- *Corresponding Author:
- Prashant Gupta
Department of Pharmaceutics, Institute of Pharmaceutical Sciences, Faculty of Pharmacy, Parul University,
Vadodara, Gujarat 391760, India
E-mail: prashantgupta_85@yahoo.com
| Date of Received | 13 December 2024 |
| Date of Revision | 11 January 2025 |
| Date of Acceptance | 30 April 2025 |
| Indian J Pharm Sci 2025;87(2):73-87 |
Abstract
Contagious infections caused by the Herpes simplex virus are globally known to be lifelong, with periodic re-activation. Available medications have limitations in terms of poor oral bioavailability and lower efficacy on topical administration. No preventive or therapeutic vaccine has been developed till date. The aim of the study is to develop and optimize Penciclovir topical formulations with enhanced drug delivery to the site and improved onset of action. Based on Penciclovir-solubility, different oils and surfactants were screened. Concentrations of mineral oil, polysorbate 20 and labrafil M1944 were chosen based on pseudoternary phase diagrams. The optimized Penciclovir- Nano emulsion (Penciclovir-Nano-emulsion) through I-Optimal design contains Smix and oil in concentrations of 16 %-21 % and 5 %-25 %, respectively. Results obtained for transmittance, droplet size, and zeta potential are 90.2 %-97.5 %, 64-450 nm, and 12-42 mV, respectively. Nanoemulsion gel was prepared using optimized Penciclovir-Nano emulsion and different types and variations in concentration levels of gelling agents. Higher concentrations of gelling agents enhance the rheological properties of Penciclovir-Nano-emulsion gel. The Penciclovir-Nanoemulsion gel formulation (Carbopol 940 at 1.8 % w/w) has shown an improved drug release profile in comparison with Penciclovir-cream and free Penciclovir-Gel. Ex vivo permeability studies were performed using human Cadaver skin, the enhancement factors of 1.87 and 1.49 were obtained against free Penciclovir-gel and Penciclovir-cream, respectively. In silico drug permeation studies were conducted using a developed human skin model, and the Logp of the optimized formulation was determined, revealing a significant change compared to the cream and free drug formulations.
Keywords
Nano-emulsion gel, pseudoternary phase diagram, surfactant screening, ex vivo study, enhanced penetration, diffusion study, flux
In the 21st century, the world has witnessed the outbreak of many severe infectious diseases that have significantly changed the lives and livelihoods of people around the globe. Among them, the Herpes Simplex Virus (HSV) is lifelong and known for periodic reactivations at the infection site. Orolabial herpes (cold sores) is HSV type 1 and transmitted through oral-oral contact, whereas genital herpes is HSV type 2 and transmitted through oral-genital contact. An estimated 67 % (3.7 billion people under the age of 50) have HSV-1 infection, and an estimated 13 % (491 million people aged 15-49) have HSV-2 infection worldwide[1]. In India, 33.3 % of individuals are seropositive for HSV type 1, and 16.6 % are seropositive for HSV type 2. Diseases caused by HSV include cold sores, genital herpes, Herpes Stromal Keratitis (HSK), eczema herpeticum, meningitis, and Herpes Simplex Encephalitis (HSE) [2]. HSV is attached to the cell surface, and capsid penetration is facilitated by interactions between cellular receptors and viral glycoproteins. Through microtubules, the nucleocapsid is transported to the nuclear membrane, where viral Deoxyribonucleic Acid (DNA) is released for replication in the nucleus[3].
Orally administrable prodrug forms such as Valacyclovir and Famciclovir have been utilized for many years for the treatment of HSV. The gastric side effects and limitations pertaining to oral drug delivery pose a major concern. Therefore, topical delivery could be a good choice for the facilitation of viral shedding while decreasing the incidence of gastrointestinal side effects. Penciclovir (PNC) and Acyclovir are being used for the management of HSV type 1 and 2 and are available for topical delivery in the form of cream. Despite the advances in the management of HSV infections, improved topical drug delivery with rapid action and enhanced bioavailability are required. Neither a preventive nor therapeutic vaccine has been developed till date for HSV Type 1 and Type 2[4].
The development of Nano-Emulsion Gels (NEGs/ Emulgel) is proven to be the most prominent approach for overcoming the problems related to conventional topical dosage forms. The drug is entrapped into the small oil droplets using suitable Solubilizer and Permeation Enhancers (PEs), and an emulsion is prepared using an aqueous continuous phase. Nano-sized drug-embedded droplets and reduced interfacial tension promote drug permeation and improved drug bioavailability[5]. Studies have shown statistically significant improvement in the time of lesion healing, lesion area, and pain with the topical treatment of PNC cream as compared to the Acyclovir cream formulation[6]. NEs formulation of PNC and then conversion into NEGs are needed to impart topical delivery of the drug on the skin surface. NEGs exhibit advantages in both emulsion and gel utilization for topical delivery[7]. To facilitate drug transport through the layers of skin, penetration enhancers are extensively studied and can be mixed in the oil phase[8]. Cellulose-based and acrylic-based polymers are widely used for the conversion of NEs to NEGs. Concentrations of polymers are selected based on the desired properties of NEGs for topical application[9].
PNC is commercially available in the cream formulation and indicated for the treatment of recurrent herpes labialis (cold sores) in adults and children (≥12 y). It was prophesied that the PNC-NEG formulation would further enhance its therapeutic effect and patient compliance. Interestingly, two reported studies have made such efforts to develop NEG formulations of PNC[10,11]. A reported study by Weiwei et al.[11] has used 30 % w/w ethanol for the preparation of micro emulsion. There are much scientific evidence demonstrating that the use of alcohols, including ethanol, for abrasive and shredded skin is not recommended because of the burning sensation[12]. Another reported study by Emmanuelle et al.[13] has used 40.5 % Labrasol: Labrafil 1944 (6:4) and 45.5 % Lauroglycol-FCC for the preparation of NE and then conversion to oral NEG. The quantity of surfactant and co-surfactant mixture used in the formulation of NEs was 86 % w/w, which is too high and needed toxicity and skin irritation assessments. The aim of the present study is to develop and optimize a NEG topical formulation of PNC for improved drug delivery through the skin surface, thereby enhancing therapeutic effects by using light Mineral Oil (MO) as the oil phase and using surfactant and co-surfactant concentrations within recommended levels. Moreover, the present study is also designed to evaluate the effects of different types of gel-forming agents (cellulose-based and acrylic-based) on PNC-NEG formulation properties. The quest of the work is to derive a composition that has all the ingredients below the toxic levels, has a scalable process, and has demonstrated stability as per The International Council for Harmonisation (ICH) recommended conditions, which were not evaluated in the reported studies.
Materials and Methods
Materials:Active pharmaceutical ingredient PNC was procured from Alembic Pharmaceuticals Limited, India; Polysorbate 20 was obtained from Avantor, China; Labrafil M 1944 from Gattefosse, France; and light MO from Sigma-Aldrich, United States of America (USA). All other chemicals and solvents used for testing were of analytical reagent grade.
Solubility of PNC in NEs formulation components:Saturation solubility of PNC was measured to screen suitable oil, surfactant, and surfactant/co-surfactant mixtures (Smix) by shaking flask method at 25°. The oil, surfactant, and cosurfactant that showed maximum solubility for PNC were used in the preparation of PNC-NEs.
