- *Corresponding Author:
- A. N. Bajaj
C. U. Shah College of Pharmacy, S.N.D.T. Woman’s University, Juhu Campus, Santacruz (W), Mumbai - 400 049, India
E-mail: bajajamrita@rediffmail.com
| Date of Acceptance | 25 October, 2007 |
| Indian J Pharm Sci,2007, 69 (5) : 717-721 |
Abstract
Introduction
Treating respiratory diseases with inhalers requires delivering sufficient drug to the lungs to bring about a therapeutic response. For optimal efficacy, drug administration must be reliable, reproducible and convenient. Enhanced powder dispersibility could be designed into microspheres and porous particles through a combination of novel formulation and process design [1]. The present work outlines the design of dry powder inhaler (DPI) formulations to achieve delivery goals. Formulation development, characterization strategies and processing methods have been discussed. Budesonide (BUD) has wide range of inhibitory activities against inflammatory mediators. Conventional BUD DPI formulations were developed and the effect of various grades of inhalable lactose on respirable fraction was studied. BUD microspheres and porous particles were developed using spray drying technology which resulted in improved respirable fraction of BUD.
Materials and Methods
Budesonide was obtained from Lupin Ltd., Mumbai; gelatin and chitosan were procured from SD Fine Chemicals, Mumbai and different grades of inhalable lactose were obtained as gift sample from DMV Int., The Netherlands.
Development of conventional BUD DPI formulations
Drug was mixed with fine lactose and this premix was dispersed over coarse lactose in geometric proportions and developed formulations were characterized (Table 1) [2]. Degree of deacetylation of chitosan was determined by IR spectroscopy [3].
| Batch Code | Assay (% w/w) | Bulk Density (g/cc) | Moisture Content (% w/w) | % Deposition in Stage ii |
|---|---|---|---|---|
| A | 109.6011 | 0.3333 | 0.0327 | 32.0487 |
| B | 103.5188 | 0.68 | 0.0109 | 31.6768 |
| C | 112.5635 | 0.3222 | 0.0020 | 30.9298 |
| D | 106.0086 | 0.625 | 0.0040 | 31.8673 |
| E | 108.5848 | 0.5875 | 0.0015 | 30.4080 |
Batch A contains Lactohale 300 and Pharmatose 150M (60:40); batch B contains Inhalac and Lactohale 100M (60:40); batch C contains Lactohale 300 and Pharmatose 150M (70:30); batch D contains Inhalac and Pharmatose 150M (70:30); batch E contains Inhalac and Lactohale 100 (70:30)
Table 1: Quality Control Tests Preformed On Conventional Bud Dpi Formulations
Development of microspheres and porous particles by spray drying
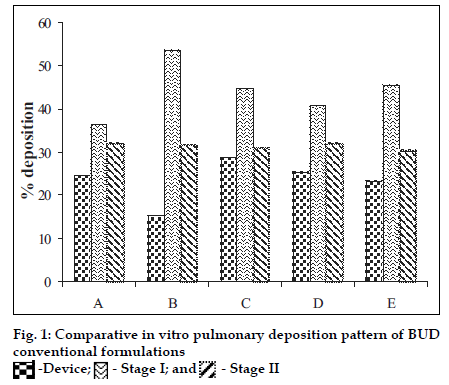
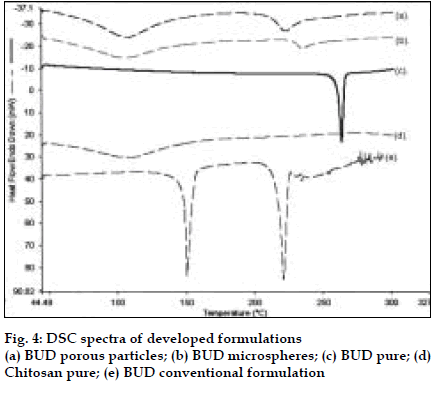
Polymers chitosan, gelatin and their combination were spray dried using a Labultima Mini Spray Dryer. Process parameters were optimized using 22 factorial design. Effect of different parameters was studied (fig. 1). Gelatin /BUD and chitosan/ BUD were spray dried in water: methanol (1:1) as 1.0% and 0.5% w/v, respectively [4]. Porous particles were generated by adding chloroform (5% v/v) as blowing agent [5]. In vitro deposition was determined using a Twin Stage Impinger apparatus [2]. Particles were also subjected to Anderson Cascade Impactor studies to determine mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD). DSC studies were performed.
