- *Corresponding Author:
- Xuju Sun
Department of Pharmacy,s Liyuan Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430077, China
E-mail: sunxuju1973@163.com
| Date of Received | 18 January 2020 |
| Date of Revision | 14 June 2021 |
| Date of Acceptance | 18 December 2021 |
| Indian J Pharm Sci 2021;83(6):1295-1303 |
Abstract
This research attempts to probe into the influence mechanism of ginsenoside Rg5 on 1-methyl-4- phenylpyridine ion induced Parkinson's disease cell model damage. Different concentrations (25, 50, 100 μmol/l) of ginsenoside Rg5 acted on human neuroblastoma SK-N-SH cells induced by 1-methyl-4- phenylpyridine ion. Adopt cell counting kit-8, flow cytometry, quantitative reverse transcription polymerase chain reaction and western blot to exam cell viability, apoptosis rate, microRNA-874-3p and thioredoxin interacting proteins expressions. Transfected microRNA-874-3p mimics and thioredoxin interacting proteins small interfering RNA to SK-N-SH cells, respectively. Then adopt the above mentioned methods to exam microRNA-874-3p overexpression or thioredoxin interacting proteins inhibition influence on SK-N-SH cells viability and apoptosis which is induced by 1-methyl-4-phenylpyridine ion. Adopt dual luciferase report experiment and western blot methods to test the regulatory function of microRNA- 874-3p on thioredoxin interacting proteins. After ginsenoside Rg5 acted on 1-methyl-4-phenylpyridine ion induced SK-N-SH cells, the cell viability and microRNA-874-3p expression increased significantly, but thioredoxin interacting proteins expression and apoptosis rate decreased significantly (p<0.05). After microRNA-874-3p over-expressing, 1-methyl-4-phenylpyridine ion induced SK-N-SH cells viability were significantly increased and apoptosis rate was remarkably reduced (p<0.05). After inhibiting thioredoxin interacting proteins expression, 1-methyl-4-phenylpyridine ion induced SK-N-SH cells viability was significantly increased, but apoptosis rate was remarkably lowered (p<0.05). MicroRNA-874-3p regulates thioredoxin interacting proteins expression negatively. MicroRNA-874-3p inhibition could attenuate the effect of ginsenoside Rg5 on the viability and apoptosis of 1-methyl-4-phenylpyridine ion induced SK-N-SH (p<0.05). Thioredoxin interacting proteins inhibition went into reverse microRNA-874-3p inhibition combined with ginsenoside Rg5 treatment on 1-methyl-4-phenylpyridine ion induced SK-N-SH activity and apoptosis (p<0.05). Ginsenoside Rg5 could alleviate 1-methyl-4-phenylpyridine ion induced Parkinson's disease cell model damage and its mechanism possibly has relationship with the up-regulation of microRNA-874-3p/thioredoxin interacting proteins molecular axis.
Keywords
Ginsenoside Rg5, Parkinson's disease, microRNA-874-3p, thioredoxin interacting proteins, 1-methyl-4-phenylpyridine ion
Parkinson's disease (PD) is a kind of progressive neurodegenerative disease. The main pathology change performs as midbrain nigra dopaminergic neuron degeneration and death. PD patients often exhibit typical motor symptoms such as resting tremor, slow and reduced movements, increased muscle tone and postural instability, which seriously affect the quality of life of millions of elderly people around the world [1]. Some previous studies have proved that oxidative stress and apoptosis make a valuable contribution towards dopaminergic neurons degeneration of PD [2]. Ginsenoside Rg5 is one of the main rare saponins produced in the process of ginseng steaming. It has multiple biological activities such as anti-cancer, anti- inflammatory, memory enhancement and so on [3]. Studies have shown that ginsenoside Rg5 can inhibit the production of active oxygen, increase mitochondrial membrane protein, reduce the apoptosis of PC12 cells induced by cobalt chloride and can reduce the cerebral infarct volume and cerebral nerve dysfunction in rats with ischemia-reperfusion injury in vivo [4]. However, the effect of ginsenoside Rg5 on cell damage in PD models is not yet known. MicroRNA-874-3p (miR- 874-3p) is with multiple regulatory functions. miR- 874-3p expression is reduced among ischemic stroke patients. miR-874-3p expression up-regulating can promote SH-SY5Y proliferation and alleviates oxygen and glucose deprivation and reperfusion which will lead to cell apoptosis and has a protective effect on ischemic stroke [5,6]. Sequence analysis revealed that there were binding sites between miR-874-3p and Thioredoxin Interacting Proteins (TXNIP). TXNIP has been confirmed to be involved in glial cell activation and neuroinflammatory response [7,8], but we haven’t known miR-874-3p and TXNIP function in PD model cell injury yet. In this study, we set SK-N-SH cells induced by 1-Methyl-4-Phenylpyridine Ion (MPP+) replicating PD neuronal injury as the model [9], to discuss the effect of ginsenoside Rg5 on PD models cell activity, apoptosis, miR-874-3p and TXNIP expressions, and analyze its possible mechanism, attempt to provide a theoretical basis for the development and application of ginsenoside Rg5 in PD prevention and treatment.
