- *Corresponding Author:
- A. Padiyar Department of Pharmacy, Industrial Pharmacy Research Lab, Shri Govindram Seksaria Institute of Technology and Science, Indore, Madhya Pradesh 452003, India E-mail: ajaysurana01@rediffmail.com
| Date of Received | 04 June 2021 |
| Date of Revision | 26 July 2023 |
| Date of Acceptance | 20 March 2024 |
| Indian J Pharm Sci 2024;86(2):625-633 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The current study aims to design, formulate, optimize and evaluate diclofenac sodium injection using a novel concept of solubilisation. The present formulation utilizes a mixed solvency concept for the development of an injectable dosage form of the sparingly water-soluble drug diclofenac sodium using solids as solubilizing compounds. Diclofenac sodium injection 75 mg/ml was formulated without using propylene glycol or any other liquid solubilize, hence can be injected through the intradeltoid route without any pain at the site of action. The developed formulation was evaluated for various parameters such as freeze-thaw study, viscosity, osmolality and physical, chemical and pH stability of formulations were studies. The outcomes of the study deciphered a successful formulation of diclofenac sodium injection using the mixed solvency concept.
Mixed solvency, diclofenac sodium, solubility, formulation
Diclofenac is a Non-Steroidal Anti-Inflammatory Drug (NSAID) known chemically as ((2,6-dichloroanilino)- 2-phenyl)-2-acetic acid[1]. The drug was developed in the 1960s by scientists at Ciba- Geigy and is sold around the world by Novartis under various trade names, including Cataflam® and Voltaren® in the United States of America[2,3]. Owing to its excellent analgesic properties, diclofenac is widely used for treating various types of pain, including both chronic and acute painful episodes. The drug is administered for the treatment of musculoskeletal and joint disorders such as rheumatoid arthritis, osteoarthritis and ankylosing spondylitis; periarticular disorders such as bursitis and tendonitis; soft tissue disorders such as sprains and strains and other painful conditions such as renal colic, acute gout, dysmenorrhea and following some surgical procedures. Migraine attacks manifest a diverse array of symptoms that must be resolved for a treatment to be deemed truly effective against migraine (instead of just treating the symptoms)[4]. In particular, the treatment must be effective against the pain, photophobia, phonophobia and nausea that are caused by migraine and it must be effective within the first 2 h of treatment, to be considered a true treatment for migraine. None of the studies reported to date suggests that a 50 mg diclofenac product could treat all of these symptoms within 2 h of treatment[5].
Materials and Methods
Diclofenac sodium was procured as a gift sample from Wilcure Remedies, Indore (Madhya Pradesh). Caffeine was procured as a gift sample from Akums Drugs and Pharmaceuticals Ltd., Haridwar. All other excipients and reagents used were procured from an industrial pharmacy research laboratory, Shri Govindram Seksaria Institute of Technology and Sciences[6-8].
Drug characterization:
Melting point determination: The melting point of diclofenac sodium was determined using the open capillary method. The diclofenac sodium sample was packed into a capillary and the filled capillary was placed in Thiel’s tube filled with liquid paraffin oil. The temperature at which diclofenac sodium melts was recorded[9]. The average of three values was considered as the melting point of the drug. Results are shown in Table 1[10-14].
| S. no | Melting point (°) | Average |
|---|---|---|
| 1 | 285° | 284.66° |
| 2 | 285° | |
| 3 | 284° |
Table 1: Melting point determination
Ultra Violet (UV) spectroscopic analysis of dicofenac sodium in Demineralised (DM) water: About 100 mg of diclofenac sodium was accurately weighed and transferred into 100 ml volumetric flasks. The drug was dissolved in an adequate amount of demineralized water and the volume was made up to 100 ml to obtain a stock solution of 1000 μg/ml. Dilution of 20 μg/ml concentration was made from the above stock solutions with DM water and the resulting drug solution was scanned on a doublebeam UV/visible spectrophotometer (Shimadzu® 1700) between wavelengths of 200 to 400 nm[15]. The UV spectra so recorded is shown in fig. 1.
