- Corresponding Author:
- M. K. Das
Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh (Assam) - 786 004, India
E-mail: du_mkd@yahoo.co.in
| Date of Submission | 30 May 2006 |
| Date of Revision | 14 January 2008 |
| Date of Acceptance | 25 January 2008 |
| Indian J Pharm Sci, 2008, 70 (1): 77-84 |
Abstract
Furosemide-loaded alginate microspheres were prepared by the ionic cross-linking technique using CaCl 2 , Al 2 (SO 4 ) 3 and BaCl 2 . The process induced the formation of microspheres with the incorporation efficiency of 65% to 93%. The effect of sodium alginate concentration, cross-linking agents and drying conditions was evaluated with respect to entrapment efficiency, particle size, surface characteristics and in vitro release behaviors. Infrared spectroscopic study confirmed the absence of any drug-polymer interaction. Differential scanning calorimetric analysis revealed that the drug was molecularly dispersed in the alginate microspheres matrices showing rough surface, which was confirmed by scanning electron microscopy study. The mean particle size and entrapment efficiency were found to be varied by changing various formulation parameters. The in vitro release profile could be altered significantly by changing various formulation parameters to give a sustained release of drug from the microspheres. The kinetic modeling of the release data indicate that furosemide release from the alginate microspheres follow anomalous transport mechanism after an initial lag period when the drug release mechanism was found to be fickian diffusion controlled.
Keywords
Sodium alginate, microspheres, furosemide, ionic cross-linking technique, anomalous transport mechanism, fi ckian diffusion controlled
Alginates, which are naturally occurring substances, found in brown algae have received much attention as a vehicle for controlled drug delivery [1-4]. Alginates can be considered as block polymers, which mainly consist of mannuronic acid (M), guluronic acid (G) and mannuronic-guluronic (MG) blocks. Dropwise addition of aqueous alginate solution to the aqueous solution containing calcium ions and/or other di and polyvalent cations cause spherical gel formation termed as alginate bead. Alginate is known to be nontoxic when taken orally and also have a protective effect on the mucous membranes of the upper gastrointestinal tract [1]. The dried alginate beads have the property of reswelling and thus they can act as controlled release system. Their reswelling property is susceptible to pH, which protects the acid-sensitive drug from gastric juice [5].
Furosemide, a potent loop diuretic, is used in the treatment of edema of hepatic, cardiac, pulmonary and renal failures and in chronic hypertension [6]. The dose related adverse effects have been observed [6] and the treatment with conventional tablets produce short period of maximum diuresis, which is inconvenient to the patients. But the treatment with sustained release tablets produces same diuretic effect as produced by conventional tablets eliminating the brief and intense diuresis, which is well tolarated to the patients [7]. However, such single unit sustained release tablets could be disastrous if they fail to release the drug at the desired rate and in the desired amount, or if they release the entire amount of drug so as to cause dose dumping. The multiunit microparticulate oral drug delivery systems can be distributed widely throughout the gastrointestinal tract providing a possibility of achieving a longer lasting and reliable release of drug at desired rate. Unwanted intestinal retention of the polymeric material and local irritation which may occur with non-disintegrating polymeric matrix tablets, can also be avoided [8].
As previously reported, the furosemide microspheres prepared by emulsion-solvent evaporation method utilize larger volume of organic solvents [9], which are costly and hazardous because of the possibility of explosion, toxicity and air pollution. The water-based ionic cross-linking technique can provide characteristic advantages over conventional microspheres preparation methods. As previously reported, this technique can be used successfully to prepare alginate microspheres containing acetaminophen [10] and nimesulide [11]. In the present study, the same procedure was applied to prepare furosemide-loaded alginate microspheres. The effects of factors, such as sodium alginate concentrations, cross-linking agents, drying conditions, on morphology of and drug release from microspheres were studied. Drug-polymer interactions in the solid state were studied by infrared spectrophotometry (IR) and differential scanning calorimetric analysis (DSC) and the surface characteristics were evaluated by scanning electron microscopy (SEM).
Materials and Methods
Furosemide was received as a gift sample from Cipla Ltd., Mumbai. Sodium alginate was procured from Loba Chemie, Mumbai. Calcium chloride (fused), barium chloride and aluminium sulphate were purchased from Ranbaxy Laboratories Ltd., New Delhi. All other chemicals and solvents were of analytical grade satisfying pharmacopoeial specifications.