Materials and Methods
Materials:
Purchase human neurocytoma cell SH-SY5Y from American Type Culture Collection; MPP+ was purchased from Sigma in the United States; Ginsenoside Rg5 (CAS No. 186763-78-0, purity ≥98 %, specification 10 mg/bottle) was purchased from Shanghai Yuanye Biological; Purcahse Cell Counting Kit (CCK-8) from Tongren Institute of Biology, Japan; Purchase Annexin-Fluorescein V-FITC (Annexin V-FITC) cell apoptosis detection kit from Shanghai Yubo Biological Company; Lipofectamine 2000, Trizol lysate, Radioimmunoprecipitation Assay (RIPA) lysate were purchased from Invitrogen, USA; miR-874-3p mimics, miR-874-3p inhibitors (anti-miR- 874-3p), TXNIP Small Interfering RNA (si-TXNIP) and corresponding controls (miR-con, anti-miR-con, si-con) and dual luciferase reporter gene vector were provided by Guangzhou Ruibo Biological Company; Purchase rabbit anti-TXNIP antibody, rabbit anti- cleaved-caspase-3 antibody, rabbit anti-beta (β)-actin antibody and goat anti-rabbit IgG from Abcam, USA.
Methods:
Cell culture, modeling and experiment grouping: Culture SK-N-SH cells in DMEM, then add 10 % fetal bovine serum and 1 % penicillin double anti- streptomycin into it, place them in 5 % carbon dioxide humidified incubator at 37°. Adopt 1 mM MPP+ to deal with SK-N-SH cells for 24 h to establish an in vitro PD cell injury model. Cells are grouped as follows: Normal Control (NC) group was SK-N-SH cells that is normally cultured; MPP+ group was that SK-N-SH cells were handled by 1mM MPP+ for 24 h to establish in vitro PD cell injury model; MPP++25 μM ginsenoside Rg5 group, MPP++50 μM ginsenoside Rg5 group and MPP++100 μM ginsenoside Rg5 group were that MPP+ medium containing 25, 50, 100 μM ginsenoside Rg5 and 1mM was used for 24 h. Collected each group of cells and detected cell viability, apoptosis and expression of miR-874-3p, TXNIP and cleaved-caspase-3 according to the following method steps.
In order to discuss how ginsenoside Rg5 works on PD cell model damage induced by MPP+ and the relationship between miR-874-3p and TXNIP, we seeded SK- N-SH cells in 6-well plates, use Lipofectamine 2000 to transfect all the vectors and controls into SK-N- SH respectively, then divided them into the several groups as below. Transfected MPP++miR-con group, MPP++miR-874-3p group, MPP++Si-Con group and MPP++Si-TXNIP group with miR-con and miR-874-3p mimics into SK-N-SH respectively, then cultured with 1 mM MPP+ for 24 h. Transfected MPP++ginsenoside Rg5+anti-miR-con and MPP++ginsenoside Rg5+anti- miR-874-3p groups with anti-miR-con and anti- miR-874-3p respectively to SK-N-SH. Then cultured them with MPP+ medium which contains 50 μM ginsenoside Rg5 and 1 mM for 24 h. Co-transfected MPP++ginsenoside Rg5+anti-miR-874-3p+si-con group and MPP++ginsenoside Rg5+anti-miR-874- 3p+si-TXNIP group with anti-miR-874-3p+Si-Con and anti-miR-874-3p+si-TXNIP to SK-N-SH, then cultured with MPP+ medium containing 50 μM ginsenoside Rg5 and 1 mM for 24 h.
CCK-8 method to exam cell viability: Placed SK- N-SH cells or transfected SK-N-SH cells into 96-well plates according to 1×105 incubated overnight and then added ginsenoside Rg5 and/or MPP+ for 24 h according to experimental groups. Each well was added 10 μl CCK-8 reagent, then continued to incubate for 2 h. Adopted the microplate reader to exam the Optical Density (OD) value at a wavelength of 450 nm.
Flow cytometry to exam cell apoptosis: Collected the above groups of cells, washed the cells twice with phosphate buffer, then added 5 μl Annexin V-FITC and 5 μl Propidium Iodide (PI) respectively to stain K-N- SH cells by following apoptosis kit directions. Adopt flow cytometry to exam apoptosis rate of each group.
Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR) detection of miR- 874-3p and TXNIP Messenger RNA (mRNA) expression: Collected the above-mentioned groups of cells add Trizol lysate to pick up cells total RNA. Complementary DNA was synthesized under the action of reverse transcriptase and use RT-qPCR to test miR-874-3p and TXNIP mRNA expression levels. The Upstream Primer Sequence (UPS) of miR-874- 3p is 5′-GAACTCCACTGTAGCAGAGATGGT-3′and the Downstream Primer Sequence (DPS) is 5′-CATTTTTTCCACT CCTCTTCTCTC-3′; U6 UPS is 5′-CTCGCTTCGGCAGCACA-3′, DPS is 5′-AACGCTTCACGAATTTGCGT-3′; TXNIP UPS is 5′-AGTTACCCGAGTCA AAGCCG-3′, DPS is 5′-TCTCGTTCTCACCTGTAGGC; The UPS of β-actin is 5'-CTTCCTTCCTGGAATC-3' and the DPS is 5'-GGCATAGAGGTCTTTACGGATG-3'. Adopt 2− ΔΔCt method to analyze miR-874-3p and TXNIP mRNA relative quantitation.
Western blot method to exam cleaved-caspase-3 and TXNIP protein expression: Collected the above groups of cells, added RIPA lysate to 4° for 1 h, centrifuge 10 min at 12 000 r/min to collect the supernatant and determined the protein concentration. Added 2× loading buffer, then place them into boiling water for 5 min to obtain denatured protein. After cooling, added 30 μg protein sample to each lane. Electrophoresis at 100 V for 100 min until bromophenol blue migrated to the bottom of the gel. 30 mA low temperature transfer membrane for 45 min, sealed the nitrocellulose membrane in 5 % skim milk at room temperature for 1 h, added diluted 1:1000 primary antibody to react for 2 h at room temperature and then added diluted 1:2000 secondary antibody to react for 1 h. After adding the chemiluminescence reagent for color development, the image processing software analyzed the gray value of the target band and calculated the relative expression of the target protein.
