- *Corresponding Author:
- Yanling Gao
Fujian Key Laboratory of Rehabilitation Technology, Fuzhou, Fujian Province 350003, China
E-mail: 32502887@qq.com
| Date of Received | 18 December 2021 |
| Date of Revision | 05 October 2022 |
| Date of Acceptance | 17 August 2023 |
| Indian J Pharm Sci 2023;85(4):1092-1098 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To analyze that glaucocalyxin B can alleviate dyskinesia, inflammatory response and oxidative stress in Parkinson's disease model rats by activating the nuclear factor erythroid 2–related factor 2/heme oxygenase-1 pathway. 150 adults were divided into 5 groups, namely, the sham operation group (healthy mice), the Parkinson's group and the low-dose group (Parkinson+20 ng/ml) glaucocalyxin B), the medium-dose group (Parkinson+40 ng/ml glaucocalyxin B) and high-dose group (Parkinson+60 ng/ml glaucocalyxin B). The number of adjustment steps, the number of forepaw movements and the number of positive reactions in each group at 0 d have no difference; on the 3rd d in the Parkinson's group, dose group were reduced than in the sham operation group; on the 7th d, the number of steps in the Parkinson's group, and dose groups were reduced than the sham operation group. The tumor necrosis factor-alpha, interleukin-1 beta and interleukin-6 in the Parkinson's disease group, and dose groups were raised than those in the sham operation group, while these in the dose groups were reduced than in the Parkinson's group. The reactive oxidative stress concentration and myeloperoxidase activity of the Parkinson's group, dose group were raised than those of the sham operation group, while in the dose group were reduced than the Parkinson's group; superoxide dismutase activity level was reduced than the sham operation group. The levels of nuclear factor erythroid 2-related factor and heme oxygenase-1 in the Parkinson's group, dose groups were raised than those in the sham operation group, while in the dose groups were raised than those in the Parkinson's group. Further, in the low, medium and high dose groups increased with the increase of dose (p<0.05). Glaucocalyxin B can alleviate the dyskinesia of Parkinson's disease model rats and reduce the inflammatory response and oxidative stress response in rats, its relevant mechanism via activating the nuclear factor erythroid 2-related factor 2/heme oxygenase-1 pathway.
Keywords
Glaucocalyxin B, nuclear factor erythroid 2, heme oxygenase, Parkinson's disease, dyskinesia, inflammatory response, depression, constipation
In middle-aged and elderly people, Parkinson's Disease (PD) causes degenerative changes to the central nervous system. Clinical features include retardation, tremor, myotonia, depression, cognitive impairment, constipation and reduced exercise[1]. In recent years, with the increase of population aging, the incidence rate of PD has been increasing, and it will gradually become one of the most serious diseases threatening mankind in the 21st century[2]. The main pathological changes of PD are in the substantia nigra and striatum, and the relevant mechanisms are not fully clear, but the progression of PD are closely related to the body's inflammatory response and Oxidative Stress (OS) response[3].
Glaucocalyxin B (GLB) is a triterpenoids isolated and purified from Rabdosia eriocalyx, which can reduce the Reactive Oxygen Species (ROS) activated by Lipopolysaccharide (LPS) and Interleukin-(IL)-1 Beta (β) and Tumor Necrosis Factor-Alpha (TNF-α) production, alleviate LPSinduced cytotoxicity, which have a certain antiinflammatory and antioxidant activity, it may have certain anti-PD effects[3-8]. This experiment takes adult male Sprague–Dawley (SD) rats as the observation subject, and aiming at analyzing that GLB can alleviate dyskinesia, inflammatory response and OS response in PD model rats via activating the Nuclear Factor Erythroid 2–Related Factor 2 (Nrf2)/Heme Oxygenase-1 (HO-1) pathway.
Materials and Methods
General information:
Experimental animals: Adult male SD rats were selected, provided by Hebei experimental animal center, the certificate number was 1306107, the average weight of the rats was (225±25) g, and the average age of the rats was about (6±1) w. We provided 22°-24° room temperature, 40 %-60 % relative humidity, and 45 dB of noise.
