- *Corresponding Author:
- K. Sonia
Department of Pharmaceutical Analysis, SRM Institute of Science and Technology, Kattankulathur, Chengalpattu 603203, Tamil Nadu, India
E-mail: soniapharm68@gmail.com
| Date of Received | 16 October 2021 |
| Date of Revision | 03 July 2023 |
| Date of Acceptance | 06 December 2023 |
| Indian J Pharm Sci 2023;85(6):1562-1573 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Blood and blood (plasma) products amuse oneself a crucial function in existence of person’s life. In the approach of rigorous science and technology development there is no course of action to produce feigned bases of human blood, hence no substitute for human blood till now. Annually, the millions of patient’s lives are safeguard by modern health care services such cases like surgeries, cancer treatment, trauma, organ transplantation and during childbirth. The role of (Centre for biological evaluation and research) which was organized by Food and Drug Administration, the World Health Organization protects the public health by secure and supply of biological products whenever is needed and also provided expedient information about biological products. Evaluation criteria for national blood regulatory system “blood regulation network” that describes about divine rights and functions of integrated blood regulations. The interior consequence includes authorization of blood firm marketing, benchmark of clinical investigation, complete data regarding patient blood management. Though, the strict or precise regulatory framework is not yet provided. The corona virus disease-2019 epidemic has wreaked havoc on several countries health-care systems. The voluntary blood donation camps were discontinued due to early government measures such as widespread lockdown and restriction strategies toward public gatherings. This study examines the recorded regulatory guidelines in case of blood and blood products, in addition of hemovigilance, as set forth by the United States food and drug administration, the Europe, and India, as well as the scenario during corona virus disease-2019.
Keywords
Blood products, corona virus disease-2019, hemovigilance, regulations
Worldwide 118.4 million blood donations are composed, out of this 40 % of blood collected from wealthier countries. According to the journal the lancet, India is now experiencing a demand for blood and blood products. In wealthier countries, blood products are typically accustomed to progesterone receptor cardiovascular surgery, transplantation, trauma resuscitation (revive), hematological diseases, treatment of cancer and medical and surgical policy. Modern Health care was accepted in 1976 and gradually it was accepted all over the world. The role of health care services, upgrade the treatment and prevention of diseases, curative of diseases and rehabilitation of physical and mental ruination. Those health care services safeguards millions of patient’s lives by alleviating at critical situations. Centre for Biological Research and Evaluation (CBER) is the key interior of Food and Drug Administration (FDA), legally considered biological products are regulated by public health service act and drugs as per federal drug and cosmetics act and get license through biological license application regulated under section (21 Code of Federal Regulations (CFRs) 600.680). Before licensing the drug must undergo specific confirmatory and laboratory tests as per regulatory guidelines to prevent contamination. CBER creates awareness and responsible for promoting safety, purity, efficacy and usage of biological products according to regulatory guidelines under section 505, 351, 361. The Blood Regulation Network (BRN) was introduced and accepted in 2006, holds main international regulatory agency with (resolution WHA63.12), the World Health Organization (WHO) urged all member nations to implement Patient Blood Management in 2010 (PBM)[1]. It focuses on three "pillars" of surgical care; peri-operative anemia identification and treatment, peri-operative blood loss reduction and harnessing and utilizing the patient's physiological reserve anemia[2]. In India, blood and blood products are regulated under Drugs and Cosmetics Act 1940 sec 2(b). The National Blood Policy (NBP) and the National Blood Transfusion Council (NACO) are working tirelessly to improve India's Blood Transfusion Services (BTS). As a result, strengthening nationwide and regional ‘blood regulatory authorities’ have been identified a vital prerequisite for securing the worldwide supply of blood products. "Assessment criteria for national" released by the BRN, is a significant step forward in this area. A policy question arises within the (national blood regulatory system) concerning in what manner to manage the blood safety and availability in the text of limited health resources and services administration and social outlooks[3]. Central Drug Standard Control Drugs (CDSCO), India is an administrative body regulatory agency for pharmaceutical drugs and medical devices which was similar to FDA. The blood system in European countries is autonomous (independent) with completely different association. In European countries the blood products regulated by European Community (EC) and European Union (EU). The suitable laws and guidelines are prepared for such cases. Based upon the certain laws and regulation the overall process has been maintained and documented. As Corona Virus Disease-2019 (Covid-19) epidemic has had a significant impact on blood transfusion[4]. As a result, the local government has been directed to take proactive measures to assure the availability of blood for clinical purposes. The acute local blood scarcity and disgruntled need were managed by allocating blood stockpiles from surrounding cities, or even provinces, through a national blood management information system[5]. Blood collection is lost owing to social closures and widespread illness, resulting in a large decline of whole blood in countries with prompt drastic output[6]. Previous pandemics taught us a variety of lessons about health-care preparedness, including the use of BTS to combat epidemics. The role of BTS in a pandemic is determined by several factors, including the pandemic’s kind, the potential for the spread of the community, the dangers of transfusion transmission, and the blood-safety intervention’s cost-effectiveness[7].
Regulation in India
In India, the cases of accidents and surgeries are frequently increasing, hence there is a large demand for blood components. The available (plasma) blood products are collected and stored in blood bank. A blood product includes the whole blood and its constituents such as plasma, cells of the blood (regional blood coordination centers), pregnant women, and children when they are in need for blood. Hence, the emergency services should work properly in this critical situation. The WHO blood regulatory network produced "assessment criteria for national blood regulatory systems" which advocates for the blood system. Entire regulatory system is based on good preparation practices, consists of transfusion of white blood cells and platelets. The death rate is high due to insufficiency of blood in case of transfusion of blood, quality system and good manufacturing practices of blood constituents[8].