In vitro release studies
These studies were conducted using Flow Through Cell assembly (USP IV) at 37° with flow rate of 16 ml/min (in 100 ml of phosphate buffer pH 7.0) [6]. Five milliliter sample was analyzed by UV Spectrophotometry at 246 nm. Mechanism of drug release was determined using various kinetic models. Coefficient of correlation from plots of Q vs. t (cumulative % drug release vs. time), log Q vs. log t and Q vs. square root of t were calculated.
Results and Discussion
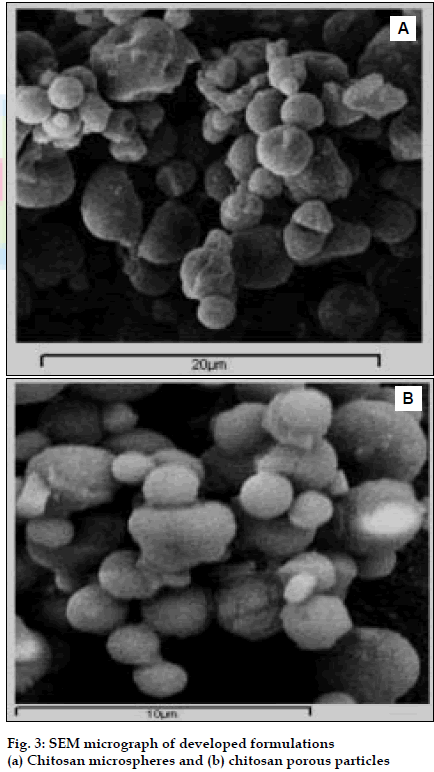
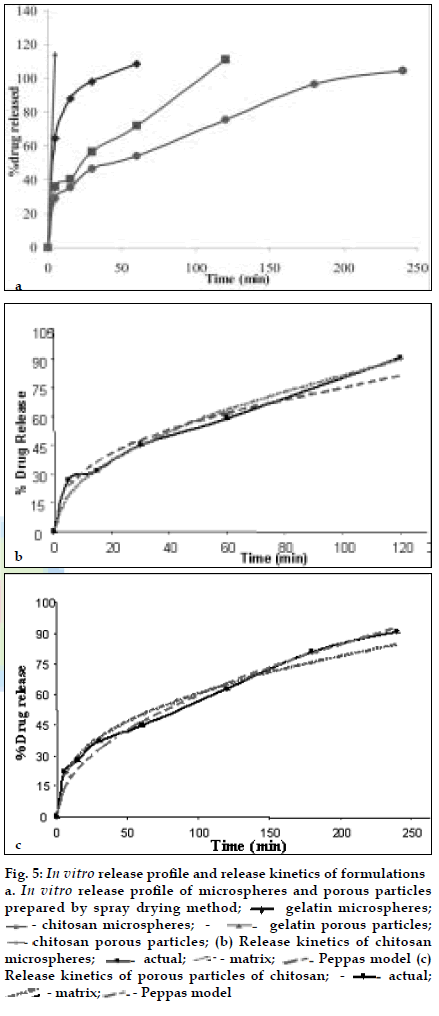
BUD DPI formulations were developed using various grades of inhalable lactose like Pharmatose, Lactohale, Inhalac and mannitol in various combinations. The effect of particle size of excipients on respirable fraction of BUD was assessed (Table 1 and fig. 1). Degree of deacetylation of chitosan was found to be 45%. Optimum process parameters were inlet temperature (130°), aspirator rate (50%), feed rate (30%) and outlet temperature (80°) (fig. 2).Microspheres and porous particles of BUD were prepared with chitosan (1:2, drug: polymer ratio) with 86% and 96% w/w entrapment efficiency, respectively. Developed chitosan microspheres and porous particles were characterized for particle size (SEM analysis - JSM- 840A-/WDS/EDS Sys- JEOL instrument, fig. 3), % drug entrapment and % FPF (Table 2). DSC studies confirmed no interaction between drug and polymer (fig. 4). In vitro release profile is shown in fig. 5a. Regression coefficients (near to 1) for zero order, matrix and Korsmeyer-Peppas kinetic equations confi rmed release by slow zero order kinetics through diffusion matrix (Table 3, fig. 5b and 5c). Korsmeyer- Peppas plot indicated good linearity (r2 = 0.9722).