Formulation of alginate microspheres
The microspheres were prepared by ionic cross-linking technique using the formulations as shown in Table 1. The alginate solutions comprising 2.5% to 4% w/v sodium alginate were prepared by initially dissolving the polymer in deionized water using gentle heat, being stirred magnetically. On complete solution, an accurately weight quantity of furosemide was added to each solution to afford homogeneous dispersions. The dispersions were sonicated for 30 min to remove any air bubbles that may have been formed during the stirring process. The sodium alginate-drug dispersions (25 ml) were added drop wise via a 20-gauge hypodermic needle fitted with a 10 ml syringe into 50 ml of 5% w/v of cross-linking agents, being stirred at 200 rpm for 10 min. The cross-linking agents were used CaCl2, Al2(SO4)3 and BaCl2. The droplets from the dispersions instantaneously gelled into discrete furosemide-alginate matrices upon contact with the solution of cross-linking agents. The formed alginate microspheres were further allowed to stir in the solution of cross-linking agents for an additional 1 h. On expiration of this period the solution of cross-linking agents was decanted and the microspheres were washed with 3 × 50 ml volumes of deionized water. The microspheres were thereafter dried at 80° for 2 h in a hot-air oven. Similarly, air-dried alginate microspheres (formulation code C1, E1 and F1) were prepared in the same way as the formulations C, E and F, respectively, by drying in air at room temperature.
| F.N.Code | Furosemide (% w/v) | Sodium alginate (%w/v) | Cross-linking agents (5%w/v) |
| A | 2.5 | 2.5 | CaCl2 |
| B | 2.5 | 3.0 | CaCl2 |
| C | 2.5 | 3.5 | CaCl2 |
| D | 2.5 | 4.0 | CaCl2 |
| E | 2.5 | 3.5 | Al2(SO4)3 |
| F | 2.5 | 3.5 | BaCl2 |
Table 1: Formulation of Alginate Microspheres
Morphology and size distribution
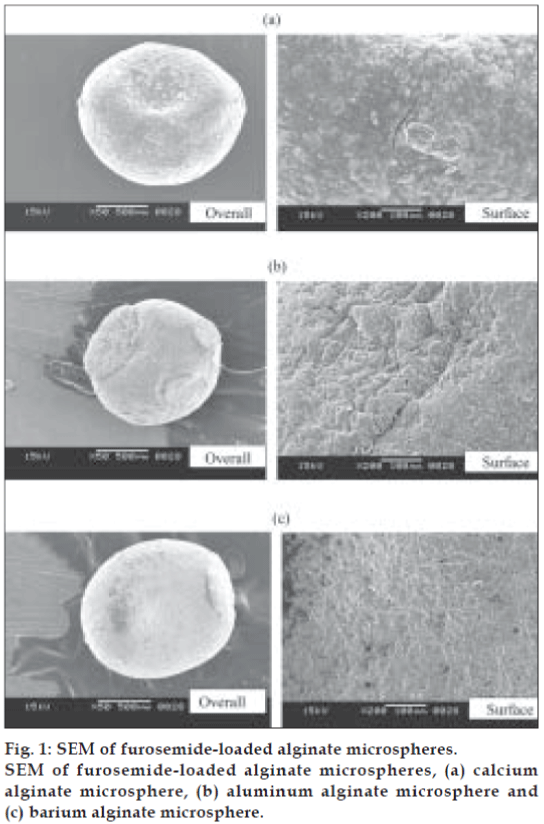
The shape and surface morphology of the alginate microspheres were investigated using Jeol, JSM- 6360, scanning electron microscope at 15 kV. Prior to examination samples were gold coated under vacuum (Fine coat, Ion sputter, JFC-1100) to render them electrically conductive. The samples include various alginate microspheres prepared using different cross-linking agents before release study. The alginate microspheres were not subjected to SEM studies after release study because they converted to gel type of matrix when dissolution was overed.