Good pharmacoepidemiology practices requirements:
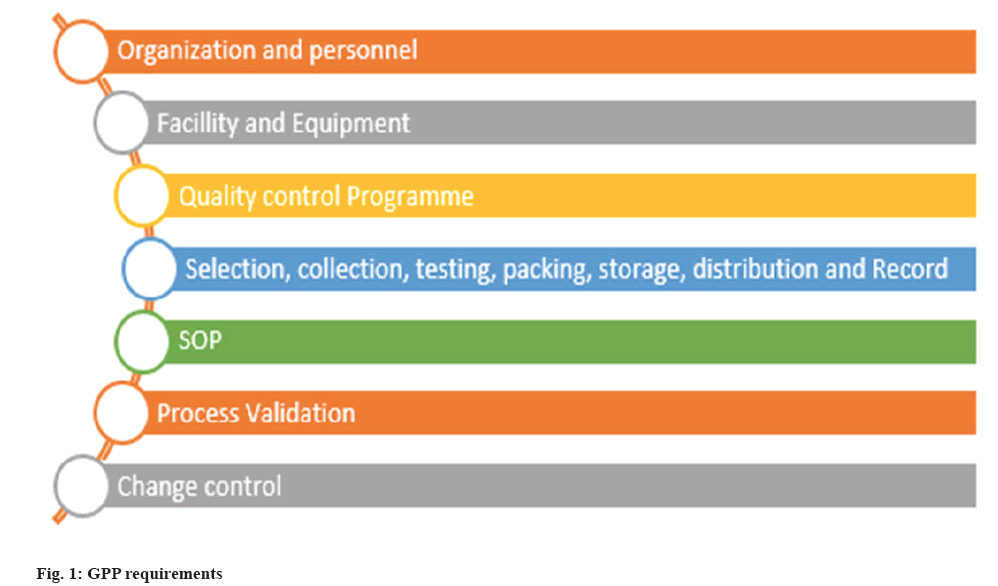
In the year 2000, the WHO declared that "blood saves lives" and that "safe blood begins with me”. In 2001, the WHO created the Quality Management Plan (QMP) and QMT programmed to ensure sufficient training and blood safety fig. 1. It was held to raise awareness about the QMP and the importance of QMT in ensuring the quality, safety, and adequacy of blood. In India, BTS are direct by the, Drugs and Cosmetics Act 1940 (D and C Act). The registration of blood donor is accepted from the age of 18 y in India. There is a backdrop for the blood transfusion system. While transfusion process, we should notice the mode of transmission of infections and other diseases. Following the process of blood transfusion, clear examination (screening of blood) should be done. The Drug Controller General of India (DGGI) was accountable for the quality and safety monitoring of blood supply in blood banks NACO (National/State AIDS Control Organization), (National/State blood transfusion council). NBP encourages the technology of BTS and improved in the field of transfusion medicine. The public private partnership model plays major role in the device implementation and management of BTS and helps to reach grass root level of healthcare system in India. eg., BTS in Gujarat[9]. In India the status of blood banks has 4 distinct types of blood center are accomplished by the NGOS, public sector, corporate sector also Indian red cross society[10].
Hospitals in south India (Puducherry) maintains ‘Stringent screening criteria’ and determined International Classification of Diseases (ICD10). The blood obtained from non-remunerated blood donor is a cherished resource which required to be efficiently managed and provided. Every patient they sign up to the date included during hospice number, gender, age, classification and number of plasma components distributed and other pertinent details. The data was recorded in Ms excel and evaluating using IBM statics for windows[11]. It is mandatory for analysing such as the Human Immune Deficiency Syndrome (HIV), Universal screening for human Chagas Disease (CD), malaria, cytomegalovirus, hepatitis B virus, hepatitis C virus, T cell lymphocyte virus type 1 and type 2 in the donor blood as per D and C Act and Rules (Rules 68A, Schedule F (Part XA, XIIB)[12,13].
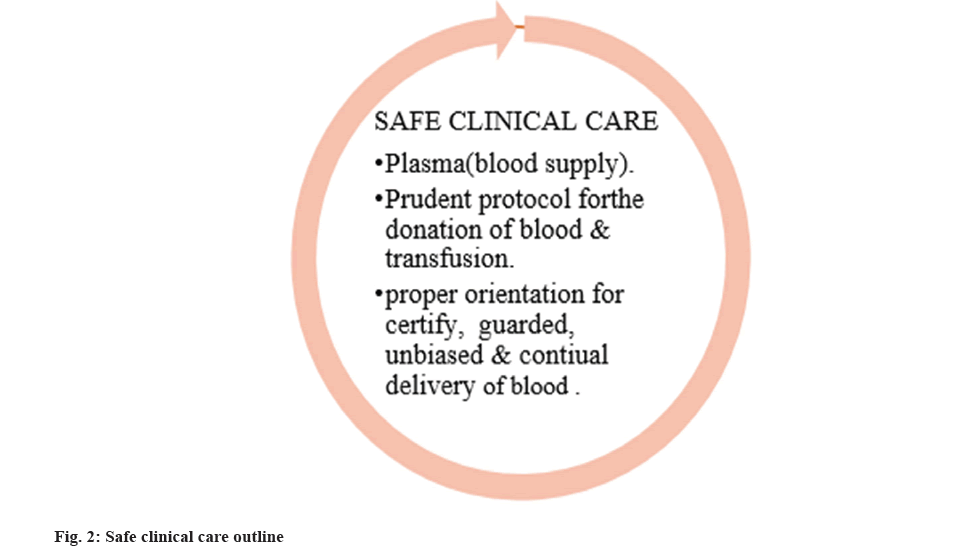
Low and Middle-Income Countries (LMICS) experiencing a shortfall in these classes (fig. 2), especially in pastoral zones. A NBP and BTS are possibly addressing and making regulatory framework for their issues, QMS in their modern perspective case is to provide quality, safety and efficacy[13]. NBP was first implemented in the year 2002 by MOHFW. The framework for NBP is given by the approach of Watt and Gibson (2009) as health policy triangle and the questions four of the ‘What’s the problem represented to be[14]. Blood transfusion has existed broadly and overviewed in medicinal practice ever since in the first part of 20th century to treat anaemia and bleeding (haemorrhage). Here it reviews about the blood transfusion process of quality of evidence and strength of recommendation under 95 % Confidence Interval (CI). Scientific data shows that the restrictive transfusion strategy is safe and sound as the standard strategy. The guidelines for restrictive blood transfusion strategy have been circulated by many systematic high societies such as the British sterol and haematology committee, the American Association of Blood Banks (AABB), the society of critical care medicine, the American college of physicians, and the American society of anaesthesiologists are among the organisations that make up the AABB[15].