Preparation of pseudo-ternary phase diagrams:Pseudo-ternary phase diagrams were constructed by using MO, Lavendor Oil (LO) and Citronella Oil (CO) as oil phase. Polysorbate-20 alone and with CAPMUL PG-8, CAPMUL MCM C8 and Labrafil M1944 in 1:1 ratio was prepared, and water is added drop wise into the mixture through titration method. Quantity of water at which solution becomes turbid is recorded. Percentage weight by weight (% w/w) values of each composition is plotted in Pseudo ternary plot diagrams (Software Chemix Ver.10.0)
Preparation, optimization and characterization of PNC-NEs formulation:From the phase diagrams, concentration ranges of MO, polysorbate 20, and Labrafil M 1944 were obtained for the preparation of NEs. The drug was added to the homogenous mixture of Polysorbate 20, Labrafil M1944, MO and stirred till it became clear. Purified water was added at a definite rate, and after the completion of the addition, batch yield was verified against the theoretical batch size.
Statistical optimization of PNC-NEs formulation components:In the present study, I-Optimal mixture design was utilized for optimization of the concentration of Smix (Factor A), Oil Phase (Factor B), and Purified Water (Factor C). Responses selected for evaluation are transmittance (percentage, %), Droplet Size (DS) (Nanometre, nm), and Zeta Potential (ZP) (millivolt, ±mV). Minimum and maximum ranges of concentrations were derived from pseudo-ternary phase diagrams.
Optimization model validation:Statistical validation of the design space and regression model was performed by Design Expert based on an Analysis of Variance (ANOVA). The models were assessed against statistically significant differences between the predicted mean and observed value of responses
Physico-chemical characterization of PNC-NEs:Transmittance: Batches of PNC-NEs were analysed for percentage transmittance in the Ultraviolet- Visible (UV) Spectrophotometer, JASCO V-730, light source D2. NEs formulations were filled in a quartz cuvette and analysed against double-distilled water as a control.
DS and ZP: Batches of PNC-NEs were analysed in triplicate for mean DS and ZP. The sample was prepared by diluting 2 ml of NEs with 100 ml of purified water. The diluted sample was tested using Zetasizer Malvern, Nano ZS, at an applied potential of 150 mV.
Preparation of PNC-NEGs formulation:The required quantity of gelling agent was dispersed in purified water at 40° under homogenization. The mixture is homogenized for 45 min to ensure uniform mixing and then allowed to cool to room temperature. The final volume was made up with the remaining quantity of purified water. Previously, prepared PNC-NEs was added to the gel dispersion and manually mixed with mild agitation using an overhead stirrer. The mixture was stirred continuously for 60 min under slow stirring to ensure the adequacy of mixing. The final pH was adjusted with an alkali solution.
Optimization of gelling agents:Grades and percentage levels of gelling agents were studied against rheological properties and in vitro and ex vivo drug diffusion studies.
Physico-chemical characterization of PNC-NEGs:Physical appearance, Homogeneity, Phase separation and pH: PNC-NEGs were examined visually for physical appearance, homogeneity, pH, and phase separation.
Potency: Percentage drug content was estimated by a validated High-Performance Liquid Chromatography (HPLC) analytical testing method through a regression equation from the linearity curve. Chromatographic parameters were: Column: Hypersil BDS C-8, 150×4.6 mm, 5 microns (make: Thermo Scientific), column oven temperature: 25°, flow rate: 1 ml/min, Wavelength: 254 nm, Inj. Volume: 20 ml (μl), mobile phase: buffer: Methanol 95:5 (v/v), 1 % orthophosphoric acid.
Viscosity: The viscosity of PNC-NEGs samples was determined in triplicate at 25°±0.5° using a Brookfield viscometer (spindle 00) (Brookfield Inc., USA) at a rotational speed of 50 rotations per minute (rpm) [14].
Bio adhesive strength: The modified balance method using fresh rat skin (hairless) was used to measure bioadhesive strength. The volume of water added to detach the slides from one face of rat skin was converted to mass and recorded as bioadhesive strength[15][16].
In-Vitro Drug Release study (IVRT):A Franz diffusion cell was used for an in vitro drug release study. Weight quantity of 0.5 grams (g), PNC-NEGs (1 % w/w), PNC-cream, and free PNC-Gel were uniformly applied onto the surface of Polyvinylidene Difluoride (PVDF) membrane (0.22 mm, μm thickness). The treatment membrane was clamped between the donor and the receptor chamber of the diffusion cell with a diffusion area of 2.5 cm2. The receptor chamber was filled with a freshly prepared phosphate buffer solution (pH 6.0). The receptor chamber was stirred by a magnetic stirrer at 50 rpm. The aliquot samples (0.5 ml) were taken at 0, 15, 30, 45, 60, 90, 120, 150, and 180 min. A filtered (0.45 μm) sample was analysed for PNC content at 254 nm. The cumulative amount of drug release per unit area (mg/cm2) through the membrane surface against time was plotted for comparison [17].
Ex vivo drug diffusion study:This study was carried out using human cadaver skin, and the set-up and processing steps were like those of an in vitro drug release study. Full-thickness human cadaver skin from the foot region of Asian subjects was obtained from Gandhi Medical College, Bhopal, M.P., India. After hydration and inspection, an integrated portion of human cadaver skin was placed between the donor and receptor compartments of the Franz diffusion cell, with the stratum corneum facing the donor compartment[18]. The average cumulative amount of drug permeated per unit surface area of the skin was studied against time. The slope of the linear portion of the plot was calculated as flux g/cm2/min, and the diffusion coefficient was calculated from the below formula:
Kp = Jss / Cv
Where, Kp is the diffusion coefficient and Cv is the total amount of drug. The enhancement of drug penetration through the skin from PNC-NEGs formulation was compared with the PNC-cream and free PNC-Gel formulation and noted as Enhancement Factor (EF). EF was calculated using the following formula:
EF = (Kp (PNC-NEGs)) / (Kp (PNC-Cream or Free PNC Gel))[19][20]
Stability:The stability studies were carried out as per the ICH guidelines Q1A. The PNC-NEGs were filled in aluminium collapsible tubes (fill weight 20.0 g±1.5 % (19.7 g to 20.3 g), sealed, and labelled appropriately. Samples withdrawn from the chamber at 40°±2°/75 %±5 % Relative humidity and 25°±2°/65 %±5 % relative humidity were tested for physical appearance, potency, pH, phase separation, the presence of lumps or crystals, and viscosity.
In silico skin permeation study:Researchers are utilizing many different methods for best prediction of skin permeation and LogP like Quantitative Structure-Activity Relationship (QSAR) modelling, Molecular Dynamics (MD) simulations, cosmetic models, Density Function Theory (DFT) based LogP estimation [21][22][23]. We have developed a model using Quantum Mechanics (QM) and MD simulations to predict LogP values and forecast the partitioning behaviour of reference and test formulations in a skin-formula system.
Molecular structure preparation:The molecular structure of drug molecule was optimized using quantum chemistry software. Geometry optimization was performed to obtain the most stable conformation of the molecule.
Electronic properties calculation:Quantum chemical calculations were employed to determine various molecular properties, including electronic energies, dipole moments, and polarizabilities.
Solvation energy calculation:Solvation energies of drug molecule in octanol and water were computed using implicit solvation models, such as the Conductor-Like Polarizable Continuum Model (CPCM) implemented in QM software. These calculations account for the interaction between the solute and solvent molecules.
LogP calculation:The partition coefficient (LogP) is calculated as the difference in solvation energies between skin membrane and formulation phases, normalized per mole of solute [24].
Mathematically, LogP = (ΔGskin - ΔGformula) / RT,
Where, ΔGskin and ΔGformula are the solvation free energies of the Application Programming Interface (API), respectively, R is the gas constant, and T is the temperature in Kelvin.