Size and size distribution of the alginate microspheres were measured by sieve analysis. The alginate microspheres were separated into different size fractions (% weight fraction) by sieving for 5 min using standard sieves having nominal mesh apertures of 1.4 mm, 1.2 mm, 1.0 mm, 0.85 mm and 0.71 mm (sieve no. 12, 14, 16, 18 and 22, respectively). The particle size distributions of the microspheres were determined and the mean particle size of microspheres were calculated using the following formula [12]:

Determination of drug incorporation effi ciency
Thirty milligrams of drug-loaded alginate microspheres from each batch was placed in 100 ml conical ß ask containing 50 ml of phosphate buffer of pH 7.4. The microspheres were magnetically stirred to promote swelling and break up of the cross-linked structure. This afforded liberation and subsequent dissolution of furosemide. The solution was filtered through a 0.45 μm membrane filter. Then the drug was quantified at 276.5 nm spectrophotometrically after appropriate dilution with phosphate buffer of pH 7.4. The incorporation efficiency was determined by the following empirical relationship: Drug incorporation efficiency (%) = (AQ/TQ) × 100, where AQ is the actual quantity of drug present in the microspheres and TQ is the 100% theoretical quantity of drug present in the microspheres (i.e., actual initial loading dose).
Infrared spectroscopy
The drug-polymer interactions were studied by infrared spectroscopy. The IR spectra were recorded between 500 to 4000 cm−1 for pure furosemide, blank alginate microspheres and furosemide-loaded alginate microspheres from KBr pellet using Perkin Elmer-883 IR spectrophotometer.
Differential scanning calorimetry
The DSC thermograms were recorded on a Universal V 2.5 H differential scanning calorimeter. The DSC studies on the samples were performed by heating samples at a heating rate of 10°/min over a temperature range of 50-300° in closed aluminium pans. The samples included pure furosemide and furosemide-loaded alginate microspheres.
In vitro dissolution testing
The dissolution studies were performed in a fully calibrated six station dissolution test apparatus (37 ± 0.5°, 50 rpm) using the USP rotating basket method in phosphate buffer media (pH 7.4, 500 ml). A quantity of alginate beads equivalent to 100 mg furosemide for each formulation was employed in all dissolution studies. The samples of 5 ml each were withdrawn at predetermined time interval and were replenished immediately with the same volume of fresh prewarmed phosphate buffer maintaining sink condition throughout the experiment. The aliquots, following suitable dilution, were analyzed spectrophotometrically at 276.5 nm. The concentrations of furosemide in the test samples were calculated using a regression equation (Absorbance = 0.007 + 0.0709 × concentration, R2 = 0.999) of the calibration curve in phosphate buffer of pH 7.4 and corrected for sampling effect using the formula reported by Hayton and Chen [13].
Kinetic modeling
In order to investigate the release mechanism, the release data (≤60%) were fitted to the following power law expression [14]: Mt/M∝ = Ktn….(1), where Mt and M∝ are the amounts of drug released at time t and the overall amount released, respectively, K is the release rate constant and n is the release exponent indicative of release mechanism. The release data were further analyzed using the modified form of the power law expression [15] to accommodate the lag time (tL) in the beginning of the drug release from the alginate beads: Mt/M∝ = K(t − tL)n ….(2). Fitness of the data into various kinetic models was assessed by determining the correlation coefficient. The value of n was calculated from the slope of the plot of log (Mt/M∝) vs. log (t) and log (Mt/M∝) vs. log (t-tL) for interpretation of release mechanism [16] (Table 3).
Results and Discussion
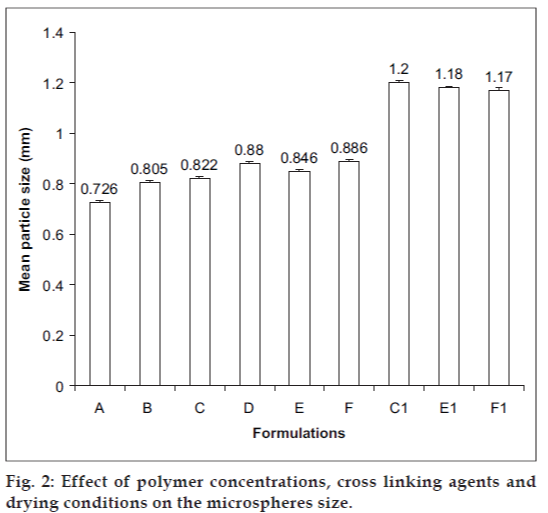
Morphology of the various formulations of alginate microspheres prepared was found to be discrete and spherical in shape. The SEM photomicrographs of the dried alginate microspheres are shown in fig. 1. The surface of the alginate microspheres was rough due to higher concentration of drug uniformly dispersed at the molecular level in the alginate matrices. The mean particle size of the various formulation of alginate microspheres were between 0.726 ± 0.007 to 1.2 ± 0.008 mm. It was found that the particle size distribution of each formulation was within a narrow range but the mean particle size was different among the formulations (fig. 2). The results indicated the proportional increase in the mean particle size of the microspheres with increasing amount of sodium alginate in the formulations A, B, C and D. This could be attributed to an increase in the relative viscosity at higher concentration of sodium alginate and formation of large droplets during addition of the polymer solution to the cross-linking agents. Air dried microspheres were of larger size than those oven dried due to incomplete dehydration as a result of air drying process. The results also indicated no significant variation in particle size of the microspheres prepared using different cross-linking agents.