Licensing for Blood in India
The central licence approving authority and the superpower of DGGI are liable for issuing licence for blood products. State/central licencing approving authorities grant the licences for operating of blood banks after inspection. The first amendment of the D and C Rules 1945 (4 Jan 2004) concerning the action of freezing to authorize the blood transfusion centres which was undertaken by Armed Force Medical Services in field and sensitive areas. Armed force transfusion services is an effective and systematic network providing support and components to personnel and their subservient[12].
Form 28. C: Licence for operating blood banks, legal paper includes a letter with a 5-rupee law court fee stamp.
Form 27. C: Challan for Rs.7500.
Location: 100 m square for operation use, 50 m square preparation of plasma components.
Collection: Collect from the certified blood depository.
Storage: Stockpiled between 2°-8°.
Master formula record: Production and control industries along with parameters.
Labelling: Consist of data like donor categorization, specific machine-readable barcodes, quantity contained and expiring particulars[16].
Implementation of Hamovigilance in India
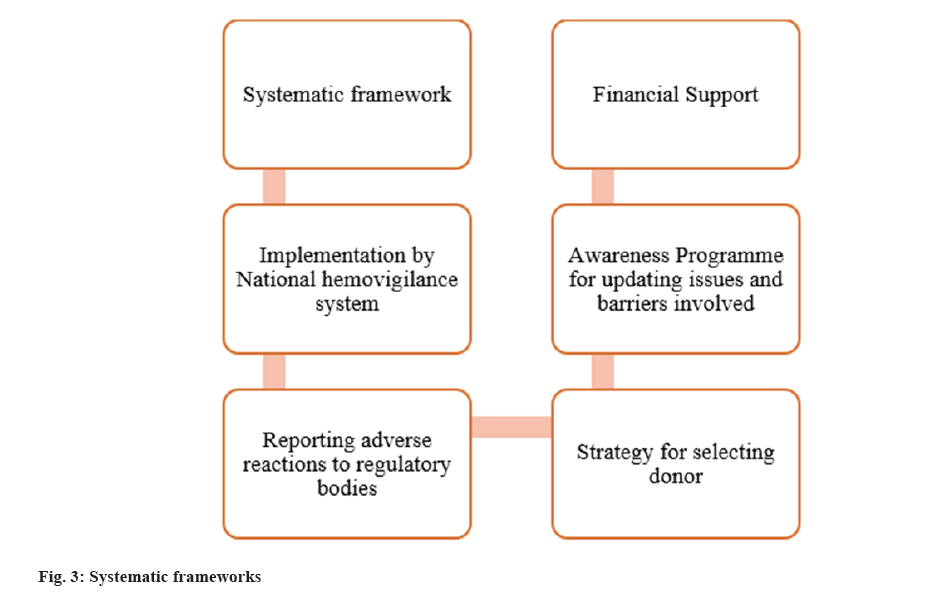
In Greek haem means blood and in Latin vigil means watchful. The concept of hemovigilance is 1st launched and present in the year 1990 in France. The ‘International Society of Blood Transfusion (ISBT)’ and the ‘international hemovigilance network’ are two organisations dedicated to improving blood transfusion safety (HN) plays a crucial role, which scrutinize the complete concatenation of transfusion. Hemovigilance is a quality control system. The QMS which able to detect the undesirable objects and improves transfusion process by organising blood transfusion program in various countries[17]. Acute haemolytic transfusion reaction is occurring due to allergic reaction and adverse events in recipients. The goal of the hemovigilance system is to improve the transfusion process while reducing wastage in order to create and promote safe transfusion of blood. Long-ago evidence shows in India, several individuals received HIV infected blood which ought to be prevented in upcoming year fig. 3[16].
Regulations in United States
Although most donors accept blood donation well from 3 %-10 % of donors will have an unpleasant reaction or damage as a result of their donation. In 2006, the FDA issued a final advisory on the newly developed uniform Donor History Questionary (DHQ) and related materials. The DHQ papers are currently used by most lifeblood facilities in United States. In the year 2007, the FDA put forward a regulation on the requirements for the transfusion of blood components, which included new or proposed changes to federal regulations regarding donor medical history assessment and a consolidated donor eligibility requirement. The demographic shifts of the donor population in the United States has increased blood donation centers' reliance on young age of 18 y old donors. Then the United States FDA (USFDA) makes no age necessities for blood donation in its recommendations and regulations[18]. Donation of blood, distribution and services for blood transfusion are provided in the United States through a system of community-based, hospital-based, and transfusion centers. The data was studied from the year 2015 National Blood Collection and Utilization Survey (NBCUS) survey to gain a better understanding of the changing dynamics of blood collection and use in the US. The survey was conducted using an electronic web-based methodology. The American blood center membership list and registration of blood establishments with the FDA database were used to locate a blood donation sites. The danger of transfusion-transmitted diseases is currently very minimal in the United States blood supply. Despite evidence showing a reduction in transfusion-related adverse effects, the very a small percentage of blood products exposed to a decrease in leukocytes in the United States is likely attributable to cost and a lack of regulatory mandate[19]. The AABB Standards for blood banks and transfusion services, as well as the USFDA, CFRs, establish general principles for blood donor screening and decision-making. A medical history, physical examination, hemoglobin screening tes, and a Confidential Unit Exclusion (CUE) protocol are used to screen donors who present for blood donation. The CUE process is voluntary and not required, according to an FDA regulation[20]. The Health and Human services convened a committee to study the prevalence of contraindications related to blood constituents and the committee suggested that multiple centers are prospectively randomized the trials by comparing the clinical results of inpatient transfusion process[21].
Life Threatening Infection
The “transfusion-related acute lung injury” and the “systemic inflammatory response syndrome” are two serious side effects of Red Blood Cell (RBC), platelet and plasma transfusions[21]. CD, commonly called American trypanosomiasis, is a life-threatening infection caused by insecticides. When an appropriate assay became available in 1989, the United States blood product advisory council advised universal screening for CD. In March of 2007, the FDA released a draught guidance suggesting universal blood donation screening. In the year 2010, the regulatory document was issued by an FDA. The ‘Safety Tripods’ idea is based on the identification of suitable and ‘low risk’ donors, the use of infection marker screening tests, and the removal of residual infections[22].