Dual luciferase reporter verifies the targeting effect of miR-874-3p on TXNIP: Insert the TXNIP Wild Type (WT) sequence containing miR-874-3p connection site and TXNIP Mutant (MUT) sequence containing miR- 874-3p connection site into pGL3 vector to construct the dual luciferase reporter gene assay WT-TXNIP, MUT-TXNIP. Co-transfected SK-N-SH cells with WT- TXNIP and MUT-TXNIP, miR-874-3p mimics and miR-NC respectively. 48 h later, use dual luciferase assay system to exam the relative luciferase activity.
Statistical analysis:
We apply Statistical Package for the Social Sciences (SPSS) 20.0 for the experimental data processing and statistical analysis. Use mean±standard deviation (x? ±s) to indicate measurement data, contrast both groups by independent sample t test, among multiple groups, we use the one-way analysis of variance, further pairwise comparisons we use the Student–Newman– Keuls (SNK)-q test. p<0.05 was supported to possess statistical significance.
Results and Discussion
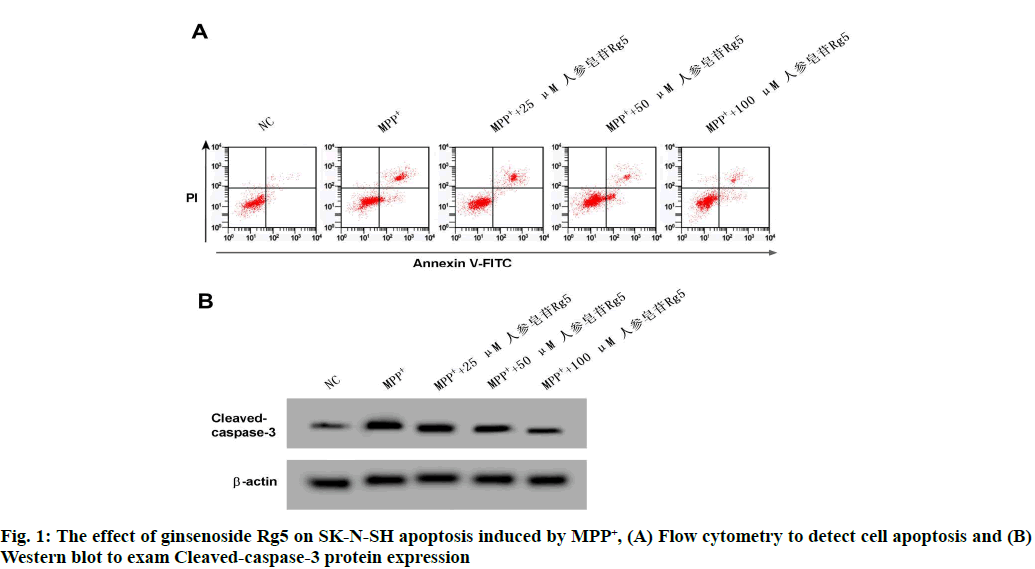
Compared with NC group, SK-N-SH cell viability of MPP+ group was remarkably reduced (p<0.05); contrast MPP+ group, SK-N-SH cells vitality was obviously increased in MPP++25 μM ginsenoside Rg5 group, MPP++50 μM ginsenoside Rg5 group and MPP+ +100 μM ginsenoside Rg5 group (p<0.05). See the following Table 1 and fig. 1.
| Grouping | Cell viability OD 450 nm |
|---|---|
| NC | 1.224±0.11 |
| MPP+ | 0.663±0.07* |
| MPP++25μM ginsenoside Rg5 | 0.712±0.06# |
| MPP++50μM ginsenoside Rg5 | 0.925±0.10# |
| MPP++100μM ginsenoside Rg5 | 1.063±0.11# |
| F | 58.534 |
| p | 0.000 |
Note: Contrast NC group mentioned above, *p<0.05; contrast MPP+ group mentioned above, #p<0.05
Table 1: The Effect of Different Levels Ginsenoside Rg5 on Sk-N-Sh Cells Activity Induced by MPP+ (x?±s, n=9)
Compared with NC group, SK-N-SH cleaved caspase-3 protein expression and apoptosis rate were remarkably enhanced in MPP+ group (p<0.05); Contrast MPP+ group, cleaved-caspase-3 protein expression and apoptosis rate of SK-N-SH cells in MPP++25 μM ginsenoside Rg5 group, MPP++50 μM ginsenoside Rg5 group and MPP++100 μM ginsenoside Rg5 group were significantly reduced (p<0.05). See the following Table 2 and fig. 2.