Infrared spectral analysis of diclofenac sodium drug sample: About 5 mg of the diclofenac sodium was triturated with approximately 100 mg of dry, finely powdered potassium bromide (Fourier-Transform Infrared Spectroscopy (FTIR) grade) and the resulted mixture was kept in a sample holder which was then placed in Diffused Reflectance Spectroscopy attachment (DRS 8000-assembly) and the spectrum was recorded on FT-IR spectrophotometer (Shimadzu® IR Affinity-1) between 500-4000 cm-1. IR spectra are shown in fig. 2 and the interpretation of FT-IR spectrum peaks of diclofenac sodium is shown in Table 2[16].
| S.no | Wave no | Interpretation |
|---|---|---|
| 1 | 3385.07 | N-H Stretching |
| 2 | 1502.55 | N-H Stretching |
| 3 | 3074.53 | C-H Stretching |
| 4 | 1450.47 | C=C Stretching |
| 5 | 1666.5 | C=0 Stretching |
| 6 | 1305.81 | O-H bending |
| 7 | 744.52 | C-Cl Stretching |
Table 2: Interpretation of infrared spectrum bands of diclofenac sodium drug sample
Differential Scanning Calorimetry (DSC) study of piroxicam drug sample: Pyris 6 DSC (Jade DSC) with thermal analyzer was used for the DSC investigation. About 3 mg of sample is accurately weighed and kept in a sealed aluminium pan before being heated at a scanning rate of 10°/min from 25°- 350° under nitrogen flow (20 ml/min). As a reference, an empty aluminium pan was used[17]. The data was analyzed and compared to the literature. The DSC spectrum of the piroxicam drug sample is shown in fig. 3.
Preformulation studies:
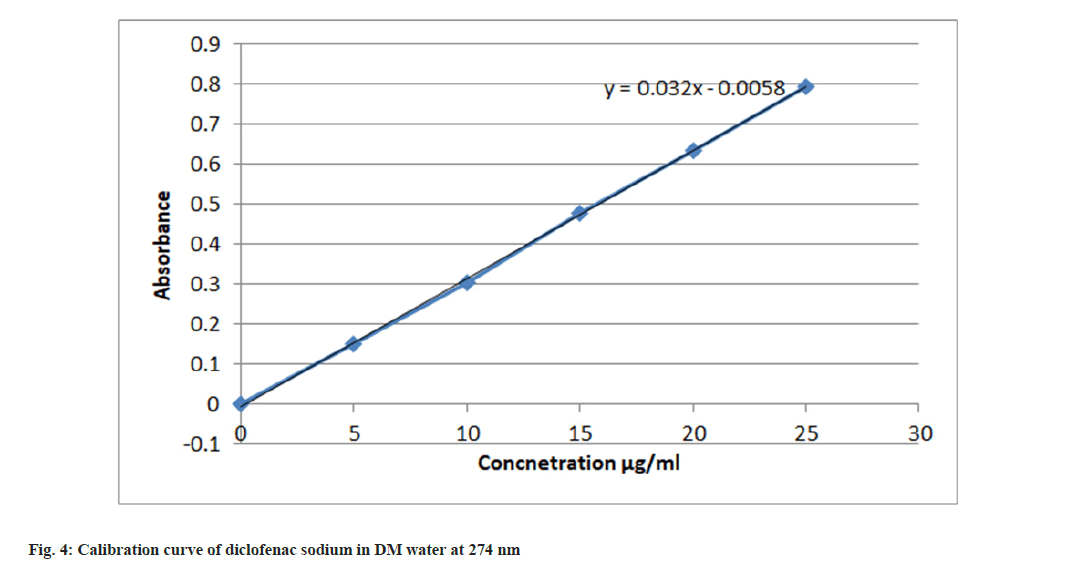
Calibration curve of diclofenac sodium in DM water using nitric acid: Accurately weighed 100 mg drug was transferred into a 100 ml volumetric flask. It was dissolved in an adequate amount of DM water and the volume was made up to 100 ml to obtain 1000 μg/ml. 10 ml of stock solution was pipetted out in a 100 ml volumetric flask and volume was made with DM water up to mark to obtain 100 μg/ ml solution. From 100 μg/ml solution, appropriate amount was taken out in a 10 ml volumetric flask to obtain a solution in a range of 5-25 μg/ml. In this 2 ml of nitric acid was added and volume was made up to mark with DM water. The absorbance of resulting solutions was noted at 380 nm against blank[18]. Absorbance data is shown in Table 3 and the calibration curve is shown in fig. 4.