Hemovigilance
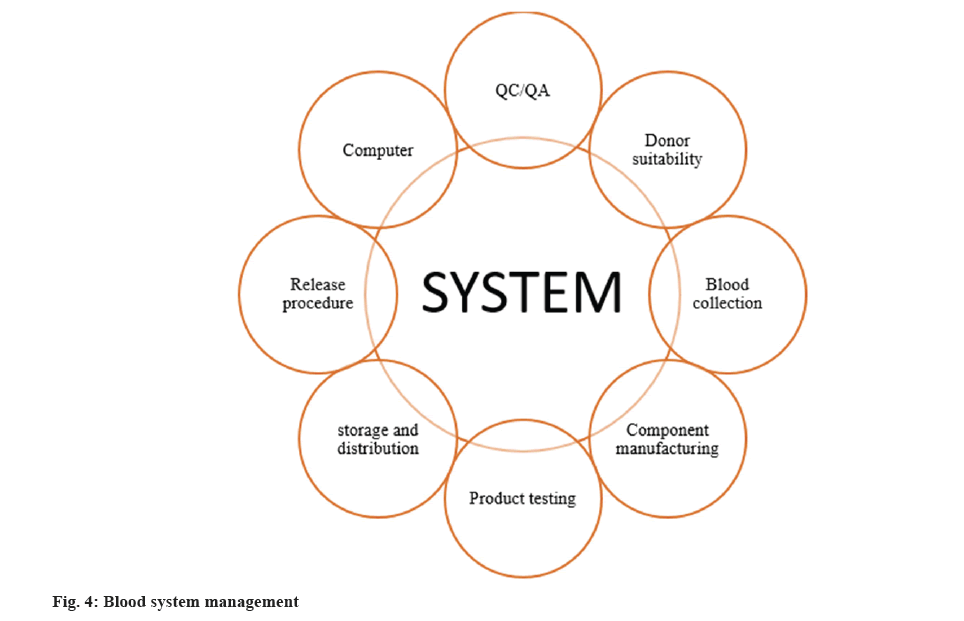
In contrast to Europe, the term hemovigilance is not often used within the United States. Nonetheless, tracking blood transfusion-related morbidity and mortality is a commonly accepted idea. The FDA has its regulatory agency, promulgates memoranda and suggests product recalls. It scrutinizes the facilities; minimize the error and accident reports. Health Care Financing Administration (HCFA) inspects blood facilities that are reimbursed via Medicos and Medicaid for blood tests and transfusions. In a nutshell, FDA inspections are meant to guarantee that blood network establishments have effectual control on their production processes and production systems as shown in fig. 4.
The FDA’s blood products advisory group offers the agency with knowledge based scientific and medical opinion on blood regulation. In United States, the FDA, AABB, HCFA, the National Institute of Health and the Center for Disease Control (CDC) and prevention are among the blood banking and transfusion medical organizations. The AABB, the college of American pathologists, another executive body, the National institute of health joint commission on accreditation of health-care organizations is a non-profit organization that authorizes health-care institution and the USFDA were all part of the medical technical team. For detecting and trending adverse responses, blood centers in the US employ a multidisciplinary approach. For “technical” occurrences, there is no unified center for data collecting, observing, and the policy intercessions[23].
Regulations in Europe
In 20th century the scientific discoveries were transformed into modern-day science due to upheaval in the use of blood. During this time, the stringent for blood components from paid promotion exponentially and scientific testimony shows that such blood components had significant rates of hepatitis infection. Despite the changing nature of the European blood market, both national and European blood policy that emphasizes the importance of unpaid donors in achieving self-sufficiency. The policy was bolstered by strong justification made by Richard Titmuss (British Sociologist on the significance of "What to be termed as the gift relationship" about blood donation, which sparked a current international and research-based discussion. Because of the regionalization of the blood transfusion service, it was impossible to expand the collection and distribution of blood on a national scale under the basic architecture of the National Health Service. Episodes of HIV/AIDS blood staining on a national level sparked scheme and regulatory measurement at the EU extend[24]. The purpose of a PBM program is to identify and limit the risk of blood transfusion while also providing a Standard Operating Procedure (SOP) for the process[25].
Since 2002, the development of EU blood supply directives has been stagnant and is currently being curtailed. The developments of EU blood supply directives has remained, since the year 2002 and are in the process of doing so through a 27-step process. The European Council, acting on support of the Parliament of EU, urged that the (Commission) panel of experts accept suitable suggestions to use a framework for a blood safety policy, and 98/463/EEC Council Recommendation was issued[26].
Scope of directive 2002/98/EC:
Regulation of blood establishing events; plasma facilities planned for the manufacturing of medical products and aspects of blood banking incidents in hospitals[26]. The European Blood Directive (EC) No. 2002/98/EC was enacted in 2002 and the directives that follow and create an overacting summary for transfusion procedure, SOP, clarification and different practices among EU member states. For more than 15 y, blood policies have been on the EC agenda. They discuss both qualitative and quantitative issues that arise to ensuring the blood quality and blood safety. By long-term blood programs, self-sufficiency in blood and its constituents was expected to be the final report[27]. In 1995, the European commission and European blood alliance collaborate. The goal is to raise public knowledge, as well as that of experts, about non-remunerated blood donation and the preparation of its components. Following directive 2002/98/EC, there are furthermore directives; Directive2004/33/EC; directive2005/61/EC; directive2005/62/EC that set out for technical implementation. There is a question raised from the implementation and conveyance of the European regulatory agenda for the components of blood. The eligibility of the donor as per the III.2.2.2 Annex III.2.2.2 Annex III.2.2.2 Annex III of Directive2004/33/EC[27]. In 1999, the Wildbad-Kreuth Initiative, which began with a seminar of 15 member state’s perspectives, formed the EC. The goal was to look into practice, assess it, and come up with a plan to make the best use of blood as a component to improve sense of security and self-sufficiency. The European Directorate for Quality of Medicine and healthcare (EDQM) of the Council of Europe (COE), which is responsible for the transmission of medical products, has been a significant supporter of the Wildbad-Kreuth initiative[28].