The incorporation efficiency increased progressively with increasing sodium alginate concentration (Table 2). Increase in the alginate concentration resulted in the formation of larger microspheres entrapping greater amounts of the drug. This may be attributed to the greater availability of active calcium-binding sites in the polymeric chains and, consequently, the greater degree of cross-linking as the quantity of sodium alginate increased [17]. The incorporation efficiencies were generally higher for the formulations cross-linked with Al2(SO4)3 and BaCl2 as compared to the beads cross-linked with CaCl2. The results tabulated in Table 2 indicate that the incorporation efficiencies were more than 90% for both oven-dried and air-dried alginate beads cross-linked with Al2(SO4)3 and BaCl2. This may be attributed to the formation of nonporous alginate beads due to increase in the apparent cross-linking density in presence of Al3+ and Ba2+ which prevent the diffusion of the drug out of the beads at the time of curing. The low incorporation efficiency of alginate beads cross-linked with Ca2+ could be attributed to the formation of porous beads ensuring the diffusion of the drug out of the beads at the time of curing.
| F.N. Code | Entrapment capacity (%w/w) (mean* ±SD) |
|---|---|
| A | 65.18 ±5.4 |
| B | 66.10 ±2.26 |
| C | 72.01 ±0.28 |
| D | 71.25 ±1.93 |
| E | 91.00 ±1.00 |
| F | 91.78 ±0.233 |
| C1 | 74.25 ±0.84 |
| E1 | 91.63 ±0.021 |
| F1 | 93.00 ±1.41 |
*n = 3
Table 2: Incorporation Efficiency Of Alginate Microspheres
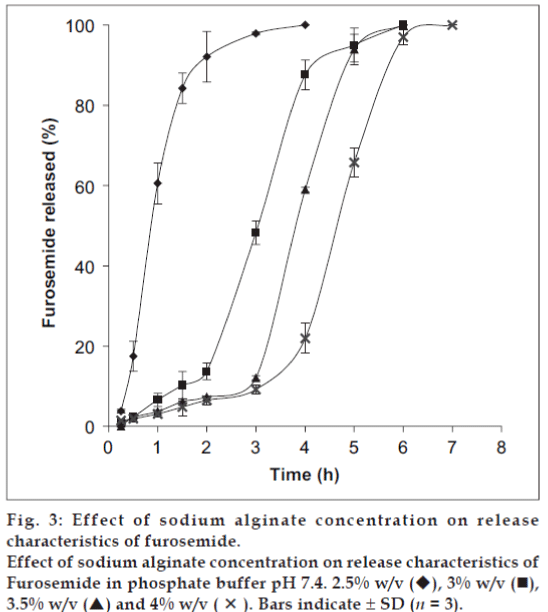
To study the effect of sodium alginate concentration on furosemide release, the sodium alginate was used at four different concentrations: 2.5 (A), 3.0 (B), 3.5 (C) and 4.0 (D) % w/v. The release profiles for these formulations are shown in fig. 3. The results indicated the more sustained effect with increase in the concentration of sodium alginate. It is observed from the fig. 3 that the steady state release was achieved after an initial lag time and it was directly proportional to the concentration of sodium alginate. This type of release behavior agreed with the pulsatile release pattern. The pulsatile or pulsed drug release is defined as the rapid release of certain amount of drug within a short time period immediately after a lag time [18]. The pulsed release pattern could be controlled by the alginate gel disintegration in phosphate buffer. The alginate disintegration was monitored by the exchange of Ca2+ with Na+ in the dissolution medium. The first phase (negligible release portion of the release graph, lag time) might be for the negligible dissociation of alginate beads in phosphate buffer and the drug release mainly based on drug diffusion through the small pores and cracks. The second phase exhibited a burst-like release pattern, which was accompanied by alginate disintegration. The sodium alginate concentration in the formulation greatly influenced the steady state release of furosemide from the alginate beads. The principle of gelation or cross-linking of sodium alginate with calcium chloride is based on the formation of tight junction between the guluronic acid residues [4]. The number of the apparent cross-linking points formed within the calcium alginate gel beads increased with increasing alginate concentration in the formulation. This increase in the apparent cross-linking density delayed the alginate gel disintegration in phosphate buffer due to the retardation of Ca2+ exchange with Na+ and eventually increasing lag time. Increased alginate gel density per unit volume was also thought to affect the decreased pore size within the gels, and thus furosemide release becomes slow.