| Grouping | Cleaved-caspase-3 | Apoptosis rate (%) |
|---|---|---|
| NC | 0.28±0.02 | 6.05±0.52 |
| MPP+ | 0.80±0.08* | 35.19±3.04* |
| MPP++25μM ginsenoside Rg5 | 0.68±0.06# | 28.33±2.23# |
| MPP++ 50μM ginsenoside Rg5 | 0.50±0.05# | 18.45±1.34# |
| MPP++ 100μM ginsenoside Rg5 | 0.40±0.04# | 14.59±1.48# |
| F | 136.303 | 319.781 |
| p | 0.000 | 0.000 |
Note: Contrast NC, *p<0.05; contrast MPP+, #p<0.05
Table 2: The Effect of Ginsenoside Rg5 on MPP+ Induced Apoptosis of SK-N-SH (x?±s, n=9)
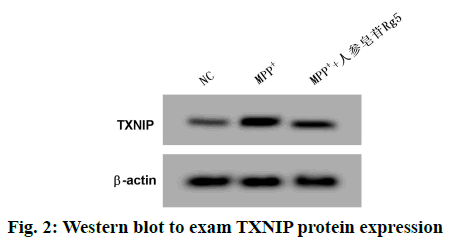
Contrast NC group, SK-N-SH cells miR-874-3p expression in MPP+ group was remarkably lowered, but TXNIP mRNA and TXNIP protein expressions were remarkably enhanced (p<0.05); Contrast MPP+ group, SK-N-SH cells miR-874-3p expression in MPP++ginsenoside Rg5 group was obviously increased, but TXNIP mRNA and TXNIP protein expressions were remarkably decreased (p<0.05). See the following Table 3 and fig. 2.
| Grouping | miR-874-3p | TXNIP mRNA | TXNIP protein |
|---|---|---|---|
| NC | 1.00±0.11 | 1.02±0.09 | 0.40±0.03 |
| MPP+ | 0.42±0.04* | 3.24±0.28* | 0.89±0.07* |
| MPP++ginsenoside Rg5 | 0.91±0.09# | 1.64±0.13# | 0.48±0.04# |
| F | 120.674 | 342.627 | 252.122 |
| p | 0.000 | 0.000 | 0.000 |
Note: Contrast NC, *p<0.05; contrast MPP+, #p<0.05
Table 3: Effects of Ginsenoside Rg5 on Mir-874-3p And Txnip Expressions in MPP+ Induced Sk-N-Sh Cells (x?±s, n=9)
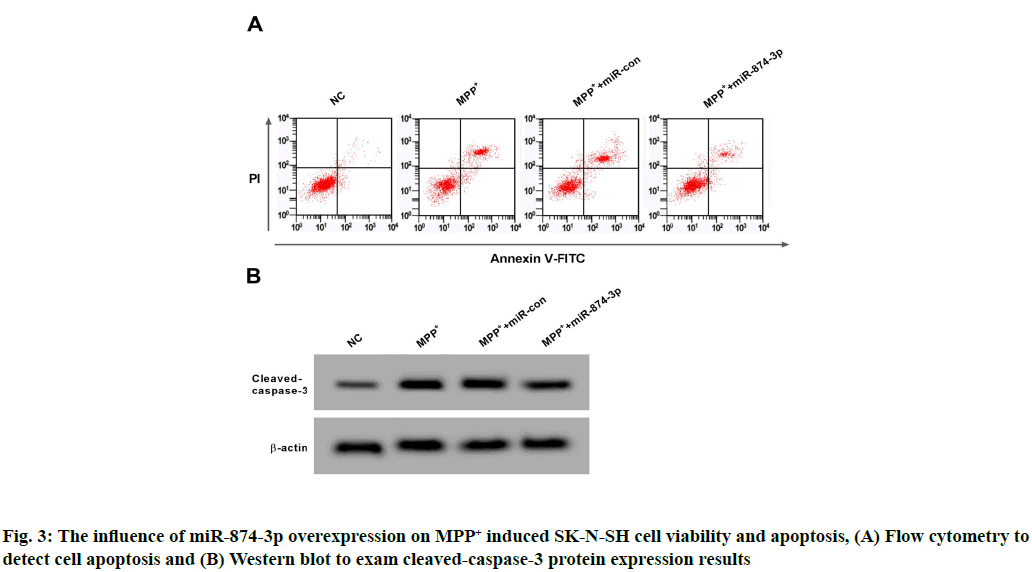
Contrast NC group, SK-N-SH cells miR-874-3p expression and cell viability in MPP+ group were significantly reduced, but apoptosis rate and cleaved- caspase-3 protein were remarkably increased (p<0.05); Compared with MPP++miR-con group, miR-874- 3p expression in SK-N-SH cells and cell viability in MPP++miR-874-3p group were remarkably enhanced, but cleaved-caspase-3 protein and apoptosis rate were remarkably lowered (p<0.05). See Table 4 and fig. 3.
