- *Corresponding Author:
- B. K. Satheeshbabu
Department of Pharmaceutics, Government College of Pharmacy, #2 P Kalinga Rao Road, Bengaluru-560 027 , India
E-mail: bksatishbabu@gmail.com
| Date of Submission | 02-Mar-2015 |
| Date of Revision | 05-Feb-2016 |
| Date of Acceptance | 28-Mar-2016 |
| Indian J Pharm Sci 2016; 78 (2): 203-209 |
Abstract
In the present research envisaged was to prepare gastroretentive beads to increase the retention time in stomach and to modulate the release pattern from the gel beads. The aim of the research work to study the compatibility between drug and other polymers by differential scanning calorimetric analysis, preparation of cod liver oil entrapped calcium pectinate gel beads of famotidine by emulsion gelation method and its evaluation. The gel beads were prepared by employing low methoxy pectin with degree of esterification less than 50% alone and with hydrophilic co polymers carbopol 934P, hydroxypropyl methylcellulose K15M and polycarbophil to study the effect of these co polymers on drug release. The prepared gel beads were subjected for various evaluations like topographical study of whole and dissected bead, percent drug entrapment efficiency, buoyancy and in vitro drug release study in conventional and modified flow through dissolution methods. The prepared beads showed ideal buoyancy, low methoxy pectin gel matrix could not sustain the drug release and presence of co polymers demonstrated sustained release. These observations suggested that cod liver oil entrapped calcium pectinate beads were promising as a carrier for intragastric floating drug delivery of famotidine.
Keywords
Famotidine, floating drug delivery systems, calcium pectinate beads, gastric residence time, modified rosette rice test apparatus
The administration of the any drug delivery to the systemic circulation by oral route is most preferred and convenient. In recent time the administration of controlled release dosage forms through oral route have gained increasing interest to achieve improved therapeutic advantages such as ease of administration, patient compliance and flexibility in formulation [1]. Many previous studies have suggested that orally administered dosage from is subject to physiological adversities such as the short gastric residence time (GRT) and the variable (unpredictable) gastric emptying time (GET), this leads to unpredictable bioavailability and time to attain peak plasma levels. For human beings gastric emptying time normally averages 2-3 h through the major absorption sites like stomach and upper part of the intestine may result in incomplete drug release from the dosage form leading to reduced efficacy of the administered dose. Thus confinement of dosage form in a specific region of GI tracts has number of advantages like enhanced bioavailability, improved therapeutic efficacy, and delivery of drugs to local region of GI tract and reduced dose size. All these considerations have led to development of oral controlled release dosage forms possessing good gastric retention capabilities so that these dosage forms can remain in the gastric region for extended period and thereby increasing the GRT significantly [2-4].
Famotidine is a histamine H2-receptor antagonist. It is widely prescribed in gastric ulcers, duodenal ulcers, Zollinger-Ellison syndrome and gastroesophageal reflux disease. In the management of benign gastric and duodenal ulceration the dose is 40 mg daily by oral route at bedtime, for 4 to 8 weeks. In gastroesophageal reflux disease the recommended dose is 20 mg by oral route twice daily for 6 to 12 weeks. Famotidine is incompletely absorbed from GI tract, the low bioavailability (40-45%) and short biological half-life (2.5-3.5 h) of famotidine following oral administration favors development of a sustained release formulation [5,6].
Materials and Methods
Famotidine was received as a gift sample from Dr. Reddy’s Laboratories Limited, Hyderabad. Low methoxy pectin with the degree of esterification less than 50% was received as a gift sample from Krishna Pectins Pvt. Ltd. Hydroxypropyl methylcellulose K15M was a gift from Colorcon Asia Pvt Ltd, Mumbai. Carbopol 934P was purchased from SD Fine Chem Ltd, Mumbai and Polycarbophil was purchased from B. F. Goodrich Chemicals Co., USA. All other reagents and chemicals used were of analytical grade.