Creation of virtual skin membrane: A multiscale modelling framework provides insight into the delivery mechanisms of drugs through skin and acts as a guiding tool in performing targeted experiments (derived from simulations) to develop a suitable delivery system. We have modelled a realistic skin subcutaneous layer at both molecular and macroscopic scales. The individual skin lipid constituents were used in this study. The equimolar bilayer structure, which was equilibrated for ~200 ns, was taken from an earlier simulation using GROMOS87 parameters[25]. A bilayer consisting of skin lipids and formulation molecules was built. The bilayer size was 4.9 nm × 4.9 nm × 11.7 nm. Density profile of individual bilayer constituents along the bilayer normal at 310 K was calculated using a 200 ns unconstrained simulation. The membrane interior is highly heterogeneous, and sampling such a multi-component interior is challenging. The diffusivity of drug molecules through the SC lipid layer was calculated using multiple atomistic MD simulations. The averaged diffusivity along the bilayer normal was used as an input for the macroscopic Computational Fluid Dynamics (CFD) model. The macroscopic model solves Fick’s 2nd law in the specified geometry (brick and mortar) with appropriate boundary conditions. To account for parallel and branched pathways in the SC (lipid region), a modified Fick’s 2nd law was used, which was developed from the first principles approach.
Results and Discussion
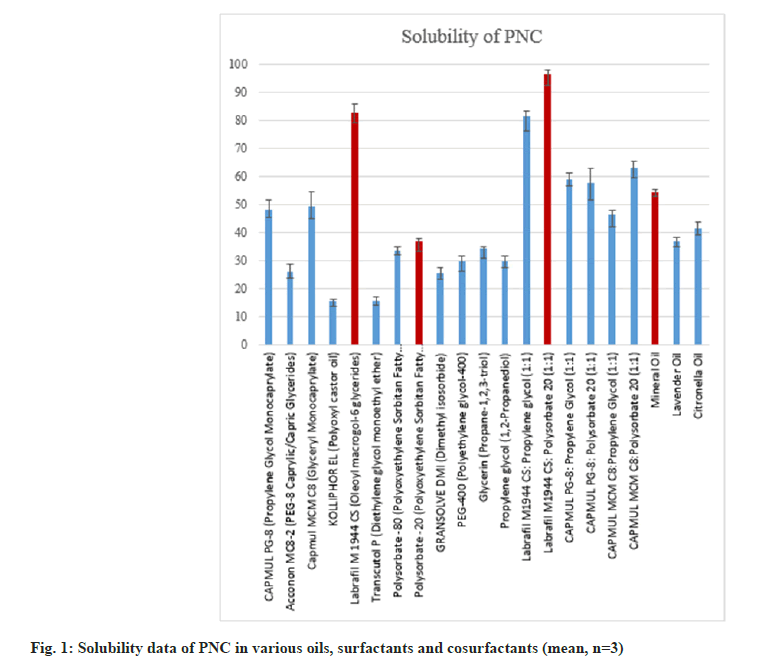
Among the oils studied, the highest solubility was observed in MO, followed by CO, and relatively low in lavender oil (fig. 1). Based on the superiority of MO usage over Citronella and lavender oil due to associated cell-related toxicity and observed solubility results, MO was selected as the oil phase [26][27]. In the previously reported studies, Labrafil M 1944 was already successfully used in the formulation of NEs[28]. Further solubility of PNC in Labrafil M 1944 is maximum as compared to studied surfactants.
Tween 20 is most widely used as a surfactant and/or co-surfactant alone and in combination in the formulation of NEs due to its non-ionic nature and high Hydrophillic-Lipophillic Balance (HLB) value (reported 16.7)[29]. In this study, we have observed the highest solubility of PNC in the Labrafil M 1944 and Tween 20 (1:1) mixture as 96.22±0.95 mg/ml. So, Labrafil M 1944 and Tween 20 in the ratio of 1:1 were selected as surfactant mixtures for the preparation of PNC-NEs.
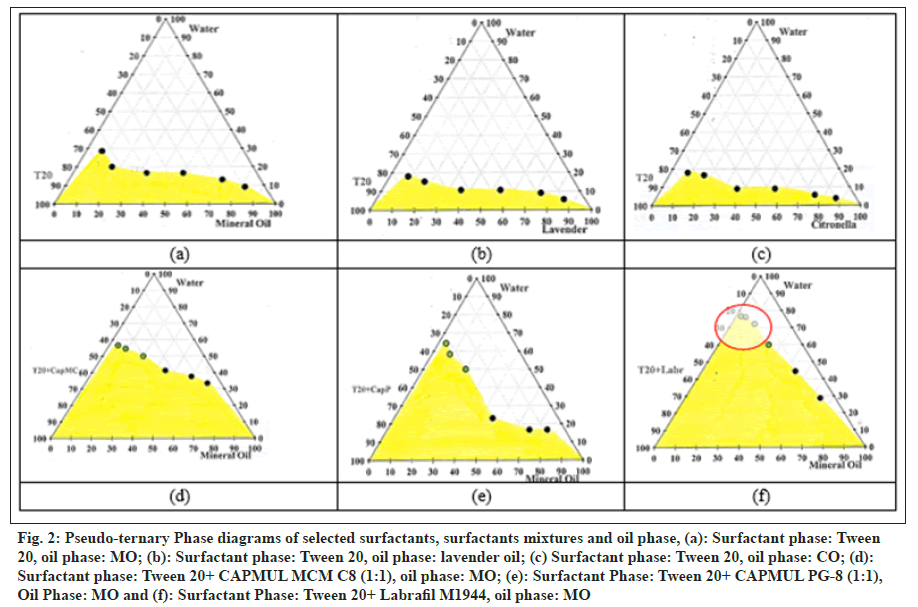
Pseudo-ternary phase diagrams were prepared by using MO, CO, and lavender oil as oil phases and Tween 20 as a surfactant (fig. 2a–fig. 2c). The micelle formation area (grey portion) was found to be relatively higher in the diagram of MO as compared to other oils. Based on observation, phase diagrams were further constructed using a mixture of CAPMUL MCM C8, CAPMUL PG-8, and Labrafil M1944 with Tween 20 in a 1:1 ratio as a surfactant (Smix) and MO as the oil phase (fig. 2d–fig. 2f).
Fig. 2: Pseudo-ternary Phase diagrams of selected surfactants, surfactants mixtures and oil phase, (a): Surfactant phase: Tween 20, oil phase: MO; (b): Surfactant phase: Tween 20, oil phase: lavender oil; (c) Surfactant phase: Tween 20, oil phase: CO; (d): Surfactant phase: Tween 20+ CAPMUL MCM C8 (1:1), oil phase: MO; (e): Surfactant Phase: Tween 20+ CAPMUL PG-8 (1:1), Oil Phase: MO and (f): Surfactant Phase: Tween 20+ Labrafil M1944, oil phase: MO
Ranges of Smix, oil phase, and purified water were derived from the phase diagram (fig. 2f) for further optimization. For the mixture of Labrafil M1944 and Tween 20 (1:1), the concentration range found is 16%–21% w/w. For MO, the concentration range found is 5%–25% w/w. As per the United States Food and Drug Administration, Inactive Ingredients Database (USFDA IID database), Tween 20 and MO for topical preparations can be used up to 10.5% and 36.95% w/w, respectively. The concentration range obtained is around 1.5 folds less than the established maximum potency or dose, as per the IID database[30]. The recommended concentrations of Tween 20, Labrafil M 1944, and MO are 1%–10%, 5%–20%, and 1%–20%[31], which are lower than our derivations from the above study.