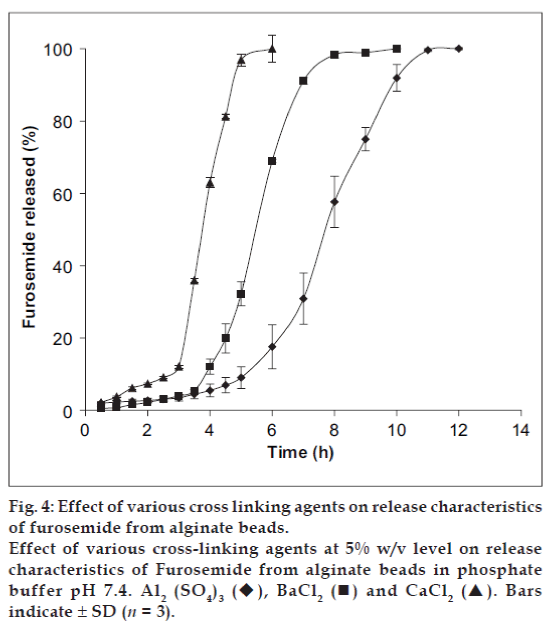
The release behaviors of alginate microspheres, produced by ionic gelation with different crosslinking agents depend upon the valency and size of the cations of the respective cross-linking agent [19]. To investigate this aspect, the sodium alginate (3.5% w/v) beads were prepared via cross-linking in 5% w/v solution of CaCl2 (C), Al2(SO4)3 (E) and BaCl2 (F), respectively. Their release profiles in phosphate buffer of pH 7.4 have been well depicted in the fig. 4. The pulsatile release pattern was observed in all cases. The steady state release was achieved after 2.45 h for Ca2+-alginate microspheres, 3.5 h for Ba2+-alginate microspheres and 5.0 h for Al3+-alginate microspheres. The results obtained can be explained on the basis of the extent of cross-linking in the microspheres. Ca2+ and Ba2+, being divalent, form two-dimensional bonding structure with sodium alginate inside the alginate matrices. But, Ba2+ has largest size (1.74 A°) as compared to the other two cations (1.14 A° for Ca2+ and 0.68 A° for Al3+), it is expected to form strong alginate microspheres with smaller voids and low water uptake [19]. Therefore, the exchange of larger Ba2+ in the microspheres with Na+ of dissolution medium (phosphate buffer, pH 7.4) and also their removal in the form of insoluble barium phosphate was hindered, thus resulting in delayed swelling of the microspheres and slow release. In case of Ca2+-alginate microspheres, the smaller size of Ca2+ as compared to Ba2+ ensure rapid removal of Ca2+ as calcium phosphate from the microspheres due to ion-exchange process with Na+ of phosphate buffer medium and thus leading to greater water uptake and rapid release. In case of Al3+-alginate microspheres, the delay was due to the ability of Al3+ to form three-dimensional bonding structure with the sodium alginate inside the microspheres. This three-dimensional bonding results in extended crosslinking through the whole microsphere producing hard alginate microspheres with low water uptake and thus leading to slow removal of Al3+ due to ion-exchange with Na+ in the phosphate buffer. As a result, the swelling of the beads are delayed leading to slow disintegration.