| Grouping | miR-874-3p | Cleaved-caspase-3 | Cell viability OD 450 nm | Apoptosis rate (%) |
|---|---|---|---|---|
| NC | 1.00±0.11 | 0.30±0.02 | 1.219±0.10 | 6.13±0.57 |
| MPP+ | 0.43±0.03* | 0.82±0.08* | 0.671±0.05* | 34.69±3.29* |
| MPP++miR-con | 0.40±0.04 | 0.84±0.09 | 0.666±0.06 | 33.24±3.02 |
| MPP++miR-874-3p | 0.89±0.09# | 0.51±0.05# | 1.079±0.08# | 14.04±1.37# |
| F | 151.930 | 139.914 | 128.369 | 326.514 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Contrast NC, *p<0.05; contrast MPP++miR-con, #p<0.05
Table 4: The Effect of Mir-874-3p Over-Expression on MPP+ Induced Sk-N-Sh Cells Activity and Apoptosis (x?±s, n=9)
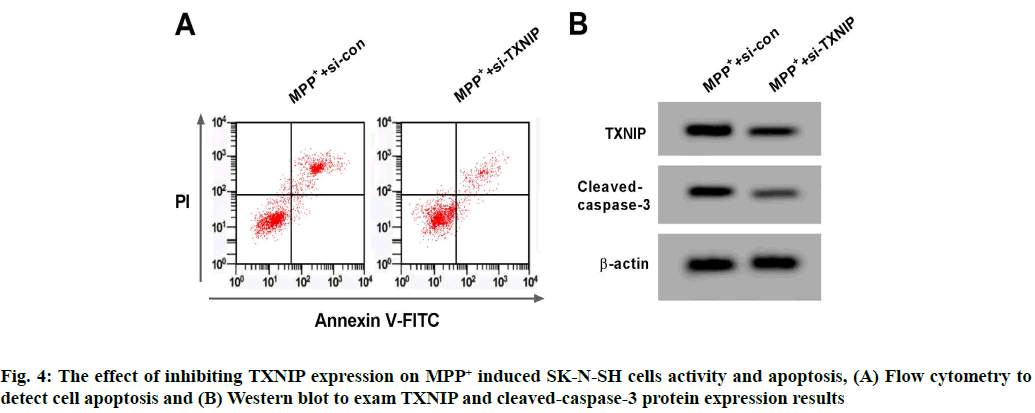
Contrast MPP++si-con group, TXNIP protein expression of the SK-N-SH cells in MPP++si-TXNIP group was significantly reduced, the cell viability was significantly increased, cleaved-caspase-3 protein and apoptosis rate were significantly reduced (p<0.05). See the following Table 5 and fig. 4.
| Grouping | TXNIP | Cleaved-caspase-3 | Cell viability OD 450 nm | Apoptosis rate (%) |
|---|---|---|---|---|
| MPP++si-con | 0.91±0.08 | 0.85±0.07 | 0.671±0.07 | 34.05±3.18 |
| MPP++si-TXNIP | 0.52±0.05* | 0.40±0.03* | 1.104±0.10* | 12.13±1.07* |
| t | 12.402 | 17.726 | 10.642 | 19.599 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Contrast MPP++si-con, *p<0.05
Table 5: The Effect of Inhibiting Txnip Expression on MPP+ Induced Sk-N-Sh Cells Activity and Apoptosis of (x?±s, n=9)
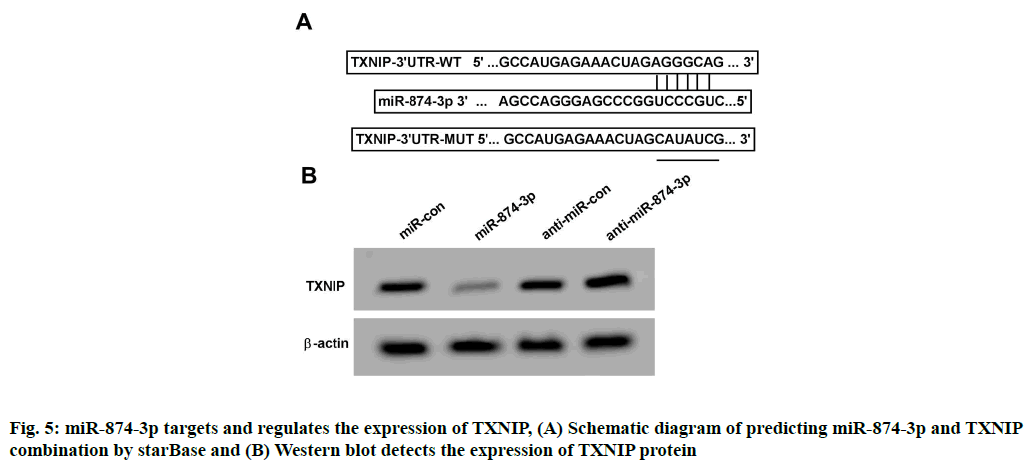
StarBase online analysis showed that miR-874-3p had a theoretical connection site with the 3'Untranslated Region (3'-UTR) region of TXNIP, as shown in fig. 5A. Compared with miR-con and WT-TXNIP cotransfection, the relative SK-N-SH cells luciferase activity was remarkably decreased after miR-874-3p mimics together with WT-TXNIP were co-transfected (p<0.05); Compared with miR-con and MUT-TXNIP co-transfection, the relative luciferase activity changes of SK-N-SH cells after miR-874-3p mimics and MUT- TXNIP co-transfection were not statistically significant, see Table 6. TXNIP protein expression of SK-N-SH cells in miR-874-3p group was remarkably lower than that in miR-con group (p<0.05); TXNIP protein expression of SK-N-SH cells in anti-miR-874-3p group was remarkably higher than that in anti-miR-con group (p<0.05), see Table 7 and fig. 5B.