In the EU, the blood pool procedure is regulated in numerous directives that dealing with its regulatory and quality issues. Also, it regulates the obligatory requirements on blood along with plasma donor eligibility. The COE, EDQM has submitted a proposal relating in order to prepare, usage, and quality assurance of blood constituents in order to lay the groundwork for SOP[29].
Pre-Hospital Blood Transfusion
Pre-Hospital Blood Products (PHBP) are used for critically damaged bleeding patients, according to evidence. This study's primary and secondary goals were to determine and estimate the optimal PHBP in 14 European countries. The European Society of Anesthesiologists' subcommittee for critical emergency performed research in numerous European nations on the use of PHBI, that concerning to establish current European practice and the basic rationale. The goal was to determine the extent of product use and to assess practitioner’s views on the use of PHBP[30].
Hemovigilance
This survey has been collecting data on hemovigilance since 2004, including reports of serious adverse events. The format for collecting hemovigilance data was devised by COE experts, submitted to the European commission, and incorporated into directive 2005/61/EC after minor adjustments by the commission. Because the majority of these serious reactions are unanticipated, unwanted side effects of blood transfusion procedures that are well-known complications from the medical literature and frequently mentioned in physician and patient "information pamphlets" reporting serious adverse reactions, as done by hemovigilance programmers, can be considered a high level of surveillance. Most people who receive blood transfusions are sick and have underlying pathologies or drugs that affect the signs and symptoms of probable transfusion responses. Unlike the EC Directives 2002/98/EC and 2005/61/EC, hemovigilance dates that aren’t triggered by blood component qualities also reported here[31].
Regulations During Covid-19
Blood and blood products are significant resources and the WHO has developed guidelines for managing blood supplies in response to the Covid-19 outbreak. The majority of Covid-19 patients do not require blood transfusions[32]. The guidance suggests reducing the risk of Covid-19 transmission by blood transfusions, in addition to the likelihood of a shortage of blood donors; regulating the blood supply; ensuring that key materials and equipment are always available and donors, recipients, all personnel, important stakeholders and the general public should all be aware of the situation.
According to the WHO, the Covid-19 pandemic reduced blood supply by 20 %-30 % in all six regions[33]. Many blood donation centers throughout the world have mostly closed during Covid-19. Donors who practice social separation, perhaps due to disease or self-quarantine, are rapidly dwindling. As alternatives dwindle, we must develop regional and national shortage plans, as well as distribute and implement PBM[34].
Implementation of Blood Management in Patients
"Implementation of PBM was strongly recommended" the European CDC (ECDC) says in its Covid-19 fast risk management dated on 12th March 2020, considering the current crisis. Furthermore, the WHO interim guidance during covid-19 pandemic that ensures efficient supply of blood, which was announced on March 20, 2020, encourages "Good PBM" to conserve blood reserves[34]. PBM methods limit allogenic blood transfusions and prevent anemia (a global health problem), resulting in better resource allocation and cost savings, as well as lower patient morbidity and death[35].
Guideliness for Blood Donations
The most recent MOHFW 2020 rules have built the criteria for blood donor selection which was little more rigorous. The government of India has published a detailed list of omission and addition criteria for blood donors in a gazette notification dated March 11, 2020[36]. We feel that there are many fears, misunderstandings and false rumors about blood donation in the minds of donors during the pandemic time. They stated that the main bother of blood donors in their investigation was the risk of contracting corona virus while donating blood. The reduction in voluntary donations has been observed in several countries around the world. To address the "blood crisis," the FDA recently reduced the deferral requirement for blood donation for males from 3 mo to 12 mo of abstinence. The Margo Clinic is leading the Covid-19 extended access programmed and is assisting all healthcare facilities, transfuse medicine providers, and other stakeholders[37].
During the donation procedure, certain protections were put in place to protect blood donors and donors. In addition, in our context, a blood donor screening questionnaire was employed to identify donors who were at high risk of being exposed to Covid-2019. The subsequent steps were implemented at both blood banks and mobile blood drives. Donors who return from any national or foreign jurisdiction will have their donations delayed for 14 d; donors who have been intimacy with confirmed Corona patients were barred from participating; donors who have Covid-19 symptoms were deferred and a body temperature of 37.5° disqualifies a donor. This was augmented by the Malaysian government, which was created to track down contacts and stratify user’s risk of contracting Covid-19[38].
The retrospective study in North India was divided into two phases; phase 1 and phase 2. The customized hospital administration software was used to retrieve information about the blood unit, which was collected both in-house and in the VBCDS. The formula for calculating the percentage of units that are obsolete beyond their expiration date[39]. No of Packed Red Blood Cells (PRBR) outdated due to expiring×100. Total number of PRBR prepared.
Polymorphism in the ABO gene is reflected in the ABO blood type trait. This heredity is linked to several extra characteristics, including Corona virus 2019 morbidity and mortality risk factors. The frequency of blood types varies by ancestral groups[40]. The BWNW (Blood Network)-increased its own recommendation that all higher-risk for remote works of employee and initiated its own emergency forethought for the potential impact on important employees. This involves talking with the medical personnel at the university’s transfusion service about sharing service and call of duty if needed[41].
Supply Chain Management
In late December, the pathogen that causes pneumonia has been discovered as a novel coronavirus strain by the WHO; this unique coronavirus has been called "Corona virus-2" means "severe acute respiratory syndrome" by the international committee on taxonomy of viruses, and the WHO has dubbed the virus linked condition Covid-19[42]. The continuing Covid-19 pandemic is a unique human disaster. In this regard, a structured literature review technique should be used to investigate the overcoming management of the supply bond strategies, and the provocation that appears due to a Covid-19 “bell-like” set of circumstances includes assuming a broad-based operative management investigation in pandemics and establishing the goals for outbreaks of epidemics; during Covid-19, practitioners in the supply chain will get access to comparative literature space and additional research possibilities and developing a theoretically integrated management of the chain of supplies; sustainability composition, durability readiness, and digital surveillance structure[43].
During the Covid-19 epidemic, pharmacy experts are measured the necessity associates, commonly saving (for example) public or resident medical response crops are also involved in repair endurance as well as society provisions. Pharmacy associations in the United States and around the world have released guidelines and warnings for pharmacists to help prepare and improve services in the Covid-19 epidemic. The international pharmaceutical federation has released a comprehensive paper that includes recommendations for pharmacy specialists in relation to Covid-19 trials in the drugstore workforce, as well as a reference list in 8 languages. The inter professional education plans and measures, with these people can also gain a better understanding of pharmacy students and specialists' abilities and capabilities[44].