| S.no | Concentration (µg/ml) | Absorbance | Average | ||
|---|---|---|---|---|---|
| Set 1 | Set 2 | Set 3 | |||
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 5 | 0.181 | 0.182 | 0.18 | 0.181 |
| 3 | 10 | 0.352 | 0.351 | 0.352 | 0.351 |
| 4 | 15 | 0.522 | 0.521 | 0.524 | 0.522 |
| 5 | 20 | 0.715 | 0.715 | 0.71 | 0.713 |
| 6 | 25 | 0.883 | 0.882 | 0.885 | 0.883 |
Table 3: Absorbance data for calibration curve in DM Water at 380 nm using nitric acid
Equilibrium solubility studies in different solvent systems: Equilibrium solubility studies in different solvent systems were achieved by adding an excess amount of drug in 10 ml of respective mediums, it was then screw capped and kept on a mechanical shaker for 24 h. It was centrifuged in centrifuge tubes at 2000 rpm for about 5 min and filtered using Whatman grade 41 filters then diluted with suitable respective medium[8,13]. The absorbance was measured at 274 nm on a double beam UV/visible spectrophotometer (Shimadzu-1700) against a reagent blank and noted in Table 4.
| S.no | Solvent system | Equilibrium solubility (mg/ml) |
|---|---|---|
| 1 | DM water | 11.224 |
| 2 | Hydrochloric acid buffer pH 1.2 | 10.087 |
| 3 | Hydrochloric acid buffer pH 2.0 | 10.194 |
| 4 | Acid phthalate buffer pH 3.0 | 10.354 |
| 5 | Acid phthalate buffer pH 4.0 | 10.567 |
| 6 | Neutralized phthalate buffer pH 5.0 0.340 Practically insoluble | 11.145 |
| 7 | Phosphate buffer pH 6.0 | 11.984 |
| 8 | Phosphate buffer pH 7.0 | 11.906 |
| 9 | Phosphate buffer pH 8.0 | 12.128 |
| 10 | Alkaline borate buffer pH 9.0 1.632 Slightly soluble | 12.346 |
| 11 | Alkaline borate buffer pH 10.0 | 12.317 |
Table 4: Equillibrium solubility of diclofenac sodium in different solvent systems
Approximate solubility of diclofenac sodium in different blends: Different concentrations of solubilizes in small quantities were used to prepare a 10 ml blend of various compositions and concentrations and labelled as blend A to blend T. Accurately measured 1 ml of blend-A solution was taken in a volumetric flask. 5 mg of drug diclofenac sodium was added and shaken vigorously for about 15-20 min on vortex. When a clear solution was formed then again the process was continued until turbidity appears[14-24]. The approximate solubility was noted in Table 5.