Compatibility study
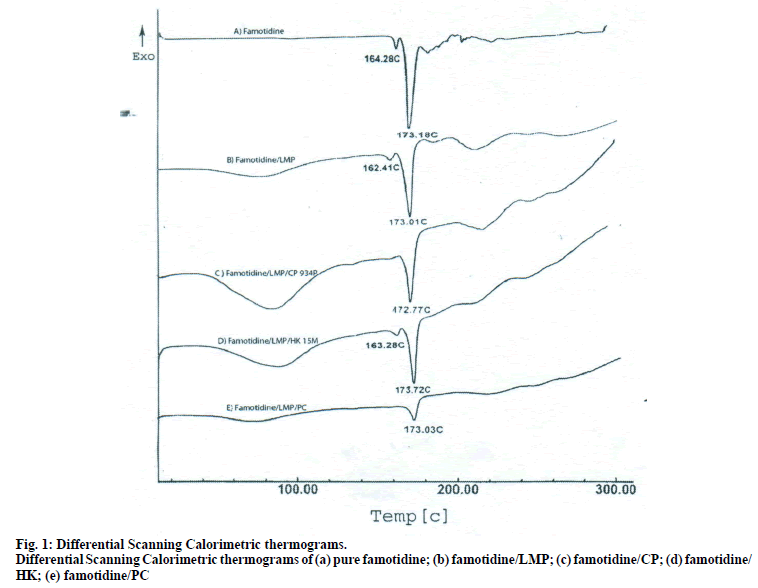
To establish the compatibility between drug and polymers used in the formulation, pure drug, polymers and physical mixture of drug and polymers were subjected to differential scanning calorimetric (DSC) analysis by placing the sample in aluminium pans (DSC-60 Shimadzu, Japan). The heating rate was kept at 10° rise/min up to 300° to better integrate the information. Nitrogen gas was used for purging.
Preparation of floating beads of famotidine
Famotidine, low methoxy pectin (LMP), carbopol 934P (CP), hydroxypropyl methylcellulose K15M (HK) and Polycarbophil (PC) were passed through sieve no 80 separately. Famotidine (20% w/w of dry polymer weight) was dissolved in distilled water. LMP (3% w/v) alone and polymer mixtures (3% w/v) containing LMP and CP, LMP and HK and LMP and PC in 3 different ratios were dissolved in above dispersion. To the above mixture cod liver oil (20% w/w) was added and stirred to form a homogeneous emulsion. The drug-loaded emulsion was extruded through a 23G syringe needle into calcium chloride solution (2% w/v) maintained under gentle agitation. The beads were allowed to remain in the same solution for 30 min to improve their mechanical strength. The formed beads were separated, washed with water and allowed to dry at room temperature overnight. Table 1 lists the formulation variables for different formulations of famotidine loaded floating beads. Blank beads without famotidine were also prepared using the same technique [7].