Preparation and optimization of PNC-NEs were carried out using I-Optimal mixture design, three factors—Smix (Factor A), oil phase (Factor B), and purified water (Factor C)—were analysed against the responses Transmittance (%), DS (nm), and ZP (±mV). Formulation trials and results are presented in Table 1.
| Run# | Factors studied | Response | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor A: Smix % | Factor B: oil phase % | Factor C: purified water | Response 1 transmittance (%) | Response 2: DS (nm) | Response 3: ZP (±mV) | ||||
| Mean | SD | ||||||||
| PG1 | 20.61 | 5.00 | 74.39 | 96.80 | 0.21 | 120 | 20 | 28 | 10 |
| PG2 | 18.80 | 13.80 | 67.40 | 93.90 | 0.13 | 265 | 25 | 22 | 8 |
| PG3 | 21.00 | 25.00 | 54.00 | 96.00 | 0.16 | 176 | 18 | 19 | 11 |
| PG4 | 16.00 | 9.00 | 75.00 | 93.80 | 0.31 | 372 | 45 | 12 | 4 |
| PG5 | 18.37 | 25.00 | 56.63 | 90.20 | 0.81 | 290 | 36 | 16 | 6 |
| PG6 | 21.00 | 15.36 | 63.64 | 97.50 | 0.12 | 64 | 22 | 42 | 14 |
| PG7 | 18.80 | 13.80 | 67.40 | 94.10 | 0.22 | 240 | 35 | 25 | 7 |
| PG8 | 16.00 | 23.12 | 60.88 | 90.80 | 0.72 | 450 | 48 | 16 | 4 |
| PG9 | 16.00 | 18.82 | 65.18 | 91.90 | 0.69 | 368 | 38 | 10 | 6 |
| PG10 | 21.00 | 20.05 | 58.95 | 97.00 | 0.18 | 90 | 15 | 32 | 8 |
| PG11 | 18.80 | 13.80 | 67.40 | 94.10 | 0.17 | 280 | 29 | 20 | 4 |
Table 1: Factor Studied and Response Obtained for Various Formulation Optimization Trials
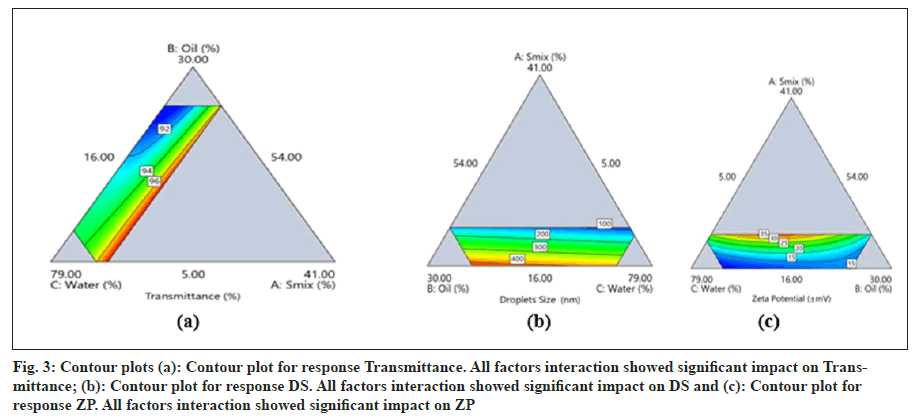
The ANOVA data for response transmittance (T) of the model is provided in Table 2a. The model was found to be significant with a p-value of <0.0001. Significant effects of interaction terms AB, AC, BC, and selected model on transmittance (%) along with an insignificant lack of fit were observed. The R2 value obtained is 0.9960 (good), and adequate precision is 43.03 (>4). Hence, this model can be used to navigate design space (fig. 3a).
The mathematical equation suggested by the software (Design Expert v-13) for T is shown in equation (1). From the equation for T, we can conclude that the increase in Smix, oil phase, and aqueous phase increases the T of PNC-NEs. Among these, the higher magnitude of the regression coefficient for factor A (Smix) shows that the effect of Smix concentration is more on the T of formulated PNC-NEs.
Transmittance = 220.73 A + 88.77 B + 94.13 C - 118.41 AB - 137.24 AC + 3.18 BC (1)
The ANOVA data for response DS of the model is provided in Table 2b. The model was found to be significant with a p-value of <0.0001. A significant effect of the linear mixture and selected model on DS, along with an insignificant lack of fit, was observed. The R2 value obtained is 0.9302 (good), and adequate precision is 16.59 (>4). Hence, this model can be used to navigate design space fig. 3b.
The mathematical equation suggested by the software for DS is provided in equation (2). From the equation for DS, we can conclude that the increase in Smix concentration decreases the DS of PNC-NEs. Whereas, increases in the oil phase and water phase have shown a negative effect on the DS.
DS = -1071.14 A + 444.37 B + 373.47 C (2)
The ANOVA data for the response ZP of the model is provided in Table 2c. The model was found to be significant with a p-value of 0.0022. Significant effects of the interaction terms AB, AC, ABC, and selected model on ZP, along with an insignificant lack of fit, were observed. The R2 value obtained is 0.9553 (good), and adequate precision is 14.43 (>4). Hence, this model can be used to navigate design space (fig. 3c).
| Source | Sum of squares | DF | Mean Square | F value | P value | |
|---|---|---|---|---|---|---|
| Model | 50.22 | 5 | 10.04 | 246.14 | <0.0001 | Significant |
| Linear mixture | 44.09 | 2 | 22.05 | 540.21 | <0.0001 | |
| AB | 2.82 | 1 | 2.82 | 69.02 | 0.0004 | Significant |
| AC | 4.15 | 1 | 4.15 | 101.75 | 0.0002 | Significant |
| BC | 0.3976 | 1 | 0.3976 | 9.74 | 0.0262 | Significant |
| Lack of fit | 0.1774 | 3 | 0.0591 | 4.43 | 0.1895 | Not significant |
| Model | 1.43E+05 | 2 | 71671.60 | 53.33 | <0.0001 | significant |
| Linear mixture | 1.43E+05 | 2 | 71671.60 | 53.33 | <0.0001 | significant |
| Lack of fit | 9933.77 | 6 | 1655.63 | 4.05 | 0.2110 | Not significant |
| Model | 834.90 | 5 | 166.98 | 21.35 | 0.0022 | Significant |
| Linear mixture | 581.32 | 2 | 290.66 | 37.17 | 0.0010 | Significant |
| AB | 109.19 | 1 | 109.19 | 13.96 | 0.0135 | Significant |
| AC | 88.64 | 1 | 88.64 | 11.33 | 0.0200 | Significant |
| ABC | 206.75 | 1 | 206.75 | 26.44 | 0.0036 | Significant |
| Lack of fit | 26.43 | 3 | 8.81 | 1.39 | 0.4441 | Not significant |
Table 2: Anova Data for Different Responses, (A): Transmittance (T); (B): DS and (C): ZP of PNC-NE Formulations
Fig. 3: Contour plots (a): Contour plot for response Transmittance. All factors interaction showed significant impact on Transmittance; (b): Contour plot for response DS. All factors interaction showed significant impact on DS and (c): Contour plot for response ZP. All factors interaction showed significant impact on ZP
The mathematical equation suggested by the software for ZP is provided in equation (3). From the equation for ZP, we can conclude that the increase in Smix, oil phase, and water phase concentrations increases the ZP of PNC-NEs. Among these, the higher magnitude of the regression coefficient for factor A (Smix) shows that the effect of Smix concentration is more on the ZP of formulated PNC-NEs.