| S.no | Blend-code | Composition of blends | Approximate solubility |
|---|---|---|---|
| 1 | Blend A | PVP K-30 (5 %) Lignocaine HCl (5 %) niacinamide (5 % sodium benzoate (5 %) | Precipitation observed |
| 2 | Blend B | PVP K-30 (5 %) niacinamide (5 %) sodium benzoate (5 %) | 37 mg/ml |
| 3 | Blend C | PVP K-30 (5 %) Sodium citrate (5 %) Niacinamide (5 %) Sodium benzoate (5 %) | 33 mg/ml |
| 4 | Blend D | PVP K-30 (5 %) niacinamide (5 %) sodium benzoate (10 %) | 38 mg/ml |
| 5 | Blend E | PVP K-30 (5 %) Sodium citate (5 %) Sodium acetate (5 %) | 11 mg/ml |
| 6 | Blend F | PVP K-30 (5 %) Caffeine (5 %) Niacinamide (5 %) Sodiumbenzoate (5 %) | 115 mg/ml |
| 7 | Blend G | Caffeine (5 %) Niacinamide (5 %) | 115 mg/ml |
| 8 | Blend H | Sodium citrate (2.5 %) Sodium benzoate (5 %) L-Arginine (10 %) Benzoic acid (5 %) Niacinamide (2.5 %) HP-β cyclodextrin (5 %) | 30 mg/ml |
| 9 | Blend I | Sodium citrate (5%) Sodium benzoate (5%) L-Arginine (10%) Benzoic acid (5%) Niacinamide (2.5%) | 30 mg/ml |
| 10 | Blend J | Sodium citrate (5 %) Sodium caprylate (5 %) HP-β cyclodextrin (5 %) L-Arginine (10 %) Benzoic acid (5 %) | 47 mg/ml |
| 11 | Blend K | Sodium citrate (5 %) Sodium caprylate (5 %) HP-β cyclodextrin (5 %) L-Arginine (10 %) Benzoic acid (5 %) | 47 mg/ml |
| 12 | Blend L | L-Arginine (10 %) Poloxamer 407 (5 %) Sodium caprylate (5 %) Sodium benzoate (5 %) Sodium citrate (5 %) Benzoic acid (5 %) L-Arginine (10 %) Poloxamer 407 |
45 mg/ml |
| 13 | Blend M | Sodium caprylate (5 %) L-Arginine (10 %) Glycine (10 %) Poloxmer 407 (5 %) Benzoic acid (5 %) | 85 mg/ml |
| 14 | Blend N | Sodium caprylate (5 %) Sodium benzoate (5 %) Sodium citrate (5 %) L-Arginine (10 %) Poloxamer 407 (5 %) Lycine Hcl (10 %) | 60 mg/ml |
| 15 | Blend O | Glycine (10 %) Poloxamer 407 (5 %) Sodium caprylate (5 %) Sodium benzoate (5 %) Sodium citrate (5 %) | 56 mg/ml |
| 16 | Blend P | L-Arginine (10 %) Glycine (10 %) | 60 mg/ml |
| 17 | Blend Q | Sodium caprylate (5 %) Sodium benzoate (5%) Sodium citrate (5 %) Niacinamide (2.5 %) | 20 mg/ml |
| 18 | Blend R | Sodium acetate (5 %) Sodium benzoate (5 %) Sodium citrate (5 %) Niacinamide (2.5 %) | 22 mg/ml |
| 19 | Blend S | Sodium acetate (5 %) Sodium citrate (5 %) | 22 mg/ml |
| 20 | Blend T | Sodium acetate (5 %) Sodium benzoate (5 %) Sodium citrate (5 %) Niacinamide (5 %) | 24 mg/ml |
Table 5: Approximate solubility of diclofenac sodium in different blends
Thin layer chromatographic study: In order to examine the possibility of interaction between drugs and solubilizers, TLC studies were performed. A plate of silica gel GF254 was activated at 110° for 1 h and then used. The methanolic solution of diclofenac sodium alone, the aqueous solution of hydrotropic solution as well as solubilized product of diclofenac sodium in blend solution were spotted on the baseline with the aid of a micro dropper. Then, the plate was left in air for 10 min to dry and transferred to a solvent jar saturated with a solvent system composed of a mixture of chloroform, acetone and formic acid solution (90:5:5). The solvent system was allowed to run for about 4 cm. Finally, the plate was allowed to air dry for 5 min and observed under UV light for visualization of spots[9-14]. The respective Retardation factor (Rf ) values were determined and recorded in Table 6.