Collection of Convalscent Plasma
Provided, equipped and authorized blood banks must collaborate with Covid-19 centers to keep track of discharged inpatients from the surrounding area and link them via phone for convalescent plasma donation. Donor enlistment for plasma recovery should not be a complication, especially given the rising number of present cases. If the need arises, transportation assistance should be offered. For a smooth workflow and to avoid unintended exposure, the recovering plasma collecting region should be separated from the rest of the blood embankment. Donor seclusion and privacy are of particular relevance when it comes to the stigma associated with this problem. The most important provocation will be weeding away qualified or trial-seeking donors in order to relieve donation facilities of needless burdens. Recapitulate clinical test that has been officially agreed must be strictly followed. Some people will be looking for ways to profit from the current contagion-chaos. Such behavior should be anticipated, closely adhered to and severely punished[45].
According to the evidence, convalescent plasma taken from Covid-19 survivors contains receptor binding dominion specific antibodies with potent antiviral activity. Meanwhile, in several countries, convalescent plasma therapy has been approved for use in patients. This has gained widespread acceptance in (red world therapeutic practice), where it is used to treat individuals suffering from a variety of Covid-19 sereness[46].
Blood Serological Testing
Because of Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) infection might be mild or asymptomatic, patients who are sick may not seek medical help. US blood donation specimens from an existing repository collected by the American Red Cross were sent to the centers for CDC for testing antibodies that reactive with SARS-COV-2, retrospectively and to check if serological analysis could provide more insight into SARS-COV-2 introduction into the US. The percentage of susceptible gifts was higher between the donors aged 40 or older among donations collected in Oregon, Washington and California[47].
To determine the exact extent of virus speed and the existence of potential antibody-mediated defense against SARS-COV-2 at the level of the community, serological studies with a large population as well as high-quality data on SARS-COV-2 antibody production in healthy freak are urgently required. Infection-induced immunity in the herd could emerge if a large number of people become infected with SARS-COV-2 and generate antibodies to the virus, in addition to persons with immunity that is not mediated by antibodies or pre-existing immunological responsiveness to the virus[48].
During the Covid-19 Pandemic, is Blood Transfusion Safe
The safeguards are very efficient in preventing blood products from being contaminated by symptomatic donors, but they do not exclude the possibility of asymptomatic people donating blood. Furthermore, it is not recommended to screen blood products from asymptomatic people. Although no cases of Covid-19 transfer during transfusion of blood have been identified, vertical blood transmission from pregnant women to their babies has been documented. Many problems remain unsolved, including how long people who have previously been infected with SARS-COV-2 should be regarded ineligible to donate blood, as a result of the gradual resumption of routine activities and the growing requirement for blood products[49]. Only one critically ill patient exhibited RNAemia from one of the 18 patients in the research who had SARS-COV-2 infection verified by RT-PCR. According to the results, among asymptomatic SARS-COV-2 infected patients, there is no risk causing of SARS-COV-2 that transmitted by blood components. However, larger studies are needed to better assess the risk of hematogenous transmission and to establish the best timing to send recovered SARS-COV-2 patients for blood donations[50]. The list included the range of donation units of blood and the number of blood products released for transfusion in a cross-sectional study conducted in the blood bank in South Arabia. To assess donor attendance, supply of blood and request management, data were examined using Statistical Package for the Social Sciences (SPSS) statistics (2.50)[42]. During Covid-19 the (CNS) Italian National blood, is the ministry of health technical and precise advising body on topics connected to blood and blood products, oversees the transfusion system through RBCs[51]. The research was presented at a (tertiary care) cancer hospital's ‘blood transfusion service’, and it included an analysis of blood contributions, packed RBC unit requirements, and packed RBC inventory during the ‘lock down’ phases. The shelf life of packed RBC units with chemical solution is 42 d. SPSS statistics were used to immediately capture data on an MS Excel spreadsheet (2.50)[52]. Platelets have a short ‘shelf life’ of only 5 d, thus they must be collected according to the conditions. They also cannot be collected as deposited like RBC units. Overall, the plateletpheresis measures remained consistent with the SOPs. In addition to the blood camp stamp, the certificate was contracted by the day by the health officer and health social employee. The public or state (blood transfusion commission) recommendations were followed in this document[53]. Even though the platelet recipient was diagnosed with very severe ‘aplastic anemia’ and was using immunosuppressant’s , the transfusion of plasma products took from diseased patients who have not yet shown signs, and symptoms of SARS-COV-2 and does not result in virus transmission. The AABB and the CDC and Prevention presently do not advise blood collection facilities to take any specific SARS-COV-2 related steps. It’s vital to think about the possibility of this infection spreading through blood transfusions[54]. The result of PBM put efforts, and advancements in surgery, blood use has decreased roughly by 30 % in the US over the last decade, blood facilities have cut donor collecting accordingly for apparent financial reasons. The majority of PBM techniques emphasize "keeping the patient's blood flowing”, which will aid in balancing supply and demand for allogenic blood components, particularly during the present epidemic[55].
Conclusion
Blood is a vital component of life that can save a person’s life. This article has compiled data from a variety of sources in order to give relevant information about the blood and blood products before and after Covid-19. During pandemics as in normal times, PBM attempts to pertinent blood supply and reducing the mortality rate. Better BTS facilities will be required by legislation. Increased public awareness of the benefits of blood donation, not just for recipients but also for the donor, may boost voluntary donations. Commitment on execution of blood safety measure is generally composite. Increase the number of blood drives to increase blood donations and lower patient mortality. Donating blood is a positive thing because it not only saves a patient's life, but it also benefits the donor.
Conflict of interests:
The authors declared no conflict of interests.
References
- Vaglio S, Gentili S, Marano G, Pupella S, Rafanelli D, Biancofiore G, et al. The Italian regulatory guidelines for the implementation of patient blood management. Blood Transfus 2017;15(4):325-8.