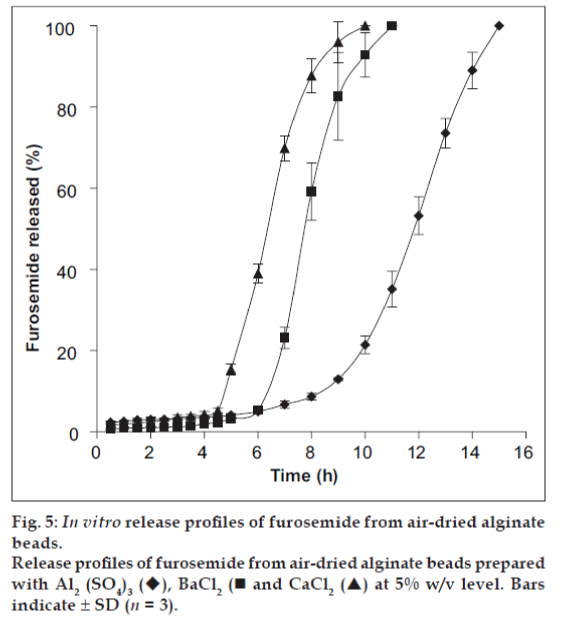
Fig. 4: Effect of various cross linking agents on release characteristics of furosemide from alginate beads.
Effect of various cross-linking agents at 5% w/v level on release characteristics of Furosemide from alginate beads in phosphate
buffer pH 7.4. Al2 (SO4)3 , BaCl2 and CaCl2 . Bars
indicate ± SD (n = 3).
The influence of the degree of dehydration on the release of furosemide was investigated by drying alginate microspheres (formulation C, E and F) at 80° for 2 h in a hot air oven, whilst the duplicate batches (formulations C1, E1 and F1) were dried in air at room temperature. The release of furosemide from oven-dried alginate microspheres was observed to take place at a faster rate as compared to the airdried alginate microspheres (figs. 4 and 5), because the steady state release was achieved after 5 h for air-dried Ca2+-alginate microspheres, where as the value was 6 h and 8.3 h for air-dried Ba2+-alginate and Al3+-alginate microspheres, respectively. The alginate microspheres that had been heated at 80° for 2 h appeared to have become fully dehydrated. The complete dehydration of alginate microspheres may develop a small degree of surface cracking which can facilitate the surface erosion of the beads upon rehydration [20] and consequently, furosemide release was more rapid from the oven-dried microspheres as compared to the air-dried microspheres. Air-drying produced partially hydrated alginate microspheres due to incomplete dehydration and the particle size of the microspheres was larger than the oven-dried microspheres. The slow release from the larger air-dried microspheres may be due to the reduction in dissolution surface area. Also, the incomplete dehydration may significantly reduce the pore size of the alginate microspheres and may prevent the surface cracking.
The in vitro dissolution data were analyzed by different kinetic models in order to find out the n value, which describe the drug release mechanism. The values of n and the coefficient of correlation (R2) obtained for the respective model are listed in the Table 3. The best fit with the highest correlation coefficient was shown in modified power law expression (Eqn. 2). The values of n for the release of furosemide from the alginate microspheres range between 0.8360 to 1.383 (from Eqn. 2), indicating that the drug release from the microspheres followed the anomalous transport and super case-II transport mechanism controlled by swelling and relaxation of the polymer chains. For the formulation E, C1, E1 and F1, the n values from power law expression (Eqn. 1) range between 0.20 to 0.54, indicating the mechanism of the initial drug release to be diffusion controlled during the lag period, when alginate dissociation was almost negligible in the dissolution medium.