| Group | Relative activity of luciferase | |
|---|---|---|
| WT-TXNIP | MUT-TXNIP | |
| miR-con | 1.00±0.11 | 1.01±0.10 |
| miR-874-3p | 0.45±0.04* | 1.03±0.09 |
| t | 14.097 | 0.446 |
| p | 0.000 | 0.662 |
Note: Contrast miR-con, *p<0.05
Table 6: Dual Luciferase Activity Test after Co-Transfection of Mir-Con or Mir-874-3p With Reporter Plasmid into Sk-N-Sh Cells (x?±s, n=9)
| Grouping | TXNIP |
|---|---|
| miR-con | 0.43±0.04 |
| miR-874-3p | 0.13±0.01* |
| anti-miR-con | 0.40±0.04 |
| anti-miR-874-3p | 0.73±0.07# |
| F | 264.402 |
| p | 0.000 |
Note: Contrast miR-con group mentioned above, *p<0.05, contrast anti-miR-con, #p<0.05
Table 7: Western Blot to Exam Txnip Expression (x?±s, n=9)
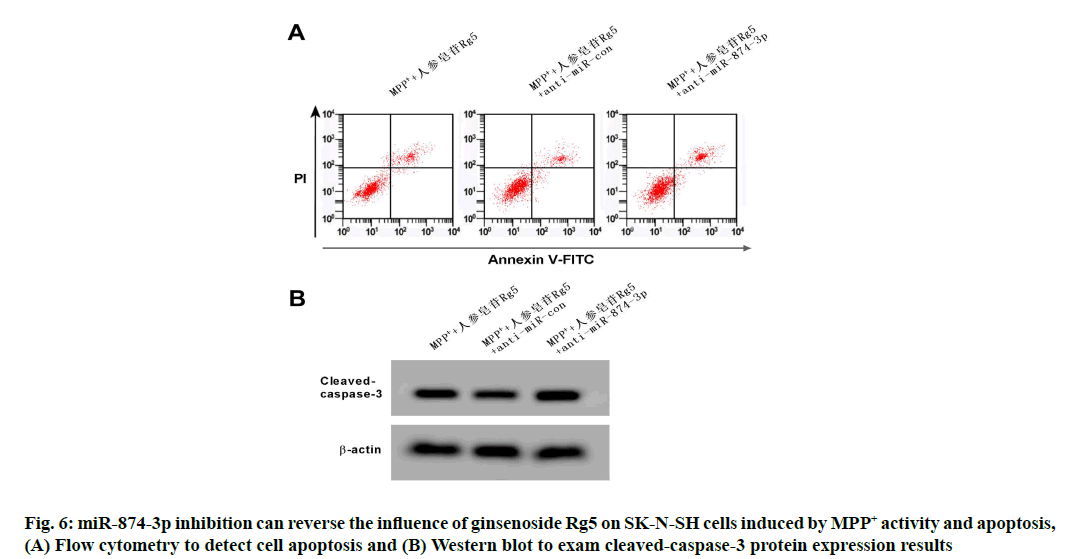
Compared with the MPP++ginsenoside Rg5+anti- miR-con group, miR-874-3p expression towards SK- N-SH cell in MPP++ginsenoside Rg5+anti-miR-874- 3p group was remarkably reduced, cell viability was significantly reduced, neither the apoptosis rate nor cleaved-caspase-3 protein expression raised remarkably (p<0.05). See Table 8 and fig. 6.
| Grouping | miR-874-3p | Cleaved-caspase-3 | Cell viability OD 450 nm | Apoptosis rate (%) |
|---|---|---|---|---|
| MPP++ ginsenoside Rg5 | 1.02±0.11 | 0.52±0.05 | 0.919±0.08 | 18.29±1.53 |
| MPP++ ginsenoside Rg5+anti-miR-con |
1.04±0.08 | 0.49±0.04 | 0.924±0.10 | 18.17±1.46 |
| MPP++ ginsenoside Rg5+anti-miR-874-3p | 0.48±0.05* | 0.88±0.08* | 0.634±0.05* | 29.68±2.72* |
| F | 129.771 | 121.114 | 39.369 | 99.40 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Contrast MPP++ginsenoside Rg5+anti-miR-con, *p<0.05
Table 8: Mir-874-3p Inhibition Can Reverse the Influence of Ginsenoside Rg5 on Sk-N-Sh Cells Induced by MPP+ Activity and Apoptosis (x?±s, n=9)
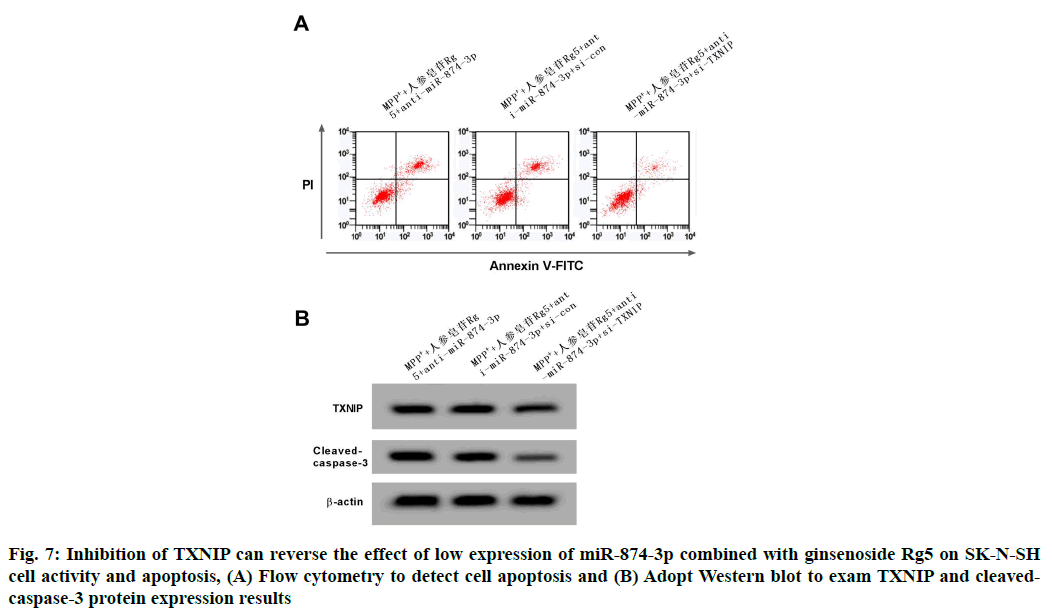
Compared with the MPP++ginsenoside Rg5+anti-miR- 874-3p+si-con group, the TXNIP protein expression of SK-N-SH cell in MPP++ginsenoside Rg5+anti-miR- 874-3p+si-TXNIP group was remarkably reduced, but the cell vitality was remarkably increased, conversely the apoptosis rate and cleaved-caspase-3 protein expressions were remarkably decreased (p<0.05). See the following Table 9 and fig. 7.