ZP = 664.0 A + 16.66 B + 9.09 C - 792.51 AB - 677.69 AC + 464.34 ABC (3)
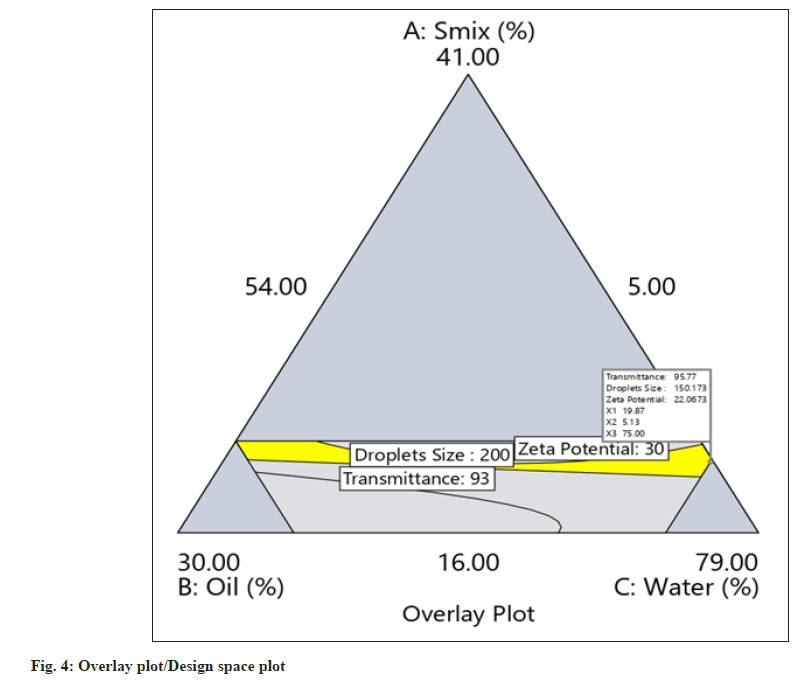
The yellow region in the overlay plot/design space plot shown in fig. 4 shows design space. Any combinations of concentrations of factors A, B, and C within the yellow region will provide PNC-NEs with predefined Critical Quality Attributes (CQA).
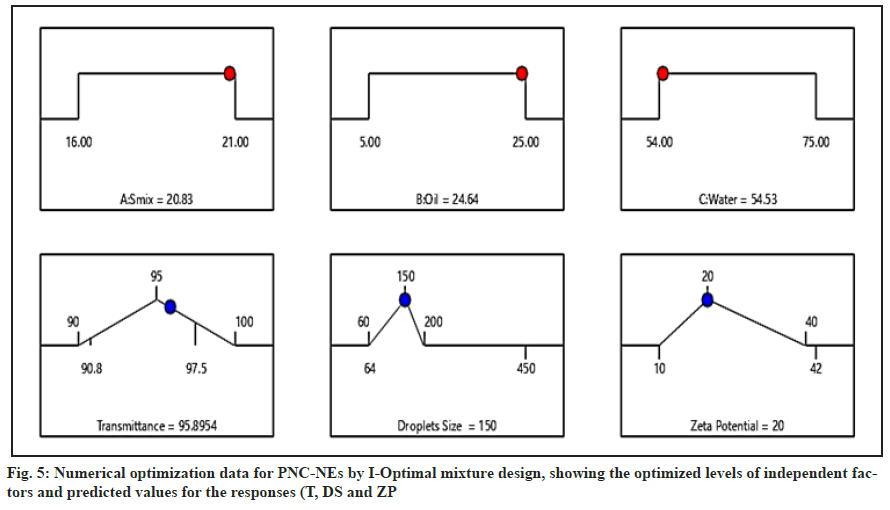
For the validation of the regression model, three batches were manufactured as suggested by the design expert software. The observed data of T, DS, and ZP was compared against the predicated mean (target) with one sample T-test to confirm the statistical similarity of the data. The p-value shown in Table 3 for all responses is >0.05 (fail to reject the null hypothesis), which indicates that the observed values are statistically similar to the predicted mean that validates the model derivation of the study (fig. 5).
| Smix (%) | Oil phase (%) | Water phase (%) | Response | Predicated mean | Observed mean (n=3) | 95 % PI low | 95 % PI high | Observed p value |
|---|---|---|---|---|---|---|---|---|
| 20.84 | 24.64 | 54.53 | Transmittance | 95.895 | 96.233 | 95.245 | 96.546 | 0.594 |
| DS | 150 | 175.3 | 51.521 | 248.48 | 0.147 | |||
| ZP | 20 | 21.7 | 10.785 | 29.215 | 0.63 | |||
| 19.88 | 5.13 | 75 | Transmittance | 95.774 | 96.1 | 95.069 | 96.479 | 0.808 |
| DS | 150 | 119 | 48.902 | 251.098 | 0.067 | |||
| ZP | 22.085 | 27 | 12.44 | 31.731 | 0.084 | |||
| 21 | 25 | 54 | Transmittance | 96.212 | 96 | 95.538 | 96.886 | 0.821 |
| DS | 141.27 | 176 | 41.608 | 240.932 | 0.076 | |||
| ZP | 19.325 | 23 | 9.6559 | 28.995 | 0.713 |
Note: Comparison of predicted and observed mean along with observed P value
Table 3: Validation of Regression Model, Formulation Trials and Responses
NEGs were prepared using an optimized formulation of PNC-NEs and three levels of concentrations (0.8, 1.3, and 1.8% w/w) of four different gel-forming agents. Formulated PNC-NEGs were observed visually for homogeneity and phase separation (Table 4). All three levels (0.8, 1.3, and 1.8% w/w) of Methocel K4M, 0.8 and 1.3% w/w levels of Carbopol 934, and 0.8% w/w levels of Methocel K15M were found to be poor in retaining homogeneity and phase. Other PNC-NEGs were analysed for potency, viscosity, pH, and bio adhesiveness.
| B. No. | Gelling agent | %w/w | Potency (%w/w) | Viscosity (Pas) | pH | Bioadhesive strength g/cm2 |
|---|---|---|---|---|---|---|
| PG17 | Carbopol 934 | 1.8 | 96.76±0.80 | 2.66±0.15 | 5.87±0.08 | 6.421±0.36 |
| PG18 | Carbopol 940 | 0.8 | 95.69±1.02 | 2.88±0.23 | 5.75±0.11 | 5.638±0.47 |
| PG19 | Carbopol 940 | 1.3 | 97.41±1.15 | 3.76±0.19 | 5.92±0.14 | 6.142±1.12 |
| PG20 | Carbopol 940 | 1.8 | 98.92±0.76 | 4.12±0.48 | 6.02±0.12 | 8.952±0.96 |
| PG22 | Methocel K15M | 1.3 | 94.25±2.12 | 3.82±0.22 | 7.68±0.09 | 6.251±1.21 |
| PG23 | Methocel K15M | 1.8 | 96.76±1.52 | 4.06±0.36 | 7.96±0.13 | 6.628±1.36 |
Note: Each value is the mean of three observations
Table 4: Results of Potency, Viscosity, PH and Bioadhesive Strength
The relatively higher drug content observed in PG20 is attributed to the high degree of homogeneity due to the viscosity of the gelling agent, Carbopol 940 (40 000 to 60 000 cPas)[32]. The viscosity of the formulation has a direct impact on drug permeation and extrudability. Drug permeation is improved due to enhanced contact with the skin and can be achieved by higher viscosity gelling agents and/or their usage in appropriate concentrations in the formulation[33][34].
PG20 and PG23 have shown good viscosities of 4.12 and 4.06, which match the recommended viscosities for gel formulations[35]. It is recommended by the researchers that topical formulations possess pH values in the range of 4–6 for the maintenance of skin health and function[36]. The primary goal is to have higher bioadhesiveness so that formulations remain adhered to the skin for a longer time, thereby enhancing the drug permeation process in a site-specific manner[37]. PG20 formulation that has Carbopol 940 in the 1.80 % w/w concentration has shown maximum bioadhesive strength; 19.694 g of water is required to separate rat skin (2.2 cm²) placed between the two glass slides.