| S. no | System | Rf |
|---|---|---|
| 1 | Drug in methanol | 0 .70 |
| 2 | Drug in Blend G | 0.71 |
| 3 | Drug in Blend M | 0.7 |
Table 6: Rf values of diclofenac sodium and solubilized product
Optimization of solubilize concentration for the development of aqueous injection: Based on solubility a study of the drug in various blends of solubilizes, proposed novel solubilizes for diclofenac sodium and their concentrations to be used to formulate a solution which is stable in freeze thaw study is determined on basis of trial and error. Two blends were considered as they provide great solubility to diclofenac sodium for the formulation of injectable solution. As a result, these mixtures were chosen for further research[15,16]. These studies were conducted based on visual appearance, which included precipitation, crystal development and clarity. Prepared blends with acceptable medication concentrations were visually observed in these experiments[17]. For clarity and precipitation, these solutions were observed. Blend-G and blend-M were used for the preparation of an aqueous topical solution of the drug as they showed maximum solubility [18].
Preparation of injection formulation using selected blends:
About 7.5 % injectable solution of diclofenac sodium using mixed solvency concept was prepared in a clean room. For this initially, all the solubilizes, were taken and weighed accurately according to quantities decided in a 100 ml clean and calibrated volumetric flask and a sufficient amount of water was added to dissolve them. When the drug dissolves totally, volume was made up with the remaining water. This gave 100 ml of blend having desired concentrations of solubilizes. Now, accurately weighed 7.5 g of diclofenac sodium drug was weighed and transferred to a 100 ml volumetric flask. After this about 60 ml of the blend was added to dissolve the drug, when a clear solution was obtained, volume was made up using the remaining blend solution. Hence, the injection using selected blends was prepared and stored in glass vials[19]. The quantities required for formulation composition are shown in Table 7 and Table 8.
| S.no | Ingredients | Quantity for 100 ml | Uses |
|---|---|---|---|
| 1 | Diclofenac sodium | 7.5 g | Active ingredient |
| 2 | Caffeine | 10 g | Solubilizer |
| 3 | Niacinamide | 10 g | Solubilizer |
| 4 | Sodium Sulfite | 0.1 g | Antioxidant |
| 5 | Benzyl alcohol | 2 ml | Preservative |
| 8 | Water for injection | q.s. 100 ml | Vehicle |
Table 7: Formulation composition of diclofenac sodium injection (GS/DS/INJ/001)
| S.no | Ingredients | Quantity for 100 ml | Uses |
|---|---|---|---|
| 1 | Diclofenac sodium | 7.5 g | Active ingredient |
| 2 | Sodium caprylate | 5 g | Solubilizer |
| 3 | L-Arginine | 10 g | Solubilizer |
| 4 | Glycine | 10 g | Solubilizer |
| 5 | Poloxamer 407 | 5 g | Solubilizer |
| 6 | Benzoic acid | 5 g | Solubilizer |
| 7 | Sodium sulfite | 0.1 g | Antioxidant |
| 8 | Benzyl alcohol | 2 ml | Preservative |
| 9 | Water for injection | q.s. 100 ml | Vehicle |
Table 8: Formulation composition of diclofenac sodium injection (GS/DS/INJ/002)
Evaluations of prepared formulation:
Freeze-thaw study of various trial formulations: Freeze-thaw study was selected as a primary screening procedure for optimization of the concentration of various solubilizes. This method was designed to simulate storage and temperature conditions and to induce any anticipated precipitation and check it in a much shorter time. The vials were kept alternately at 40°±1° and 4°±1° for 24 h each and shaken every day for 5 min on a touch-type vortex mixer. Two vials of formulation were taken, one of which was kept at 40°±1° and the other at 4°±1° for 1 d, followed by subsequent temperature cycling and shaking as described. After 7-7 such cycles at 4°±1° and 40°±1° (alternately), the vials were observed to check turbidity and precipitation[20,21].