| Formulation code | LMP : CP (3% w/v) |
LMP : HK (3%w/v) |
LMP : PC(3%w/v) | Oil (% w/w) |
Floating duration (h) | Mean Diameter ± SD (mm |
% EE |
|---|---|---|---|---|---|---|---|
| F Blank | 10:0 | 10:0 | 10:0 | - | NF | 1.675±0.012 | -- |
| F | 10:0 | 10:0 | 10:0 | 10 | NF | 1.693±0.015 | 36.98 |
| 10:0 | 10:0 | 10:0 | 15 | NF | |||
| 10:0 | 10:0 | 10:0 | 20 | 24 | |||
| F1 | 9:1 | - | - | 20 | 24 | 1.740±0.020 | 49.87 |
| F2 | 8:2 | - | - | 20 | 24 | 1.700±0.026 | 58.38 |
| F3 | 7:3 | - | - | 20 | 24 | 1.793±0.015 | 52.79 |
| F4 | - | 9:1 | - | 20 | 24 | 1.720±0.030 | 50.94 |
| F5 | - | 8:2 | - | 20 | 24 | 1.727±0.021 | 53.28 |
| F6 | - | 7:3 | - | 20 | 24 | 1.723±0.015 | 47.50 |
| F7 | - | - | 9:1 | 20 | 24 | 1.707±0.015 | 49.68 |
| F8 | - | - | 8:2 | 20 | 24 | 1.783±0.021 | 64.13 |
| F9 | - | - | 7:3 | 20 | 24 | 1.730±0.020 | 53.23 |
Table 1: Formulation Variables and Floating Properties Various Famotidine Bead Formulations
Evaluation of floating beads
The prepared beads were evaluated for drug entrapment efficiency (EE). An accurately weighed sample of beads (200 mg) was crushed in a mortar and added to 100 ml of 0.1N hydrochloric acid (HCl) buffer (pH 1.2). This mixture was kept overnight under stirring to elute complete drug from the polymer matrix. The mixture was filtered and analysed spectrophotometrically at a wavelength of 265nm (UV spectrophotometer, 1601, Shimadzu, Japan) against blank bead mixture, which was treated similarly. The percentage drug entrapment efficiency (% EE) of each bead formulation was calculated using the following equation, Entrapment efficiency= (Actual drug content/theoretical drug content)×100 [8]. The mean surface diameter of the beads was determined in dry state using a dial thickness meter.
Floating properties
The time between the introduction of the floating drug delivery dosage system (FDDS) into the medium and its buoyancy to the upper one third of the dissolution vessel is floating lag time and the time for which the formulation constantly floated on the surface of the medium is floating duration were measured simultaneously as a part of dissolution studies [9].
Swelling study
The swelling behaviour of the calcium pectinate beads was studied by adopting the following method. The beads were placed in dissolution baskets and weighed (W1). The baskets along with the beads were immersed in 0.1 N HCl and periodically withdrawn and noted down the weight (W2), the study was conducted for 8 h. The swelling index (SI) of each batch was calculated using the following Eqn., % SI=(W2–W1)/W1×100.
In vitro drug release study
The prepared beads were subjected for in vitro release studies. The study was done by using conventional and USP XIV dissolution testing apparatus 2 (paddle method) and modified flow through dissolution method. For conventional method 500 ml of 0.1 N HCl buffer as dissolution medium maintained at 37±0.5° was used. The contents were stirred at 50 rpm and 5 ml aliquot of the solution was withdrawn at predetermined time intervals for 8 h and fresh 5ml dissolution media was replaced to maintain sink condition. The sample aliquots were analyzed spectrophotometrically at a wavelength of 265 nm [10].
The Rosette Rice flow through dissolution apparatus was further modified by including the outer water glass jacket through which hot water at 37±2° was circulated during the study. A dissolution fluid reservoir was mounted above the apparatus to deliver fresh dissolution medium at flow rate of 10 ml/30 min. 0.1N HCl (pH 1.2) was used as dissolution medium and content were stirred at 75 rpm on a magnetic stirrer. Samples were collected every 30 min till 8 h and analysed spectrophotometrically at a wavelength of 265 nm [11].