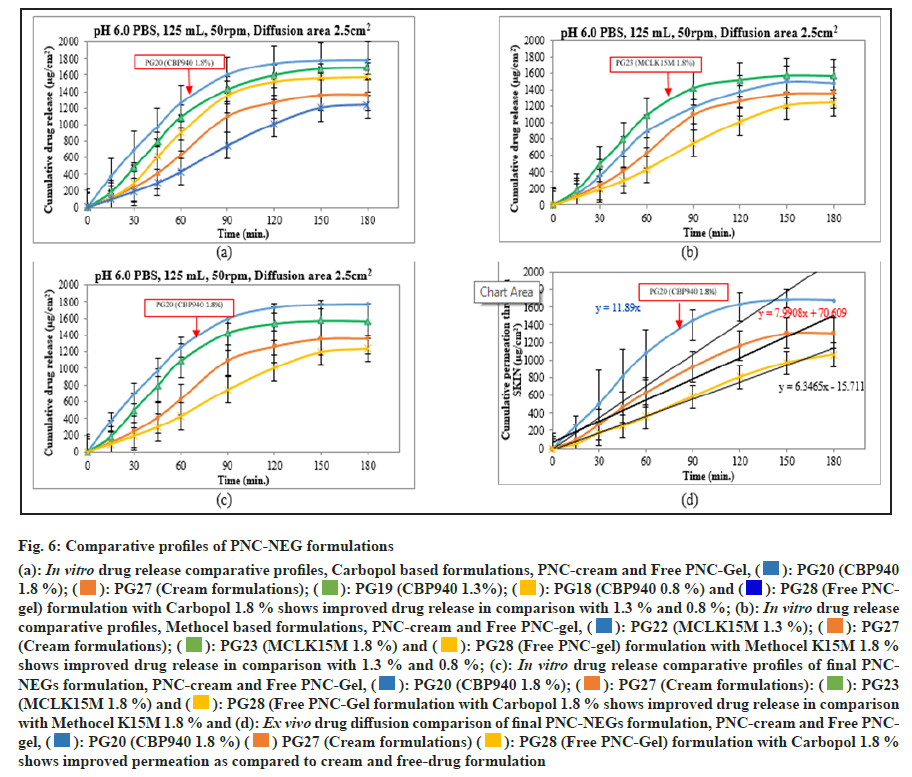
The in vitro drug release results showed a significant improvement in PNC release in the PNC-NEGs optimized formulation. At the end of 60 min, PNC release from PNC-cream and free PNC-Gel was 31.5 % and 21.4 %, whereas from the PNC-NEG formulation of three different levels of Carbopol 940, release was 45.1 %, 54.6 %, and 62.8 %. Drug release at 60 min was found to increase up to 3-fold in the PNC-NEGs with a 1.8 % w/w concentration of Carbopol 940 as compared to free PNC-Gel. Changes in the drug release were found in the order of change in concentration of Carbopol 940, i.e., 0.8 % < 1.3 % < 1.8 %, which is in line with the reported studies where drug release patterns were assessed against different concentrations of gelling agents[34]. At the end of 180 min, PNC release from PNC-Cream and free PNC-Gel was 68 % and 62 %, whereas drug release from the PNC-NEG formulation of three different levels of Carbopol 940 was 78.9 %, 84.4 %, and 88.9 %. Significant improvement in drug release was observed in the PNC-NEG formulation as the drug is in dissolved form in Nano-sized oil droplets in the presence of surfactants (fig. 6). The approach of encapsulating poorly water-soluble drugs in oil droplets to substantially increase dissolution is widely studied[38].
The smaller DS increases the surface area, enhancing contact with the skin and improving permeability through the stratum corneum (the skin’s outermost layer). The embedding of water-soluble drugs within the oil phase of the NE enables better penetration into deeper layers of the skin. Once the NE droplets reach the target skin layer, they release the drug, improving its local availability and therapeutic action. NEGs bypass the limitations of creams, which often struggle with limited skin penetration. Enhanced drug retention at the target site prolongs the duration of action, reducing the need for frequent reapplication. NEGs address challenges such as poor solubility, low bioavailability, and potential irritation, which are common with conventional formulations. Their therapeutic impact would be reduced viral shedding time by improving drug penetration and retention, shorten the time during which the virus is actively shed, potentially reducing transmission rates. Enhanced delivery and prolonged action can accelerate the healing process for lesions and improve patient outcomes.
The most acceptable assessment tool by the USFDA for comparison of the dissolution profiles is the similarity factor (f2). An f2 value greater than 50 indicates similar release profiles[39]. Thus, in the present study, the observed f2 values of 24 and 31 show a significant difference between the in vitro release profiles of free PNC-Gel vs. optimized PNC-NEG (PG20) and PNC-Cream vs. optimized PNC-NEG (PG20).
In addition, PNC-NEG methocel formulations were also evaluated for drug release studies (fig. 6b). In the present study, the f2 values of 31 and 42 observed show a significant difference between the in vitro release profiles of free PNC-Gel vs. PNC-NEG (PG23) and PNC-Cream vs. optimized PNC-NEG (PG23). Drug release profiles of 1.8 % w/w concentrations of Carbopol 940 and Methocel K15M were also compared (fig. 6c) and found to be dissimilar with an f2 value of 49 (<50). However, improved drug release was observed in Carbopol 940 at 1.8 % w/w PNC-NEGs, and that forms the basis for finalizing it for the ex vivo drug diffusion study and stability study.
Fig. 6: Comparative profiles of PNC-NEG formulations
(a): In vitro drug release comparative profiles, Carbopol based formulations, PNC-cream and Free PNC-Gel,  : PG20 (CBP940
1.8 %);
: PG20 (CBP940
1.8 %);  : PG27 (Cream formulations);
: PG27 (Cream formulations);  : PG19 (CBP940 1.3%);
: PG19 (CBP940 1.3%);  : PG18 (CBP940 0.8 %) and
: PG18 (CBP940 0.8 %) and  : PG28 (Free PNCgel)
formulation with Carbopol 1.8 % shows improved drug release in comparison with 1.3 % and 0.8 %; (b): In vitro drug release
comparative profiles, Methocel based formulations, PNC-cream and Free PNC-gel,
: PG28 (Free PNCgel)
formulation with Carbopol 1.8 % shows improved drug release in comparison with 1.3 % and 0.8 %; (b): In vitro drug release
comparative profiles, Methocel based formulations, PNC-cream and Free PNC-gel,  : PG22 (MCLK15M 1.3 %);
: PG22 (MCLK15M 1.3 %);  : PG27
(Cream formulations);
: PG27
(Cream formulations);  : PG23 (MCLK15M 1.8 %) and
: PG23 (MCLK15M 1.8 %) and  : PG28 (Free PNC-gel) formulation with Methocel K15M 1.8 %
shows improved drug release in comparison with 1.3 % and 0.8 %; (c): In vitro drug release comparative profiles of final PNCNEGs
formulation, PNC-cream and Free PNC-Gel,
: PG28 (Free PNC-gel) formulation with Methocel K15M 1.8 %
shows improved drug release in comparison with 1.3 % and 0.8 %; (c): In vitro drug release comparative profiles of final PNCNEGs
formulation, PNC-cream and Free PNC-Gel,  : PG20 (CBP940 1.8 %);
: PG20 (CBP940 1.8 %);  : PG27 (Cream formulations):
: PG27 (Cream formulations): : PG23
(MCLK15M 1.8 %) and
: PG23
(MCLK15M 1.8 %) and  : PG28 (Free PNC-Gel formulation with Carbopol 1.8 % shows improved drug release in comparison
with Methocel K15M 1.8 % and (d): Ex vivo drug diffusion comparison of final PNC-NEGs formulation, PNC-cream and Free PNCgel,
: PG28 (Free PNC-Gel formulation with Carbopol 1.8 % shows improved drug release in comparison
with Methocel K15M 1.8 % and (d): Ex vivo drug diffusion comparison of final PNC-NEGs formulation, PNC-cream and Free PNCgel,  : PG20 (CBP940 1.8 %)
: PG20 (CBP940 1.8 %)  PG27 (Cream formulations)
PG27 (Cream formulations)  : PG28 (Free PNC-Gel) formulation with Carbopol 1.8 %
shows improved permeation as compared to cream and free-drug formulation
: PG28 (Free PNC-Gel) formulation with Carbopol 1.8 %
shows improved permeation as compared to cream and free-drug formulation
In the ex vivo diffusion study, the permeation of PNC through human cadaver skin when presented as PNC-NEGs in comparison with PNC-Cream and free PNC-Gel was evaluated. Permeation was calculated in terms of flux (μg/cm²/min), Kp (diffusion coefficient), and EF. The flux was found to be 11.89 (Kp 5.95 × 10-6), 7.99 (3.99 × 10-6) and 6.35 (Kp 3.18 × 10-6) μg/cm²/min for PNC-NEGs, PNC-cream, and free PNC-Gel formulations, respectively. The flux from PNC-NEGs was significantly higher (p < 0.05) than the flux from PNC-cream and Free PNC-Gel (fig. 6d). This highlighted the importance of the newly developed PNC-NEG. The EF of 1.87 was obtained for PNC-NEG against free PNC-Gel and 1.49 was obtained against PNC-cream. Overall, the diffusion studies confirmed the effectiveness of the PNC-NEG formulation and its ability to deliver the drug through the skin.