| Grouping | TXNIP | Cleaved-caspase-3 | Cell viability OD 450 nm | Apoptosis rate (%) |
|---|---|---|---|---|
| MPP++ ginsenoside Rg5+anti-miR-874-3p | 0.83±0.08 | 0.89±0.08 | 0.627±0.06 | 30.25±2.92 |
| MPP++ ginsenoside Rg5+anti-miR-874-3p+si-con | 0.80±0.07 | 0.85±0.09 | 0.631±0.07 | 29.36±2.81 |
| MPP++ ginsenoside Rg5+anti-miR-874-3p+si-TXNIP | 0.52±0.05* | 0.42±0.04* | 1.057±0.09* | 10.22±0.94* |
| F | 57.196 | 113.870 | 99.323 | 199.78 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Contrast MPP++ginsenoside Rg5+anti-miR-874-3p+si-con, *p<0.05
Table 9: Inhibition of Txnip Can Reverse the Influence of Mir-874-3p Low Expression Combined With Ginsenoside Rg5 on Sk-N-Sh Cell Activity and Apoptosis (x?±s, n=9)
Ginseng has thousands of years’ usage history in China and ginsenoside is its main active ingredient. Research has shown that Rg5 can protect cisplatin-induced renal epithelial cell toxicity by regulating cell apoptosis [10]. Rg5 can also reduce the kidney damage in diabetic mice by inhibiting the activation of oxidative stress and Nod- Like Receptor Protein 3 (NLRP3) inflammasomes [11]. In addition, Rg5 can resist the neuronal apoptosis induced by heat stress by regulating the cognitive function damage mediated by oxidative stress and heat stress, and is a potential natural neuroprotective agent [12]. This study showed that after different concentrations of ginsenoside Rg5 acted on MPP+ induced SK-N-SH cells, cell activity was remarkably raised, cell apoptosis rate was remarkably reduced and the expression of apoptosis marker cleaved-caspase-3 was significantly reduced. It is suggested that ginsenoside Rg5 can reduce the damage of PD cell model induced by MPP+. In addition, this study showed that MPP+ treatment can reduce SK-N-SH cells miR-874-3p expression and increase expression level of TXNIP, while ginsenoside Rg5 can promote miR-874 in SK-N-SH cells induced by MPP+ -3p expression inhibits TXNIP expression, indicating that ginsenoside Rg5’s protective effect on PD model cell damage possibly have relationship on miR-874-3p and TXNIP expression regulation.
As a short non-coding RNA, miR-874-3p regulates downstream target gene expression at the post- transcriptional level by interacting with specific sequences in the 3' UTR of downstream target genes and participates in regulating biological functions such as differentiation, cell proliferation and apoptosis; it plays a major role in a multiple human diseases. miR-874-3p low expression can improve the malignant progression of esophageal cancer and epithelial ovarian cancer and promote the chemotherapy resistance of colon cancer cells [13-15]. miR-874-3p expression down-regulation can reduce the I/R-treated PC12 cells viability as well, induce apoptosis and aggravate brain I/R damage [16]. In addition, miR-874-3p is also a potential biomarker of acute myocardial infarction [17]. We attempts to discover the potential effect of miR-874-3p towards PD model cell damage, in this research miR-874-3p expression up-regulation in MPP+ induced SK-N-SH cells by transfecting miR-874-3p mimics and found that cell viability was significantly increased, the rate of cell apoptosis was significantly reduced, suggesting that overexpression of miR-874-3p can effectively inhibit PD model cell damage induced by MPP+.
TXNIP is an endogenous reactive oxygen species scavenging inhibitor, which promotes oxidative stress and induces cell apoptosis by binding to the cysteine residues of thioredoxin [18]. Studies have shown that TXNIP regulates Parkin/PTEN-Induced Putative Kinase 1 (PINK1)-mediated mitochondrial autophagy under high glucose conditions. Down-regulating TXNIP can reduce reactive oxygen level in PC12 cells and also reduce the cells induced by high glucose. Injury is a potential treatment strategy to reduce the risk of PD under high glucose conditions [19]. TXNIP can also inhibit the flow of autophagy, induce the accumulation of α-nucleoprotein and promote the loss of dopamine neurons in the midbrain of mice [20]. Laquinimod has a therapeutic effect on PD neuroinflammation by inhibiting the expression of TXNIP [21]. In order to discuss the potential function of TXNIP in PD model cell injury, this study inhibited TXNIP expression in MPP+ induced SK-N-SH cells by transfecting si-TXNIP and found that cell viability was remarkably increased and apoptosis rate was remarkably reduced. In addition, miR-874-3p has a targeted negative regulatory effect on TXNIP, suggesting that miR-874-3p/TXNIP molecular axis has important effect on MPP+ induced PD model cell damage. Recovery experiments show that miR- 874-3p inhibition can reverse the effect of ginsenoside Rg5 on MPP+ induced SK-N-SH cell activity and apoptosis, and inhibition of TXNIP expression can also reverse the inhibition of miR-874-3p combined with ginsenoside Rg5 treatment The effect of MPP+ induced SK-N-SH cell activity and apoptosis further indicates that the protective effect of ginsenoside Rg5 on MPP+ induced PD model cell damage concerns the up-regulation of miR-874-3p/TXNIP molecular axis.