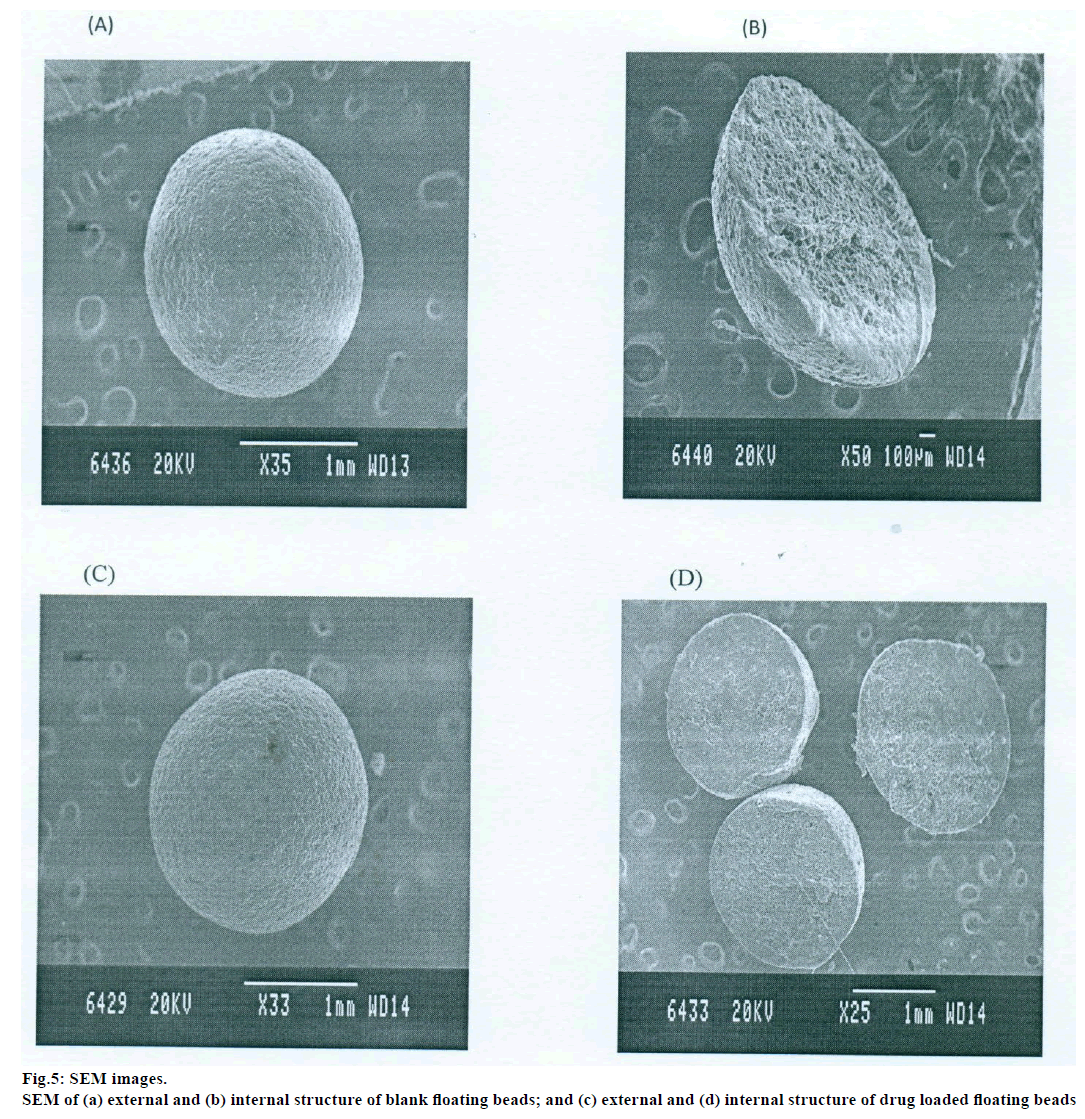
Scanning electron microscopy
The prepared beads were subjected for topographical study of external surface characteristics and internal structure of the dried beads of both drug loaded and blank beads by scanning electron microscope (SEM, Jeol, JSM-840A). For study the internal structure the bead was dissected into two halves with a sharp steel blade, sample were gold coated prior to scanning [7].
Topographical examination of the surface and internal structure of the dried beads (Both drug loaded and blank beads) was performed in a SEM. The samples were gold coated prior to the scanning. For examination of the internal structure of the beads, they were cut in half with a steel blade [7].
Stability study
Stability studies were carried out according to ICH guidelines by storing the Formulations F7 at 40±2° and relative humidity 75±5% for a period of two months in a programmable environmental test chamber (CHM-10S, Remi Instruments Ltd., Mumbai). The samples were withdrawn at 30 and 60 days and analysed for the drug content, floating behaviour and in vitro drug release.