The optimized molecular structure of PNC is subjected to solvation energy calculations in octanol and water using the DFT software package. The calculated solvation energies are used to determine the partition coefficient (logP) of PNC in the octanol-water system. The obtained logP value provides insights into the lipophilicity of PNC, which influences its absorption and distribution properties in biological systems.
The quantum chemistry-based LogP calculation method yields a precise estimation of PNC partition coefficient in the skin-formula system (fig. 7). The obtained LogP value can be compared with experimental data or pharmacokinetic properties of PNC.
The partition coefficient signifies the amount of active partitioning into the subcutaneous (SC) layer from the formulation. The accuracy of the LogP prediction depends on various factors, including the level of theory, solvation model, and conformational sampling. Sensitivity analysis and validation studies can be conducted to evaluate the robustness and reliability of the methodology. In this study, we have performed Multiscale Simulation (MD) of the skin SC layer. The obtained release profile of different formulations through the SC model shows good qualitative agreement with experiments. A realistic model of skin’s SC layer having an equimolar mixture of ceramide, cholesterol, and free fatty acid was used. Several constrained MD simulations were performed to calculate the diffusion of three formulations, namely P20, PG27 (cream formulations), and PG28 (Free PNC-Gel). As shown in Table 5, diffusivity of the formulations changed with the formulation matrix. The higher the LogP values, the better the permeation of active ingredients from the formulation to skin. In the PG20, the diffusivity was ~1.5 times higher than that of PG28. This model also helps to understand the impact of each formulation excipient on API permeation by assessing it in terms of binding and energy contributions.
| Test parameters | Acceptance criteria | Initial | 40°±2°/75± 5 % RH | 25°±2°/65±5 % RH 6 mo | ||
|---|---|---|---|---|---|---|
| 1 mo | 3 mo | 6 mo | ||||
| Potency (%) | 90-110 % | 98.24±0.66 | 96.41±0.81 | 95.25±1.11 | 96.05±0.92 | 97.48±45 |
| pH of gel | 5.0 to 6.5 | 6.35±0.20 | 6.27± 0.18 | 6.11± 0.36 | 5.96±0.25 | 6.30±0.12 |
| Density (g/ml) | 1.15±0.05 | 1.18±0.08 | 1.14±0.07 | 1.16±0.03 | 1.17±0.02 | 1.14±0.17 |
| Phase separation | Should be single phase | No phase separation | No phase separation | No phase separation | No phase separation | No phase separation |
| Change in color | Translucent | Translucent | Translucent | Translucent | Translucent | Translucent |
| Lump/Crystals | Free from lumps and crystals | Free from lumps and crystals | Free from lumps and crystals | Free from lumps and crystals | Free from lumps and crystals | Free from lumps and crystals |
Note: Accelerated and long term stability results of optimized PNC-NEG (Batch no. PG20)
Table 5: Stability Data
Stability of optimized PNC-NEGs was performed under accelerated conditions (40°±2°/75 %±5 % Relative humidity) and long-term conditions (25°±2°/65 %±5 % relative humidity). The results of potency, pH, and density were shown in Table 6. No change with respect to phase separation, color, lumps, or crystal formation was observed until the evaluation period of 6 mo.
| Formulation | Logp_skin_formula | Flux J ss (µg/cm2/min) | Drug release 60 min |
|---|---|---|---|
| PG20 | -0.597 | 11.89 | 1256 |
| PG27 (Cream Formulation) | -0.618 | 7.99 | 630 |
| PG28 (Free PNC-Gel) | -0.664 | 6.35 | 428 |
Table 6: Computational Logp Results
PNC-NEs were formulated and optimized by I-optimal design using percentages of Smix, oil, and purified water as the variable factors and T, DS, and ZP as responses. The optimized PNC-NEs contained Smix, oil, and water in concentrations of 20.84, 24.64, and 54.52 %, respectively. The optimized PNC-NEs have T, DS, and ZP values close to the prediction. The concentration range of different excipients obtained is around 1.5 times less than the established maximum potency or dose, as per the USFDA IID database[25]. Reported recommended concentrations of Tween 20, Labrafil M 1944, and MO are 1 %-10 %, 5 %-20 %, and 1 %-20 %, respectively. The concentrations of excipients in the optimized PNC-NE are well within these ranges. The optimized formulation has been deemed non-toxic and safe for administration based on current composition selection. This establishes a foundation for advancing to more detailed testing like drug irritation testing to assess any potential irritation caused by the drug or formulation, likely through animal models or alternative in vitro methods, and drug retention testing to evaluate how effectively the drug remains at the application site over time, possibly through histopathological analysis of the skin.
The Nano-emulsion gel was prepared from the PNC-NE by the spontaneous emulsification method and evaluated for potency, bio adhesive strength, viscosity, pH, in vitro drug release, and ex vivo diffusion. Further, improved drug permeation through skin was observed from the PNC-NEG when compared with the PNC-cream and free PNC-gel formulations. The results of the study demonstrated the superiority of the developed PNC-NEG formulation over PNC-cream and free PNC-Gel. The PNC-NEG formulation was found to be encouraging and can be further evaluated by in vivo studies to establish its clinical supremacy through pharmacokinetic/pharmacodynamics parameters for therapeutic use in HSV-associated infections. The study positions PNC-NEG as a novel formulation for HSV treatment, potentially overcoming the limitations of existing therapies like poor skin penetration, stability, or irritation.
Acknowledgments:
The author acknowledges Dr. Vishvesh Joshi, Vicepresident, Alembic Labs LLC, New Jersey, USA for his entire guidance and support.
Conflict of interests:
The authors declared no conflict of interests.
References
- Charlotte J, Manale H, Welton NJ, Katherine MT, Abu-Raddad LJ, Gottlieb SL, et al. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull World Health Organ 2020;98(5):315-29.
[Crossref] [Google Scholar] [PubMed]
- Shuyong Z, Viejo-Borbolla A. Pathogenesis and virulence of herpes simplex virus. Virulence 2001;12(1):2670-702.
[Crossref] [Google Scholar] [PubMed]
- Agelidis AM, Shukla D. Cell entry mechanisms of HSV: What we have learned in recent years. Future Virol 2016;10(10):1145-54.