Evaluations of prepared formulation:
Freeze-thaw study of various trial formulations: Freeze-thaw study was selected as a primary screening procedure for optimization of the concentration of various solubilizes. This method was designed to simulate storage and temperature conditions and to induce any anticipated precipitation and check it in a much shorter time. The vials were kept alternately at 40°±1° and 4°±1° for 24 h each and shaken every day for 5 min on a touch-type vortex mixer. Two vials of formulation were taken, one of which was kept at 40°±1° and the other at 4°±1° for 1 d, followed by subsequent temperature cycling and shaking as described. After 7-7 such cycles at 4°±1° and 40°±1° (alternately), the vials were observed to check turbidity and precipitation[20,21].
Viscosity study of optimized trials: The viscosity parameter is selected as a secondary screening procedure for optimized formulation of suitable solubilize concentration. Viscosity was determined on Brook field viscometer DV 2+ pro using spindle no.00 Viscosity at maximum torque was recorded in Table 9[18,22].
| S.no | Formulation | Viscosity | RPM | Torque | Temperature |
|---|---|---|---|---|---|
| 1 | F7 | 2.56 CPS | 80 | 0.905 | 27.6° |
| 2 | F11 | 7.98 CPS | 120 | 0.981 | 28° |
| 3 | F12 | 8.77 CPS | 60 | 0.798 | 28° |
| 4 | F22 | 3.13 CPS | 160 | 0.836 | 26.3° |
Table 9: Viscosity result formulations
PH study of optimized trial: PH stability of developed formulations was determined using a digital pH meter (Cyber scan 510), previously calibrated using standard buffer solution[23,24]. Results are shown in Table 10.
| Formulation | pH |
|---|---|
| GS/DS/INJ/001 | 8.6 |
| GS/DS/INJ/002 | 8.94 |
Table 10: PH Result of various optimized formulations
Stability studies:
As soon as a product is developed, it is subjected to ageing. Its physical properties, chemical composition and even biological availability may change. The prepared formulations were subjected to standard conditions as per International Council for Harmonisation (ICH) to observe the stability of the drug on a proposed formulation. Samples were withdrawn at 1 mo, 3 mo, 6 mo and analyzed using Shimadzu UV Spectrophotometer. Percent drug residuals at definite time intervals were recorded using a colorimetric method for diclofenac sodium injection[25]. Results are shown in Table 11.
| Storage condition | Percent content of formulation at different time interval | |||||
|---|---|---|---|---|---|---|
| Initial | 15 d | 1 mo | 2 mo | 3 mo | 6 mo | |
| Room temp. | 100.00 | 100.00 | 100.00 | 100.00 | 99.97 | 98.01 |
| 40°/75 % RH | 100.00 | 99.45 | 98.99 | 98.01 | 97.56 | |
| 30°/65 % RH | 100.00 | 99.87 | 98.95 | 98.01 | 97.64 | |
Table 11: Results of stabilitysStudies (GS/DS/INJ/001)
Physical stability testing of formulated injections: The vials samples of formulations (20 samples of each batch) were kept at Room Temperature (RT), 40°/75 % RH and 2°-4°. For physical stability studies, the samples were observed at definite time intervals for colour change and clarity (to observe any turbidity or precipitation)[26]. Results are shown in Table 12.
| Batch No. | pH | ||||
|---|---|---|---|---|---|
| Initial | 15 d | 30 d | 60 d | 90 d | |
| GS/DS/INJ/001 | 8.515 | 8.517 | 8.523 | 8.6 | 8.675 |
| GS/DS/INJ/002 | 8.49 | 8.499 | 8.5 | 8.521 | 8.679 |
Table 12: PH stability data of diclofenac sodium injection formulation
pH stability study of formulation: Injection formulations were subjected to pH stability study for a period of 3 mo. pH was measured using a digital pH meter (Cyberscan 510), which was calibrated using a standard buffer solution at each time interval. Results are shown in Table 12.