Results and Discussion
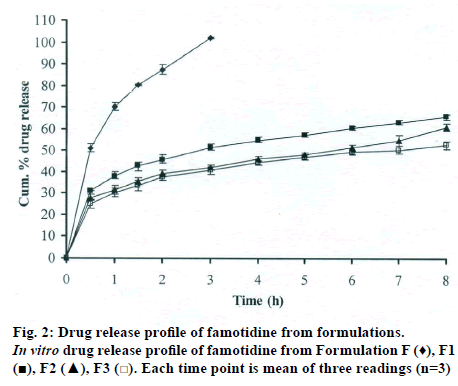
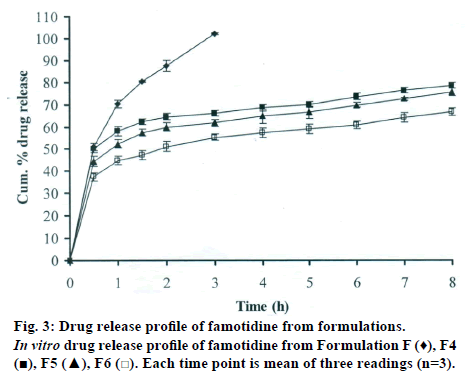
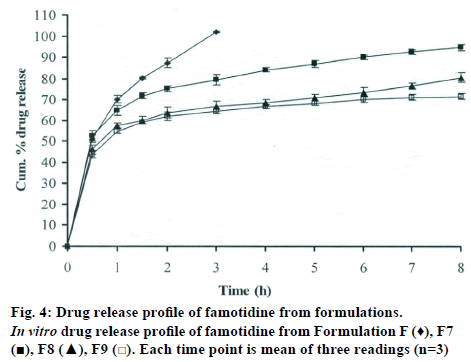
The DSC thermographs for pure famotidine and famotidine with polymers were shown in fig. 1. There were two endothermic peaks observed for pure famotidine at 164.28° and at 173.18° and thermographs for physical mixture of drug and polymer showed no characteristic peak of the polymers and drug peaks were still present but slightly shifted from their original positions. These findings indicate that the drug and polymers were compatible with each other. The prepared beads were spherical and translucent. The mean surface diameter of 10 formulations was between 1.675±0.012 (SD) and 1.793±0.015 mm and results were shown in Table 1. The percent entrapment efficiency for all the formulations was determined and it was found to between 37-64% and results were given in Table 1. The buoyancy properties of the prepared beads was evaluated along with dissolution studies, the beads without oil sank instantaneously beads with sufficient of cod liver oil demonstrated instantaneous and excellent floating ability and results were shown in Table 1. The swelling behaviour of beads from all formulations were performed in 0.1 N HCl, there was no swelling takes place during study period and no significant changes in swelling ratio of the beads was observed. The beads of all the formulations were subjected for in vitro release studies in both conventional dissolution apparatus and modified rosette flow through dissolution apparatus using 0.1N HCl (pH 1.2) as dissolution medium for the period of 8 h, the formulation consists of only LMP could not sustain drug release up to 8 h the complete release was observed at the end of 3 h whereas formulations consists of CP F1, F2 and F3 released 65.62, 60.76 and 52.41% of drug at the end of 8 h, respectively. In Formulations F4, F5 and F6 HK was included drug released was observed was 78.28, 75.27 and 66.51% of the drug at the end of 8h, respectively. The formulations prepared by including PC F7, F8 and F9 were shown drug release 94.39, 80.38 and 71.37% of the drug at the end of 8h, respectively. For in modified rosette flow through dissolution cell the drug release from formulation F 63.79±1.40% of the drug within 1h but could not sustain the drug release over the following 7 h and released 100% drug at the end of 5 h 30 min. the release from all the formulations F1 to F9 was 56.81, 50.99, 48.39, 75.84, 71.47, 67.76, 81.55, 74.82 and 68.95% of the drug at the end of 8h, respectively. Whereas formulation comprised only LMP could not sustain the famotidine release up to 8 h the complete drug release was observed drug at the end of 5.5 h. The release profile was given in figs. 2, 3 and 4 for conventional dissolution. The scanning electron micrographic analysis for blank and drug loaded beads was carried out to study topography and internal structure of the beads and micrographs are shown in fig. 5. In view of potential utility stability studies were carried out for formulation F7 for two months and conditions maintained were as per ICH guidelines. At the end of or each month the drug content, floating behaviour and in vitro release studies were carried out and results are shown in Table 2.