[Crossref] [Google Scholar] [PubMed]
- Richard W, Baines J. Clinical management of herpes simplex virus infections: Past, present, and future. F1000Res 2018;7( Rev):1-9.
[Crossref] [Google Scholar] [PubMed]
- Gupta P, Patel DH. A review on nano-emulsion based gel formulations for dermatological application: emulgel, technology and recent advances. Int J Pharma Sci Nanotechn 2022;15(1):5741-62.
[Crossref]
- Schmid-Wendtner MH, Korting HC. Penciclovir cream-improved topical treatment for herpes simplex infections. Skin Pharmacol Physiol 2004;17(5):214-8.
[Crossref] [Google Scholar] [PubMed]
- Ashara KC, Paun JS, Soniwala MM, Chavada JR, Mori NM. Micro-emulsion based emulgel: A novel topical drug delivery system. Asian Pacific J Trop Dis 2014;4(Suppl 1):S27-32.
- Chen Y, Quan P, Xiaochang L, Wang M, Fang L. Novel chemical permeation enhancers for transdermal drug delivery. Asian J Pharma Sci 2014;9(2):51-64.
- Zheng Y, Ouyang WQ, Wei YP, Syed SF, Hao CS, Wang BZ, et al. Effects of Carbopol® 934 proportion on nanoemulsion gel for topical and transdermal drug delivery: A skin permeation study. Int J Nanomedicine 2016;11:5971-87.
[Crossref] [Google Scholar] [PubMed]
- Hosny KM, Sindi AM, Alkhalidi HM, Kurakula M, Alruwaili NK, Alhakamy NA, et al. Oral gel loaded with penciclovir-lavender oil Nano-emulsion to enhance bioavailability and alleviate pain associated with herpes labialis. Drug Deliv 2021;28(1):1043-54.
[Crossref] [Google Scholar] [PubMed]
- Zhu W, Guo C, Yu A, Gao Y, Cao F, Zhai G. Microemulsion-based hydrogel formulation of penciclovir for topical delivery. Int J Pharm 2009;378(1-2):152-8.
[Crossref] [Google Scholar] [PubMed]
- Lachenmeier DW. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J Occup Med Toxicol 2008;3(26):1-16.
[Crossref] [Google Scholar] [PubMed]
- Lémery E, Briançon S, Chevalier Y, Bordes C, Oddos T, Gohier A, et al. Skin toxicity of surfactants: Structure/toxicity relationships. Colloids and Surfaces A: Phsicochemical and Engineering Aspects 2015;469:166-79.
- Nigam P, Gupta AK, Vats A. Film forming transemulgel-A novel drug delivery system. IP Int J Comp Adv Pharmacol 2020;5(3):100-4.
- Chavda V, Rupapara V. Formulation and evaluation of naproxen emulgel for topical delivery by a modified method. Pharmacie Globale Int J Comp Phar 2013;7(3):1-4.
- Varma VNSK, Maheshwari PV, Navya M, Reddy SC, Shivakumar HG, Gowda DV. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm J 2014;22(6):591-99.
[Crossref] [Google Scholar] [PubMed]
- Kanfer I, Rath S, Purazi P, Mudyahoto NA. In-vitro release testing of semi-solid dosage forms. Dissolution Technol 2017:52-60.
- Bharot BS, Parejiya PB, Patel HK, Gohel MC, Shelat PK. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: Optimization of formulation using D-optimal design. AAPS PharmSciTech 2012;13(1):184-92.
[Crossref] [Google Scholar] [PubMed]
- Stanos SP. Topical agents for the management of musculoskeletal pain. J Pain Symptom Manage 2007;33(3):342-55.
[Crossref] [Google Scholar] [PubMed]
- Rao M, Sukre G, Aghav S, Kumar M. Optimization of metronidazole emulgel. J Pharm 2013;2013:1-10.
[Crossref] [Google Scholar] [PubMed]
- Kwon S, Bae H, Jo J, Yoon S. Comprehensive ensemble in QSAR prediction for drug discovery. BMC Bioinformatics 2019;20(521):2-12.
[Crossref] [Google Scholar] [PubMed]
- Bhatnagar N, Kamath G, Chelst I, Potoff JJ. Direct calculation of 1-octanol-water partition coefficients from adaptive biasing force molecular dynamics simulations. J Chem Phys 2012;137(1):014502.
[Crossref] [Google Scholar] [PubMed]
- Buggert M, Cadena C, Mokrushina L, Smirnova I, Maginn EJ, Arlt W. COSMO-RS calculations of partition coefficients: different tools for conformational search. Chem Eng Technol 2009;32(6):977-86.
- Gajula K, Gupta R, DB S, Rai B. In-silico skin model: A multiscale simulation study of drug transport. J Chem Inf Model 2017;57(7):1-19.
[Crossref] [Google Scholar] [PubMed]
- Pronk S, Pall S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013;29(7):845-54.
[Crossref] [Google Scholar] [PubMed]
- Prashar A, Locke IC, Evans CS. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif 2004;37(3):221-29.
[Crossref] [Google Scholar] [PubMed]
- Sinha S, Jothiramajayam M, Ghosh M, Mukherjee A. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem Toxicol 2014;68:71-7.
[Crossref] [Google Scholar] [PubMed]
- Wang Z, Mu HJ, Zhang XM, Ma PK, Lian SN, Zhang FP, et al. Lower irritation micoremulsion-based rotigotine gel: formulation optimization and in vitro and in vivo studies. Int J Nanomedicine 2015;10(1):633-44.
[Crossref] [Google Scholar] [PubMed]
- Li X, Tu ZC, Sha XM, Ye YH, Li ZY. Flavor, antimicrobial activity, and physical properties of composite film prepared with different surfactants. Food Sci Nutr 2008;8(7):3099-109.
[Crossref] [Google Scholar] [PubMed]
- United States Food and Drug Administration 2025. Inactive ingredient search for approved drug products.
- Paul SJ, Bruno HC, Gary MP, David GJ. Handbook of Pharmaceutical Excipients. 9th ed. Washington DC: Pharmaceutical Press and American Pharmacists Association; 2020.
- Sasutjarit RA, Sirivat A, Vayumhasuwan P. Viscoelastic properties of Carbopol 940 gels and their relationships to piroxicam diffusion coefficients in gel bases. Pharm Res 2005;22:2134-40.
[Crossref] [Google Scholar] [PubMed]
- Binder L, Mazál J, Petz R, Klang V, Valenta C. The role of viscosity on skin penetration from cellulose ether‐based hydrogels. Skin Res Technol 2019;25(5):725-34.
[Crossref] [Google Scholar] [PubMed]
- Batheja P, Sheihet L, Kohn J, Singer AJ, Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J Control Release 2011;149(2):159-67.
[Crossref] [Google Scholar] [PubMed]
- Nurman S, Yulia R, Irmayanti, Noor E, Candra Sunarti T. The optimization of gel preparations using the active compounds of arabica coffee ground nanoparticles. Scientia Pharmaceutica. 2019;87(4):32.
- Lukić M, Pantelić I, Savić SD. Towards optimal ph of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021;8(3):69.
- Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996;17(16):1553-61.
[Crossref] [Google Scholar] [PubMed]
- Algahtani MS, Ahmad MZ, Ahmad J. Nanoemulgel for improved topical delivery of retinyl palmitate: formulation design and stability evaluation. Nanomaterials 2020;10(5):848.
[Crossref] [Google Scholar] [PubMed]
- Diaz DA, Colgan ST, Langer CS, Bandi NT, Likar MD, Van Alstine L. Dissolution similarity requirements: How similar or dissimilar are the global regulatory expectations? AAPS J 2016;18:15-22.
[Crossref] [Google Scholar] [PubMed]