Osmolarity: The osmolarity of prepared formulations and the marketed product was determined using a freezing-point based osmometer[18], advance instruments (Model 33201) was used for the study. Results are shown in Table 13.
| S.No | Formulation | Osmolarity |
|---|---|---|
| 1 | Dynapar AQ | 1559 mosm/kg |
| 2 | Voveran | Samples do not freeze |
| 3 | GS/DS/INJ/001 | 470 mosm/kg |
| 4 | GS/DS/INJ/002 | 568 mosm/kg |
Table 13: Osmolarity result of various marketed and prepared formulation
Results and Discussion
The drug was characterized using melting point, UV spectroscopy, IR spectroscopy and DSC studies. Results obtained confirm the purity of diclofenac sodium. Drug analysis was done using a colourimetric method at 380 nm. The results of the Thin Layer Chromatography (TLC) study revealed that there is no considerable change in Rf values of diclofenac sodium solubilized in methanol and diclofenac sodium solubilized in solubilize blend solution. From the results of the TLC study, it can be concluded that there is no salt formation or complication of the drug and solubilizer molecule. Both formulations were found to be stable during the entire freeze thaw study. No precipitation or turbidity were found in the formulations. Viscosities of formulation were found satisfactory, hence both formulations were considered as optimized formulations. Both the injection formulations were found to be unaffected in respect of colour stability at all taken temperature conditions for a period of 30 d. No visual colour change or precipitate was observed in the developed formulations. The pH values of both formulations were found to be in the range as provided in the Indian Pharmacopoeia 2014 monograph. The osmolarity of proposed formulations was found to be less as compared to the osmolality of the reference product.
The mixed solvency technique can be successfully utilized in the formulation development of injection dosage form of poorly water-soluble drug diclofenac sodium and to reduce the concentration of individual solubilize to minimize the side effects. Every substance has solubilizing power and hence various solid solubilizes can be screened for their use in various pharmaceutical formulations. Novel solubilizers such as amino acids and proteins can be used for the solubility enhancement of various drugs.
Acknowledgments:
The Authors would like to acknowledge Willcure Remedies and Akums Drugs and Pharmaceuticals Ltd., for providing drug and excipients. We also thank Sri Aurobindo Institute of Pharmacy for providing a research facility to conduct stability studies.
Conflict of interests:
The authors declare no competing interests.
References
- Bakshi R, Ezzet N, Frey L, Lasry D, Salliere D. Efficacy and tolerability of diclofenac dispersible in painful osteoarthrosis. Clin Rheumatol 1993;12(1):57-61.
[Crossref] [Google Scholar] [PubMed]
- Bakshi R, Jacobs LD, Lehnert S, Picha B, Reuther J. A double-blind, placebo-controlled trial comparing the analgesic efficacy of two formulations of diclofenac in postoperative dental pain. Curr Ther Res 1992;52(3):435-42.
[Crossref] [Google Scholar] [PubMed]
- Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS. Diclofenac sodium: A review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs 1980;20:24-48.
[Crossref] [Google Scholar] [PubMed]
- Dahlof C, Bjorkman R. Diclofenac-K (50 and 100 mg) and placebo in the acute treatment of migraine. Cephalalgia 1993;13(2):117-23.
[Crossref] [Google Scholar] [PubMed]
- Degen PH, Dieterle W, Schneider W, Theobald W, Sinterhauf U. Pharmacokinetics of diclofenac and five metabolites after single doses in healthy volunteers and after repeated doses in patients. Xenobiotica 1988;18(12):1449-55.
[Crossref] [Google Scholar] [PubMed]
- Indian Pharmacopoeia. Goverment of India ministry of health and family Welfare. 4th ed. The Controller of Publication; Delhi: 1996. p. 332.
- United States Pharmacopoeia 24 and National Formulary 19 2000. The official compendia of standards, Asian ed. The United States Pharmacopeial Convention Inc., Rockville.