| F.N.Code | Mt/M∝ = Ktn model | Mt/M∝ = K(t-tL)n model | Drug release mechanism | ||
|---|---|---|---|---|---|
| R2 | n | R2 | n | ||
| A | 0.9308 | 1.997 | 0.9290 | 0.9980 | 0.5 <n >1.0 Anomalous transport, super case II transport |
| B | 0.9910 | 1.553 | 0.9630 | 0.8360 | 0.5 <n >1.0 Anomalous transport, super case II transport |
| C | 0.9350 | 0.9360 | 0.9840 | 0.9650 | 0.5 <n <1.0 Anomalous transport |
| D | 0.9360 | 0.9160 | 0.9820 | 0.9350 | 0.5 <n <1.0 Anomalous transport |
| E | 0.8970 | 0.3050 | 0.9870 | 1.06 | 0.5 >n ³1.0 Fickian diffusion, case II/super case II transport |
| F | 0.9600 | 1.17 | 0.9820 | 1.40 | n >1.0 Super case II transport |
| C1 | 0.8490 | 0.542 | 0.9870 | 1.383 | 0.5 £n >1.0 Fickian diffusion, super case II transport |
| E1 | 0.8310 | 0.20 | 0.9920 | 1.110 | 0.5 >n >1.0 Fickian diffusion, super case II transport |
| F1 | 0.8480 | 0.3440 | 0.9690 | 1.179 | 0.5 >n >1.0 Fickian diffusion, super case II transport |
R2 is the correlation coefÞ cient and n is the release exponent
Table 3: Furosemide Release Kinetic Data
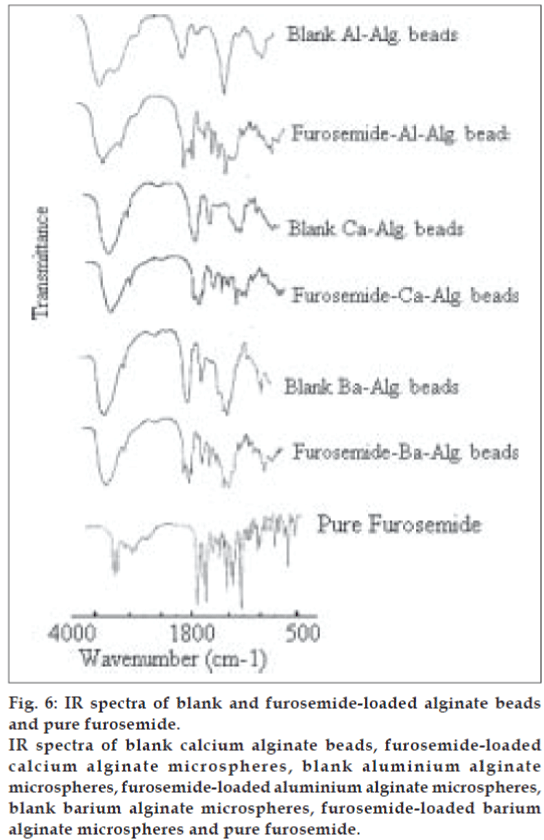
The stability of furosemide in the alginate microspheres was investigated by infrared spectroscopy study (IR). The study of IR spectra of furosemide (fig. 6) demonstrated that the characteristics absorption bands for N-H stretching vibration of secondary amine, C = O stretching vibration and S = O stretching vibration of sulphonamide group and C-Cl stretching vibration appeared at 3398, 1675, 1593, 1325 and 578 cm−1, respectively. The almost identical absorption bands were obtained from furosemide-loaded alginate microspheres, but with lower intensity as shown in fig. 6. The above observed absorption bands were similar to the reported values [7]. Thus, the IR study indicates the stable nature of furosemide in the freshly prepared alginate microspheres.
Fig. 6: IR spectra of blank and furosemide-loaded alginate beads and pure furosemide.
IR spectra of blank calcium alginate beads, furosemide-loaded calcium alginate microspheres, blank aluminium alginate
microspheres, furosemide-loaded aluminium alginate microspheres,
blank barium alginate microspheres, furosemide-loaded barium
alginate microspheres and pure furosemide.
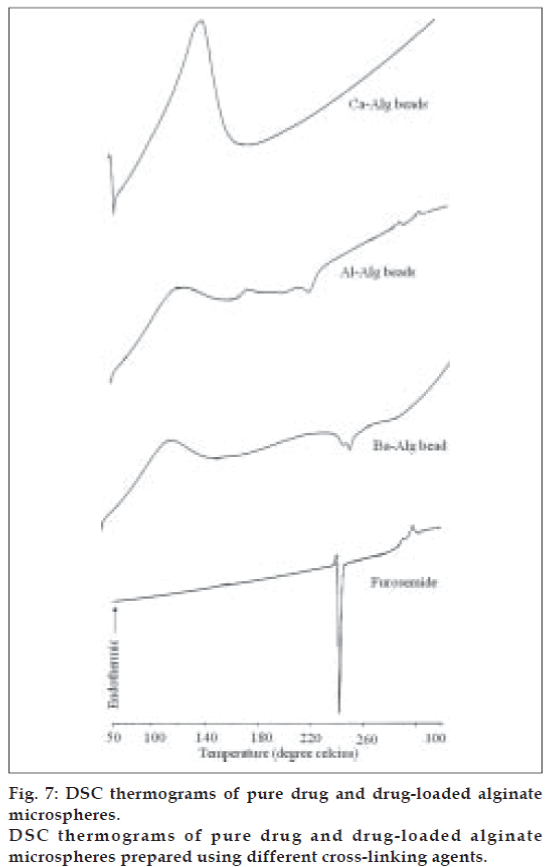
The DSC thermograms of pure drug and drugloaded alginate microspheres prepared with different cross-linking agents are shown in fig. 7. Furosemide exhibited a sharp endothermic peak at 220.8°C corresponding to its melting point. The peak of the drug did not appear in the thermogram of any type of the prepared microspheres containing the drug. It may indicate that the drug was uniformly dispersed at the molecular level in the microspheres as observed by SEM analysis (fig. 1).