[Google Scholar] [PubMed]
- Klein AA, Arnold P, Bingham RM, Brohi K, Clark R, Collis R, et al. AAGBI guidelines: The use of blood components and their alternatives 2016. Anaesthesia 2016;71(7):829-42.
[Crossref] [Google Scholar] [PubMed]
- Epstein JS. Best practices in regulation of blood and blood products. Biologicals 2012;40(3):200-4.
[Crossref] [Google Scholar] [PubMed]
- Stanworth SJ, New HV, Apelseth TO, Brunskill S, Cardigan R, Doree C, et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol 2020;7(10):e756-64.
[Crossref] [Google Scholar] [PubMed]
- Cai X, Ren M, Chen F, Li L, Lei H, Wang X. Blood transfusion during the COVID-19 outbreak. Blood Transfu 2020;18(2):79.
[Crossref] [Google Scholar] [PubMed]
- Barriteau CM, Bochey P, Lindholm PF, Hartman K, Sumugod R, Ramsey G. Blood transfusion utilization in hospitalized COVID‐19 patients. Transfusion 2020;60(9):1919-23.
[Crossref] [Google Scholar] [PubMed]
- Dhiman Y, Patidar GK, Arora S. Covid‐19 pandemic‐response to challenges by blood transfusion services in India: A review report. ISBT Sci Ser 2020;15(4):365-73.
- Swathi J, Natarajan J, Senthil V. A Review on regulations and legislations for blood and blood products in India. J Pharm Sci Res 2021;13(2):124-7.
- Pal R, Kar S, Zaman FA, Pal S. The quest for an Indian blood law as of blood transfusion services regulatory framework. Asian J Transfus Sci 2011;5(2):171-4.
- Bhatia V, Raghuwanshi B, Sahoo J. Current status of blood banks in India. Global J Transfus Med 2016;1(2):72-4.
- Ambroise MM, Ravichandran K, Ramdas A, Sekhar G. A study of blood utilization in a tertiary care hospital in South India. J Nat Sci Biol Med 2015;6(1):106-10.
[Crossref] [Google Scholar] [PubMed]
- Dhot PS. Amendments to Indian drugs and cosmetics act and rules pertaining to blood banks in armed forces. Med J Armed Forces India 2005;61(3):264-6.
[Crossref] [Google Scholar] [PubMed]
- Jenny HE, Saluja S, Sood R, Raykar N, Kataria R, Tongaonkar R, et al. Access to safe blood in low-income and middle-income countries: Lessons from India. BMJ Global Health 2017;2(2):bmjgh-2016.
- Malik P. Comparative health policy analysis: National blood policy of India, 2007 and national blood policy of Bhutan, 2007. Manipal J Med Sci 2019;4(1):4.
- Yaddanapudi S, Yaddanapudi LN. Indications for blood and blood product transfusion. Indian J Anaesth 2014;58(5):538-42.
- Mano V, Kumar RS. Implementing haemovigilance in India as a national perspective. Appl Clinical Res Clin Trials Regulat Aff 2020;7(1):30-6.
- Mukherjee S, Maiti R. Haemovigilance: A current update in Indian perspective. J Clin Diagn Res 2016;10(11):EE05.
[Crossref] [Google Scholar] [PubMed]
- Eder A, Goldman M, Rossmann S, Waxman D, Bianco C. Selection criteria to protect the blood donor in North America and Europe: Past (dogma), present (evidence), and future (hemovigilance). Transfus Med Rev 2009;23(3):205-20.
[Crossref] [Google Scholar] [PubMed]
- Ellingson KD, Sapiano MR, Haass KA, Savinkina AA, Baker ML, Chung KW, et al. Continued decline in blood collection and transfusion in the United states–2015. Transfusion 2017;57(2):1588-98.
[Crossref] [Google Scholar] [PubMed]
- Newman B. Blood donor suitability and allogeneic whole blood donation. Transfu Med Rev 2001;15(3):234-44.
[Crossref] [Google Scholar] [PubMed]
- Valeri CR, Ragno G. An approach to prevent the severe adverse events associated with transfusion of FDA-approved blood products. Transfu Apher Sci 2010;42(3):223-33.
[Crossref] [Google Scholar] [PubMed]
- Angheben A, Boix L, Buonfrate D, Gobbi F, Bisoffi Z, Pupella S, et al. Chagas disease and transfusion medicine: A perspective from non-endemic countries. Blood Transfu 2015;13(4):540.
[Google Scholar] [PubMed]
- Menitove JE. Hemovigilance in the United States of America. Vox Sang 1998;74(S2):447-55.
[Crossref] [Google Scholar] [PubMed]
- Farrell AM. Is the gift still good? Examining the politics and regulation of blood safety in the European Union. Med Law Rev 2006;14(2):155-79.
[Crossref] [Google Scholar] [PubMed]
- Theusinger OM, Felix C, Spahn DR. Strategies to reduce the use of blood products: A European perspective. Curr Opin Anesthesiol 2012;25(1):59-65.
[Crossref] [Google Scholar] [PubMed]
- Robinson EA. The European union blood safety directive and its implications for blood services. Vox Sang 200;93(2):122-30.
[Crossref] [Google Scholar] [PubMed]
- Hansen-Magnusson H. Governance in the European union: The European Blood Directive as an evolving practice. Clin Lab Med 2010;30(2):489-97.
[Crossref] [Google Scholar] [PubMed]
- Berger K, Klein HG, Seitz R, Schramm W, Spieser JM. The Wildbad Kreuth initiative: European current practices and recommendations for optimal use of blood components. Biologicals 2011;39(3):189-93.
[Crossref] [Google Scholar] [PubMed]
- De Kort W, Mayr W, Jungbauer C, Vuk T, Kullaste R, Seifried E, et al. Blood donor selection in European Union directives: Room for improvement. Blood Transfus 2016;14(2):101-8.
[Google Scholar] [PubMed]
- Thies KC, Truhlar A, Keene D, Hinkelbein J, Rutzler K, Brazzi L, et al. Pre-hospital blood transfusion–an ESA survey of European practice. Scand J Trauma Resusc Emerg Med 2020;28:1-8.
[Crossref] [Google Scholar] [PubMed]
- Nikitin IK, Thay SV. Collection, testing and use of blood and blood components in Europe (review of the data of the European council). Gematol Transfuziol 2011;56(6):39-44.