| Time | Drug content ±SD (mg) |
Floating behavior | Drug release at the end of 8 h | |
|---|---|---|---|---|
| FLT (min) | Floating duration (h) | |||
| Zero month | 2.908±0.057 | 0 | 24 | 81.55±1.61 |
| First Month | 2.923±0.096 | 0 | 24 | 79.91±1.78 |
| Second Month | 2.911±0.079 | 0 | 24 | 81.49±2.05 |
Table 2: Stability Study of Formulation F7
The DSC thermograph of pure famotidine showed two endothermic peaks, the DSC thermographs of physical mixture of famotidine and polymers showed no characteristic peaks of polymers and pure famotidine peaks were still present but slightly shifted from the original positions which could be possibly due to an ionic interaction and this characteristic features of drug melting suggested that compatibility between drug and polymers, but Some modification of drug peak, such as changes in area, shape or peak temperature were found, they arose simply from mixing the components. The pectin with low degree of esterification (DE) was form gel by ionotropic gelation with divalent calcium ions. To get gel beads an emulsion of cod liver oil consisting pectin was dropped into calcium chloride solution through needle a spherical gel beads were formed instantaneously due to intermolecular cross-links were formed between the divalent calcium ions and negatively charged carboxyl groups of the pectin molecules. It was found that the oil layer was separated from the pectin solution in spit being mixed by stirrer [7]. To avoid the leakage of oil homogenisation must.
During homogenisation process pectin helped to emulsify the mixture of water and oil, this property was limited when the oil proportion was increased to more than 30%w/w but in this proportion leakage of oil from the bead were taken place. It was found that a minimum of 20% w/w cod liver oil was necessary to impart satisfactory buoyancy to the beads. Beads from all the formulations were spherical and the mean particle diameter of the blank oil entrapped calcium pectinate beads without drug and containing drug and other co polymers found that mean diameter increased as compared with beads without drug, even process parameters were kept constant the reason for increased mean diameter due to added materials to the formulations. The drug entrapment efficiency of the famotidine loaded beads prepared with only LMP was low as compared with beads prepared with other co polymer where entrapment efficiency was more, this may be attributed to the highly porous nature of the calcium pectinate matrix in the absence of other co polymer due to which drug may diffused back into the cross linking solution during curing period. It was observed that the prepared beads with 20% w/w of cod liver oil demonstrated instantaneous and excellent floating ability. It was observed that varying the polymer and co polymer proportion did not affect the floating lag time or the floating duration. The floating ability of the beads was directly related to the amount of oil entrapped in the polymer matrix. During the study beads were floated till 24 h. The swelling behaviour of the gel beads was performed in 0.1 N HCl buffer, it was observed that there was no change in the swelling ratio and during dissolution study beads were not swollen or eroded. These observations suggested that the drug release was not under the control of the swelling but rather dissolution controlled and diffusion through the polymer matrix [12]. The calcium pectinate beads exhibited a biphasic release as an initial rapid drug release followed by a slower and sustained and gradually increasing drug release phase after 1 h. The formulation contain only LMP could not sustain the release and maximum percent of drug released in 1 h and 100% release at the end of 3 h this could be due to the large gel porosity of the calcium pectinate matrix and it was observed that calcium pectinate alone not suitable for oral controlled release and this property was attributed to use of co polymers in the formulations. The incorporation of these co polymers imparts the sustainability to the formulations by enhancing the viscosity and gel strength of the matrix. As the proportions of co polymers increased correspondingly sustainability was increased. The compression of percentage of drug release in both studies showed that the conventional method shown high percentage of drug release than the flow through dissolution method the reason could be due to high volume of dissolution medium and this observation was contradicted the findings of Gohel et al. who have claimed a higher drug release from modified dissolution method [11]. The in vitro release data of all formulations were fitted to zero order, first order. Huguchi and Korsemeyer and Peppas equations, it was observed that formulation F followed first order release and formulations F1 to F9 followed Higuchi model. As the n values of the Korsemeyer and Peppas model for all formulations was found to be less than 0.5 which suggested that drug release from the bead matrices was Fickian diffusion.