It can be concluded from the above investigation that the proper selection of formulation conditions are very important to achieve high encapsulation efficiency and to control the release of furosemide from alginate microspheres. The pulsatile release pattern was observed from all the formulations investigated. The alginate microspheres swelled and eventually disintegrated in phosphate buffer of pH 7.4. Consequently, 100% of furosemide was released in the dissolution medium. Therefore, more formulation studies are needed to design the best controlled release formulation.
Acknowledgements
The authors are grateful to All India Council of Technical Education, New Delhi, for granting scholarship and contingency to P. C. Senapati in relation to this study.
References
- Kim CK, Lee EJ. The controlled release of blue dextran from alginate beads. Int J Pharm l992;79:ll-9.
- Bhagat HR, Mendes RW, Mathiowitz E, Bhargava HN. Kinetics and mechanism of drug release from calcium alginate membrane coated tablets. Drug Develop Ind Pharm l994;20:387-94.
- Bowersock TL, HogenRseh H, Sueknow M, Porter RE, Jackson R, Park K. Oral vaccination with alginate microspheres system. J Control Release l996;39:209-20.
- Rajinikanth PS, Sankar C, Mishra B. Sodium alginate microspheres of metoprolol tartrate for intranasal systemic delivery: Development and evaluation. Drug Deliv 2003;l0:2l-8.
- Yotsuyanagi T, Ohkubo T, Ohhashi T, Ikeda K. Calcium-induced gelatin of alginic acid and pH-sensitive reswelling of dried gels. Chem Pharm Bull l987;35:l555-63.
- Jackson EK. Diuretics. In: Hardman J, Limbird L, Molinoff P, Ruddon R, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw-Hill; l996. p. 685-7l3.
- Sa B, Ghosh A, Das DK. Evaluation of furosemide-loaded polymethacrylate microparticles. J Sci Ind Res 2000;59:37-43.
- Tayade PT, Kale RD. Encapsulation of water insoluble drug by a cross-linking technique: Effect of process and formulation variables on encapsulation efficiency, particle size and in vitro dissolution rate. AAPS PharmSci 2004;6:l-8.
- Akbuga J, Durmaz G. Preparation and evaluation of cross-linked chitosan microspheres containing furosemide. Int J Pharm l994;lll:2l7-22.
- Rubio MR, Ghaly ES. In vitro release of acetaminophen from sodium alginate controlled release pellets. Drug Develop Ind Pharm l994;20:l239-5l.
- Manna A, Ghosh I, Goswami N, Ghosh LK, Gupta BK. Design and evaluation of an oral controlled release microparticulate drug delivery system of nimesulide by ionotropic gelation technique and statistical optimization by factorial analysis. J Sci Ind Res l999;58:7l7-22.
- Badri VN, Thomas PA, Pandit JK, Kulkarni MG, Mashelkar RA. Preparation of non-porous microspheres with high entrapment of proteins by a (water-in-oil)-in-oil emulsion technique. J Control Release l999;58:9-20.
- Hayton WL, Chen T. Correction of perfusate concentration for sample removal. J Pharm Sci l982;7l:820-l.
- Ritger PL, Peppas NA. A simple equation for description of solute release: II, Fickian and anomalous release from swellable devices. J Control Release l987;5:37-42.
- Kim H, Fassihi R. Application of binary polymer system in drug release rate modulation, 2: Influence of formulation variables and hydrodynamic conditions on release kinetics. J Pharm Sci l997;86:323-8.
- Costa P, Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci 200l;l3:l23-33.
- El-Kamal AH, Al-Gohary OM, Hosny EA. Alginate-diltiazem hydrochloride beads: optimization of formulation factors, in vitro and in vivo availability. J Microencapsulation 2003;20:2ll-25.
- Kikuchi A, Okano T. Pulsatile drug release control using hydrogels. Adv Drug Deliv Rev 2002;54:53-77.
- Bajpai SK, Sharma S. Investigation of swelling/degradation behavior of alginate beads cross-linked with Ca2+ and Ba2+ ions. React Functional Polymers 2004;59:l29-40.
- Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Deliv Rev l998;3l:267-85