability study was conducted on optimized formulation as per ICH guidelines. The parameters evaluated were color, odor, hardness, friability, disintegration time, % drug release and drug content. The optimized formulation was found stable after evaluation of these parameters at different stability conditions and the results are given in Table 4.
| Formulation parameter | Ambientcondition | 30±2º/65±5% RH | 40±2º/75±5% RH |
|---|---|---|---|
| Colour | White | White | White |
| Odour | No | No | No |
| Hardness | 3?3.5 | 3?3.5 | 3.5 |
| Friability | 0.367±0.10 | 0.394±0.12 | 0.417±0.14 |
| Drug content (%) | 100.96±0.73 | 99.15±0.13 | 98.88±0.11 |
| Disintegration time (sec) | 18.85±0.34 | 18.68±0.27 | 18.74±0.44 |
| % Drug release | 72.53±0.32 | 71.52±0.29 | 70.42±0.36 |
Table 4: stability study of optimized Formulation
The directly compressible ODTs of quetiapine fumarate with shorter disintegration time, greater drug release and low friability (good mechanical strength) were obtained using Indion 414, camphor and other excipients at tested concentrations. The formulation F3 was selected as optimum owing to less DT, greater drug release and other evaluation parameters using minimum concentration of superdisintegrant and subliming agent. The results of 32 factorial design revealed that selected variables, Indion 414 and camphor have significant effect on dependant responses. Hence, using experimental design optimum formulation can be developed in shorter time. Sublimation would be an effective approach for the development of ODTs.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
References
- Battu SK, Michael AR, Soumyajit M, Madhusudan RY. Formulationand evaluation of rapidly disintegrating fenoverine tablets: Effect of superdisintegrants. Drug DevInd Pharm 2007;33:1225-32.
- Hirani JJ, Dhaval RJ, Vadalia RK. Orally Disintegrating Tablets: A Review. Trop J Pharm Res 2009;8:161-72.
- Chang RK, Guo X, Burnside BA, Couch RA. Fast-dissolving tablets. Pharm Tech 2000;24:52-9.
- Koizumi K. Watanabe Y, Morita K, Utoguchi N, Matsurnoto M. New method of preparing high-porosity rapidly saliva soluble compressed tablets using mannitol with camphor, a subliming material. Int J Pharm 1997;152:127-31.
- Badgujar BP, Mundada AS. The technologies used for developing orally disintegrating tablets: A review. Acta Pharm 2011;61:117-39.
- FDA. Draft Guidance for Industry: Orally disintegrating tablets Rockville. U. S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research; 2008 MD:1-3.
- Rang PH, Dale MM. Pharmacology in antipsychotic drugs. 6th ed. Philadelphia: Elsevier publication; 2007.
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National comorbidity survey replication (NCS-R). Arch Gen Psychiatry 2005;62:617-27.
- U.S. Census Bureau Population Estimates by Demographic Characteristics. Table 2: Annual estimates of the population by selected age groups and sex for the United States: April 1, 2000 to July 1, 2004 (NC-EST2004-02) Source: Population Division, U.S. Census Bureau Release Date: June 9, 2005. Available from: http://www.census.gov/ popest/national/asrh/ [Last accessed on 2013 Feb 04].
- Robins LN, Regier DA. Psychiatric disorders in America: The Epidemiologic Catchment Area Study. New York: The Free Press; 1991.
- Roser BJ, Blair J. Rapidly soluble oral dosage form, methods of making the same and composition thereof. 1998, US patent 5762961.
- Carter SJ, editors. Copper and Gun’s: Tutorial Pharmacy. New Delhi: CBS Publishers and Distributors; 1998.
- Aulton ME, editors. Pharmaceutics: The science of Dosage form Design. London: Churchill Livingstone; 1998.
- Lachman L, Lieberman HA. Pharmaeutical Dosage Forms of Tablets. United States: Marcel Dekker; 1981.
- Indian Pharmacopoeia. Vol. 2. Ghaziabad: Indian Pharmacopeal Convention; 1996.
- Basak SC, Selvin CDS, Sabapathy R. Formulation and in vitroevaluation of amoxicillin dispersible tablets. Indian Pharmacist 2006;5:71-4.
- Sinco PJ, editor. Martin’s Physical Pharmacy and Pharmaceutical Sciences. New Delhi: Lippincott Williams and Wilkins; 1994.
- Masaaki S, Toru M, Shinji N, Koji M, Hiroyuki Y. Factors affecting the characteristics of rapidly disintegrating tablets in the mouth prepared by the crystalline transition of amorphous sucrose. Int J Pharm 2005;296:64-72.
 ), F2( ? ), F3 (
), F2( ? ), F3 ( ), F4 (
), F4 ( ), F5 (
), F5 ( ), F6 (? ), F7 ( ?), F8 ( ??) and F9 ( ??).
), F6 (? ), F7 ( ?), F8 ( ??) and F9 ( ??).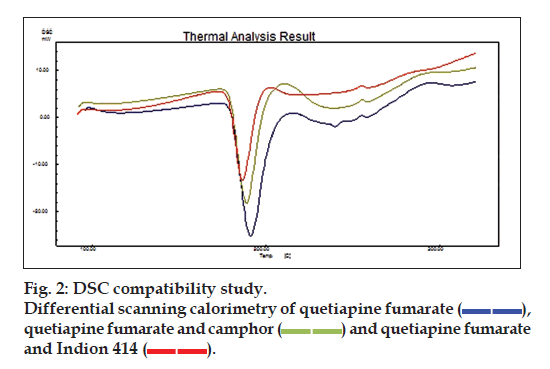
 ), quetiapine fumarate and camphor (
), quetiapine fumarate and camphor ( ) and quetiapine fumarate and Indion 414 (
) and quetiapine fumarate and Indion 414 (  ).
).