In summary, this study confirmed that ginsenoside Rg5 can reduce the damage of MPP+ induced PD cell model and its mechanism may have something to do with miR-874-3p/TXNIP molecular axis up-regulation. Therefore, ginsenoside Rg5 has potential application value in the prevention and treatment of PD.
Conflict of interests:
Conflict of interests:
References
- Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386(9996):896-912.
- Mullin S, Schapira AH. Pathogenic mechanisms of neurodegeneration in Parkinson disease. Neurol Clin 2015;33(1):1-7.
- Kim EJ, Jung IH, Van Le TK, Jeong JJ, Kim NJ, Kim DH. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J Ethnopharmacol 2013;146(1):294-9.
- Cheng Z, Zhang M, Ling C, Zhu Y, Ren H, Hong C, et al. Neuroprotective effects of ginsenosides against cerebral ischemia. Molecules 2019;24(6):1102.
- Bam M, Yang X, Sen S, Zumbrun EE, Dennis L, Zhang J, et al. Characterization of dysregulated miRNA in peripheral blood mononuclear cells from ischemic stroke patients. Mol Neurobiol 2018;55(2):1419-29.
- Jiang D, Sun X, Wang S, Man H. Upregulation of miR-874-3p decreases cerebral ischemia/reperfusion injury by directly targeting BMF and BCL2L13. Biomed Pharmacother 2019;117:108941.
- Hu L, Zhang H, Wang B, Ao Q, He Z. MicroRNA-152 attenuates neuroinflammation in intracerebral hemorrhage by inhibiting thioredoxin interacting protein (TXNIP)-mediated NLRP3 inflammasome activation. Int Immunopharmacol 2020;80:106141-51.
- Feng L, Zhang L. Resveratrol suppresses Aβ-induced microglial activation through the TXNIP/TRX/NLRP3 signaling pathway. DNA Cell Biol 2019;38(8):874-9.
- Yuan Q, Zhao H, Ma Y. Protective Effect of Rutin on SH-SY5Y Cells Injured by MPP+. Chin J Exp Tradit Med Formula 2018;24(16):109-14.
- Li W, Yan MH, Liu Y, Liu Z, Wang Z, Chen C, et al. Ginsenoside Rg5 ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of inflammation, oxidative stress and apoptosis. Nutrients 2016;8(9):566-76.
- Zhu Y, Zhu C, Yang H, Deng J, Fan D. Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol Res 2020;155:104746-56.
- Choi SY, Kim KJ, Song JH, Lee BY. Ginsenoside Rg5 prevents apoptosis by modulating heme-oxygenase-1/nuclear factor E2-related factor 2 signaling and alters the expression of cognitive impairment-associated genes in thermal stress-exposed HT22 cells. J Ginseng Res 2018;42(2):225-8.
- Yuan RB, Zhang SH, He Y, Zhang XY, Zhang YB. MiR-874-3p is an independent prognostic factor and functions as an anti-oncomir in esophageal squamous cell carcinoma via targeting STAT3. Eur Rev Med Pharmacol Sci 2018;22(21):7265-73.
- Xia B, Lin M, Dong W, Chen H, Li B, Zhang X, et al. Upregulation of miR?874?3p and miR?874?5p inhibits epithelial ovarian cancer malignancy via SIK2. J Biochem Mol Toxicol 2018;32(8):e22168.
- Que K, Tong Y, Que G, Li L, Lin H, Huang S, et al. Downregulation of miR-874-3p promotes chemotherapeutic resistance in colorectal cancer via inactivation of the Hippo signaling pathway. Oncol Rep 2017;38(6):3376-86.
- Liu B, Xu T, Meng Y. IncRNA NEAT1 aggravates cerebral ischemia/reperfusion injury by sponging miR-874-3p. J Biol Regul Homeost Agents 2020;34(2).
- Yan Y, Song X, Li Z, Zhang J, Ren J, Wu J, et al. Elevated levels of granzyme B correlated with miR-874-3p downregulation in patients with acute myocardial infarction. Biomark Med 2017;11(9):761-7.
- Mohammad Alhawiti N, Al Mahri S, Azhar Aziz M, Shafi Malik S, Mohammad S. TXNIP in metabolic regulation: physiological role and therapeutic outlook. Curr Drug Targets 2017;18(9):1095-103.
- Su CJ, Shen Z, Cui RX, Huang Y, Xu DL, Zhao FL, et al. Thioredoxin-Interacting Protein (TXNIP) Regulates Parkin/PINK1-mediated Mitophagy in dopaminergic neurons under high-glucose conditions: implications for molecular links between Parkinson’s disease and diabetes. Neurosci Bull 2020:1-3.
- Su CJ, Feng Y, Liu TT, Liu X, Bao JJ, Shi AM, et al. Thioredoxin?interacting protein induced α?synuclein accumulation via inhibition of autophagic flux: Implications for Parkinson's disease. CNS Neurosci Ther 2017;23(9):717-23.
- Zhang X, Jin J, Xie A. Laquinimod inhibits MMP+ induced NLRP3 inflammasome activation in human neuronal cells. Immunopharmacol Immunotoxicol 2020;42(3):264-71.