The topographical study reveals that the external surface was smooth with slightly rougher surface or shrinkage which could be due to drying. The internal surface of the blank beads showed sponge like structure with little droplets of entrapped oil which imparts buoyance and in drug loaded beads drug and rate controlling polymers were dispersed uniformly in polymer matrix. Results of stability showed no change in the drug content, floating behaviour and in vitro drug release characteristics of the formulation F7 which suggested the formulation was stable.
In the present research work cod liver oil entrapped floating calcium pectiante gel bead of famotidine were prepared to prolong the residence time in the stomach and to achieve sustainability of the drug release. The gel beads prepared with 20% cod oil showed excellent buoyancy properties for the study period. Presence of co polymers effectively sustains the drug release from the gel matrices.
Financial Assistance
None.
Conflict of Interests
None declared.
References
- Nayak AK, Maji R, Das B. Gastroretentive drug delivery systems: a review. Asian J Pharm Clin Res 2010;1:2-9.
- Singh BN, Kwon H. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention, J Control Release 2000;63:235-59.
- Dave BS, Amin AF, Patel MM. Gastroretentive drug delivery system of ranitidine hydrochloride: Formulation and in vitro evaluation. AAPS PharmSci Tech 2004;5:77-82.
- Arora S, Ali J, Ahuja A, Khar RK, Baboota A. Floating drug delivery system: A review. AAPS PharmSci Tech 2005;6: E372–90.
- Available from: htpp://www.parpharm.com/pdf/product/0608.pdf. [Last accessed on Nov 3, 2010].
- Jaimini M, Rana AC, Tanwar YS. Formulation and evaluation of famotidine floating tablets. Current Drug Delivery 2007;4:51-5.
- Sriamornsak P, Thirawong N, Puttipipakhachorn S. Morphology and buoyancy of oil entrapped calcium pectinate beads. AAPS PharmSci Tech 2004;6:65-71.
- Halder A, Maiti S, Sa B. Entrapment efficiency and release characteristics of polyethyleneimine-treated or untreated calcium alginate beads loaded with propranolol- resin complex. Int J Pharm 2005;302:84-94.
- Shishu, Gupta N, Aggarwal N. Stomach specific drug delivery of 5 fluorouracil floating alginate beads. AAPS Pharmscitech 2007;8(2): E143–9.
- Rajinikanth PS, Mishra B. Preparation and in vitro characterization of gellan based floating beads of acetohydroxamic acid for eradication of H. pylori. Acta Pharm 2007;57:413-27.
- Gohel MC, Mehta PR, Dave RK, Bariya NH. A more relevant dissolution method for evaluation of floating drug delivery system. J Dissol Tech 2004;11:21-5.
- Piyakulawat P, Praphairaksit N, Chantarasiri N, Muangsin N. preparation and evaluation of chitosan/carrageenan beads for controlled release of sodium diclofenac. AAPS PharmSciTech 2007;8(4):E97.