- Fan BE, Ong KH, Chan SS, Young BE, Chong VC, Chen SP, et al. Blood and blood product use during COVID‐19 infection. Am J Hematol 2020;95(7):E158.
[Crossref] [Google Scholar] [PubMed]
- Loua A, Kasilo OM, Nikiema JB, Sougou AS, Kniazkov S, Annan EA. Impact of the COVID‐19 pandemic on blood supply and demand in the WHO African region. Vox Sang 2021;116(7):774-84.
[Crossref] [Google Scholar] [PubMed]
- Shander A, Goobie SM, Warner MA, Aapro M, Bisbe E, Perez-Calatayud AA, et al. Essential role of patient blood management in a pandemic: A call for action. Anesth Analg 2020;131(1):74-85.
[Crossref] [Google Scholar] [PubMed]
- Baron DM, Franchini M, Goobie SM, Javidroozi M, Klein AA, Lasocki S, et al. Patient blood management during the COVID–19 pandemic: A narrative review. Anaesthesia 2020;75(8):1105-13.
[Crossref] [Google Scholar] [PubMed]
- Das K, Raturi M, Agrawal N, Kala M, Kusum A. Indian blood donor selection guidelines: Review in the context of the ongoing COVID-19 pandemic. Transfus Clin Biol 2021;28(2):213-6.
[Crossref] [Google Scholar] [PubMed]
- Sahu KK, Raturi M, Siddiqui AD, Cerny J. “Because every drop counts”: Blood donation during the COVID-19 pandemic. Transfus Clin Biol 2020;27(3):105.
[Crossref] [Google Scholar] [PubMed]
- Tan PP, Chang CT, Noor SM. Blood supply management during the Covid-19 pandemic: experience in a tertiary referral hospital in Malaysia. Transfus Apher Sci 2021;60(1):102982.
[Crossref] [Google Scholar] [PubMed]
- Raturi M, Kusum A. The blood supply management amid the COVID-19 outbreak. Transfus Clin Biol 2020;27(3):147-51.
[Crossref] [Google Scholar] [PubMed]
- Zietz M, Zucker J, Tatonetti NP. Associations between blood type and COVID-19 infection, intubation, and death. Nat Commun 2020;11(1):5761.
[Crossref] [Google Scholar] [PubMed]
- Pagano MB, Hess JR, Tsang HC, Staley E, Gernsheimer T, Sen N, et al. Prepare to adapt: Blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID‐19) pandemic affecting Washington State. Transfusion 2020;60(5):908-11.
[Crossref] [Google Scholar] [PubMed]
- Yahia AI. Management of blood supply and demand during the COVID-19 pandemic in King Abdullah Hospital, Bisha, Saudi Arabia. Transfus Apheresis Sci 2020;59(5):102836.
[Crossref] [Google Scholar] [PubMed]
- Farooq MU, Hussain A, Masood T, Habib MS. Supply chain operations management in pandemics: A state-of-the-art review inspired by COVID-19. Sustainability 2021;13(5):2504.
- Aruru M, Truong HA, Clark S. Pharmacy Emergency Preparedness and Response (PEPR): A proposed framework for expanding pharmacy professionals’ roles and contributions to emergency preparedness and response during the COVID-19 pandemic and beyond. Res Social Adm Pharm 2021;17(1):1967-77.
[Crossref] [Google Scholar] [PubMed]
- Arcot PJ, Kumar K, Mukhopadhyay T, Subramanian A. Potential challenges faced by blood bank services during COVID-19 pandemic and their mitigative measures: The Indian scenario. Transfus Apheresis Sci 2020;59(5):102877.
- Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020;371:m3939.
[Crossref] [Google Scholar] [PubMed]
- Basavaraju SV, Patton ME, Grimm K, Rasheed MA, Lester S, Mills L, et al. Serologic testing of US blood donations to identify severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–reactive antibodies: December 2019–January 2020. Clin Infect Dis 2021;72(12):e1004-9.
[Crossref] [Google Scholar] [PubMed]
- Slot E, Hogema BM, Reusken CB, Reimerink JH, Molier M, Karregat JH, et al. Low SARS-CoV-2 seroprevalence in blood donors in the early COVID-19 epidemic in the Netherlands. Nat Commun 2020;11(1):5744.
[Crossref] [Google Scholar] [PubMed]
- Bassil J, Rassy E, Kattan J. Is blood transfusion safe during the COVID-19 pandemic? Future Sci OA 2020;6(9):FSO626.
- Corman VM, Rabenau HF, Adams O, Oberle D, Funk MB, Keller‐Stanislawski B, et al. SARS‐CoV‐2 asymptomatic and symptomatic patients and risk for transfusion transmission. Transfusion 2020;60(6):1119.
[Crossref] [Google Scholar] [PubMed]
- Franchini M, Farrugia A, Velati C, Zanetti A, Romanò L, Grazzini G, et al. The impact of the SARS‐CoV‐2 outbreak on the safety and availability of blood transfusions in Italy. Vox Sang 2020;115(8):603-5.
[Crossref] [Google Scholar] [PubMed]
- Gupta AM, Ojha S, Nagaraju P, Poojary M, Sh S, Sathyan V, et al. Impact of the novel coronavirus disease and lockdown on the packed red blood cells inventory management: An experience from a tertiary care oncology center in Western India. HematolTransfus Cell Ther 2021;43:126-32.
[Crossref] [Google Scholar] [PubMed]
- Ojha S, Gupta AM, Nagaraju P, Poojary M, Sumathi SH. Challenges in platelet inventory management at a tertiary care oncology center during the novel COVID-19 pandemic lockdown in India. Transfus Apher Sci 2020;59(5):102868.
[Crossref] [Google Scholar] [PubMed]
- Cho HJ, Koo JW, Roh SK, Kim YK, Suh JS, Moon JH, et al. COVID-19 transmission and blood transfusion: A case report. J Infect Public Health 2020;13(11):1678-9.
[Crossref] [Google Scholar] [PubMed]
- Gehrie EA, Frank SM, Goobie SM. Balancing supply and demand for blood during the COVID-19 pandemic. Anesthesiology 2020;133(1):16-8.
[Crossref] [Google Scholar] [PubMed]