| Parameters | Observations | |
|---|---|---|
| BUD Microspheres | BUD Porous particles | |
| Apperance | Hollow, Porous | Hollow, Porous,free flowing microspheres |
| Particle Size | 1-10µm | 1-10µm |
| %yield | 20-30 | 20-30 |
| %moisture content | 0.3770 | 0.3280 |
| %drug entrapment | 86.00(RSD=0.4890) | 96.00(RSD=0.2616) |
| %FPF | 35.6785% | 46.8199% |
| MMAD | 4.60µm | 4.30µm |
| GSD | 1.75µm | 2.54µm |
FPF is Þ ne particle fraction; MMAD is mass median aerodynamic diameter and GSD is geometric standard deviation
Table 2: Results Of Optimized Dpi Formulations Prepared By Spray Drying Technology
| Formulations | R2 | ||
|---|---|---|---|
| Zero order | Korsmeyer-peppas | Matrix | |
| Gelatin microspheres | 0.4272 | 0.9920 | 0.9166 |
| Chitosan microspheres | 0.8338 | 0.9722 | 0.9898 |
| Gelatin porous particles | 1.0000 | 1.0000 | 1.0000 |
| Chitosan porous particles | 0.8315 | 0.9877 | 0.9906 |
Table 3: Regression Coefficients For Formulations
Fig. 5: In vitro release proÞ le and release kinetics of formulations
a. In vitro release proÞ le of microspheres and porous particles
prepared by spray drying method; ![]() - gelatin microspheres;
- gelatin microspheres; ![]() - chitosan microspheres;
- chitosan microspheres;![]() - gelatin porous particles;
chitosan porous particles; (b) Release kinetics of chitosan
microspheres;
- gelatin porous particles;
chitosan porous particles; (b) Release kinetics of chitosan
microspheres; ![]() actual;
actual;![]() - matrix;
- matrix; ![]() Peppas model (c)
Release kinetics of porous particles of chitosan;
Peppas model (c)
Release kinetics of porous particles of chitosan; ![]() - actual;
- actual; ![]() - matrix;
- matrix;![]() - Peppas model
- Peppas model
Microspheres and porous particles were engineered to be both hollow and porous and these exhibited excellent flow and dispersion from passive DPIs. In vitro characterization predicted highly efficient lung delivery. The results indicated that spray drying technology can be used to generate inhalable particles like microspheres and porous particles with improved pulmonary deposition as compared to conventional DPI formulations
References
- Ashurst I, Malton A, Prime D, Sumby B (2000). Latest advances in the development of dry powder inhalers. Pharm Sci Tech Today;3:246-56.
- Feddah MR, Brown KF, Gibbs EM, Davies NM (2000). In vitro characterization of Metered dose inhaler versus dry powder inhaler Glucocorticoid products- influence of inspiratory flow rates. J Pharm Pharm Sci;3:317-24.
- Baxter A, Dillon M, Taylor KD, Roberts GA (1992). Improved method for IR determination of the degree of N-acetylation of chitosan. Int J Biol Macromol;14:166-9.
- Corrigan DO, Healy AM, Corrigan OI (2006). Preparation and release of salbutamol from chitosan and chitosan co-spray dried compacts and multiparticulates. Eur J Pharm Biopharm;62:295-305.
- He P, Davis SS, Illum L(1999). Chitosan microspheres prepared by spray drying. Int J Pharm;187:53-65.
- Davies NM, Feddah MR, A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm 2003;255:175-87.