- Higuchi T, Shih FM, Kimura T, Rytting JH. Solubility determination of barely aqueous-soluble organic solids. J Pharm Sci 1979;68(10):1267-72.
[Crossref] [Google Scholar] [PubMed]
- Boylan JC. Liquids, In: Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy, 3rd ed. Varghese Publishing House: Bombay: 1991. p. 466.
- Maheshwari RK, Joshi G, Gehlot S, Mahajan SC. Novel application of hydrotropy in thin layer chromatography. Indian Pharm 2010;8(10):57-9.
- Sonali J, Kamaldeep Y, Bhumika S, Sanjay J, Kumar MR. Hydrotropy-A novel approach in estimation of poorly aqueous soluble drugs by TLC. Int J Pharm Pharm Sci 2013;5(2):176-8.
- Mangal A, Bhadoriya SS, Verma A, Mishra KK. Novel application hydrotropic solubilization phenomenon in the thin layer chromatrography analysis of omeprazole. Int J Curr Pharm Res 2011;8(1):15-6.
- Maheshwari RK, Jagwani Y. Mixed hydrotropy: Novel science of solubility enhancement. Indian J Pharm Sci 2011;73(2):179.
[Crossref] [Google Scholar] [PubMed]
- Maheshwari R, Chaturvedi S, Jain N. Novel application of hydrotropic solubilization in the analysis of some NSAIDs and their solid dosage forms. Indian J Pharm Sci 2007;69(1):101-6.
- Padiyar A, Maheshwari RK. Formulation development of diclofenac sodium lotion using mixed solvency concept and in vitro evaluation. Indian Drugs 2022;59(5):24-8.
- Padiyar A, Maheshwari RK. Novel dry injection for reconstitution of aspirin using solid solubilisers. J Drug Deliv Ther 2017;7(7):44-5.
- Nagwanshi K, Maheshwari RK, Padiyar A. Formulation and development of topical dosage form of ibuprofen using mixed solvency concept and its evaluations. Ann Pharm Res 2022;10(1):610-24.
- Padiyar A, Maheshwari RK, Jain S. Formulation and development of high concentration diclofenac sodium injection using mixed solvency concept and its evaluation. Int J Adv Pharm 2016;6(2):78-84.
- Maheshwari RK, Shilpkar RA. Formulation development and evaluation of injection of poorly soluble drug using mixed solvency concept. Int J Pharm Biosci 2012;3(1):179-89.
- Solanki SS, Soni LK, Maheshwari RK. Study on mixed solvency concept in formulation development of aqueous injection of poorly water soluble drug. J Pharm 2013;2013:678132.
[Crossref] [Google Scholar] [PubMed]
- Pawar PB, Rawat S, Mahajan YY, Galgatte UC, Maheshwari RK. Formulation development and evaluation of aqueous injection of poorly soluble drug made by novel application of mixed solvency concept. Int J Drug Deliv 2013;5(2):152.
- Soni LK, Solanki SS, Maheshwari RK. Solubilization of poorly water soluble drug using mixed solvency approach for aqueous injection. Br J Pharm Res 2014;4(5):549-68.
- Solanki SS. Development of parenteral formulation of poorly water soluble drugs: The role of novel mixed-solvency concept. Asian J Pharm 2017;11(1):1-10.
- Khan MA. Enhancement of solubility of poorly water soluble drugs diclofenac sodium by mixed solvency approach. Res J Pharm Dosage Forms Technol 2013;5(1):39-41.
- Soni LK, Solanki SS, Maheshwari RK. Evaluation of analgesic, anti-inflammatory and ulcerogenic liability of oral solution (syrup) formulation developed by novel mixed solvency concept. Adv Pharmacol Toxicol 2015;16(2):21.
- Jat P, Maheshwari RK. Formulation development of dry powder injection for reconstitution of poorly water-soluble drug, indomethacin, using mixed solvency concept and their evaluations. Int J Pharm Res Appl 2021;6(2):314-26.