Experimental equipment: -70° ultra-low temperature refrigerator was provided by Qingdao Haier Biomedical Co., Ltd.; microplate reader provided by Wuhan Clod-clone diagnostic reagent research Institute Co., Ltd.. The electric heating constant temperature blast drying box was provided by Shanghai Chenlian biological technology development Co., Ltd.; the ultraspeed refrigerated centrifuge provided by Beijing Image Trading Co., Ltd.. The vacuum drying oven was provided by Shanghai Chenlian Biological Technology Development Co., Ltd.; the fluorescence quantitative Polymerase Chain Reaction (PCR) instrument was provided by Shanghai Shiwei Experimental Instrument Technology Co., Ltd. The microscope was provided by Olympus (Beijing) Sales Service Co., Ltd.; the electrophoresis instrument was provided by Shanghai Fuze Trading Co., Ltd.; the transfer tank was provided by Shanghai Yihui Biological Technology Co., Ltd.; the double vertical protein electrophoresis apparatus was provided by Hangzhou Noted Scientific Equipment Co., Ltd.
Experimental reagents: GLB was provided by Beijing Yiqiao Shenzhou Technology Co., Ltd.; high-purity total Ribonucleic Acid (RNA) rapid extraction kit was provided by Beijing Bioteke Biotechnology Co., Ltd. The ROS kit was provided by Shanghai Jianglai Biotechnology Co., Ltd.; the Superoxide Dismutase (SOD) kit, Myeloperoxidase (MPO) kit, and the internal control antibody β-actin were provided by Shanghai Hengfei Biotechnology Co., Ltd.; (Radioimmunoprecipitation Assay (RIPA)) lysis buffer was provided by Shanghai Global Bio resource Center Biotechnology Co., Ltd. Rabbit anti-Nrf2 was provided by Xiamen Yanke Biotechnology Co., Ltd. and rabbit anti- HO-1 was provided by Beijing Biolab Technology Co., Ltd.
Method:
PD animal model construction: 150 healthy male rats were taken and adaptively fed for 1 w, and then they were divided into 5 groups, namely, the sham operation (healthy mice), the PD (Parkinson), and the low-dose (Parkinson+20 ng/ml GLB), the medium-dose (Parkinson+40 ng/ml GLB) and the high-dose (Parkinson+60 ng/ml GLB) group. All rats were anesthetized by intraperitoneal injection of 10 % chloral hydrate and were in the prone position. The drapes were routinely disinfected, and the skin was cut along the midline and the frontal parietal bones were exposed. The stereotactic coordinates of rat striatum were determined according to the "rat brain stereotactic atlas", and a bone hole with a diameter of around 1 mm on the surface of the skull was drilled using a mini dental drill. The rats in the PD, low-dose, middle-dose and high-dose group were injected with a microinjection pump at a speed of 1 μl/ min. 2 μl of 5 mg/ml LPS was injected to induce a rat PD model. The sham operation group was given an equal volume of corresponding sterile normal saline. After the LPS injection completed, the stereoscopic position of lateral ventricle was determined according to the brain atlas and a bone hole with a diameter of around 1 mm on the surface of the skull was drilled. The injection speed of the microinjection pump was adjusted to 0.5 μl/min, and 5 μl of GLB solution of different concentrations was given through the lateral ventricle of the brain by single local injection. The low-dose group was given 20 ng/ml GLB solution, the middle-dose group was given 40 ng/ml GLB solution, the high-dose group was given 60 ng/ml GLB solution, and the sham operation group was given an equal volume of solvent.
Observation indicators:
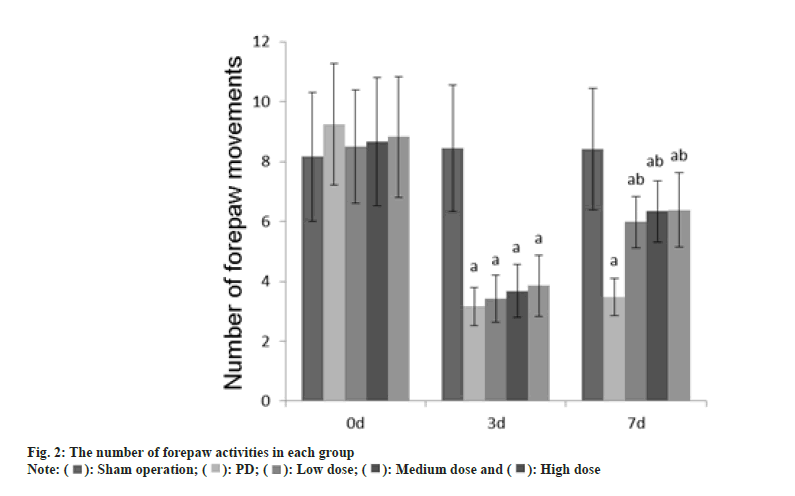
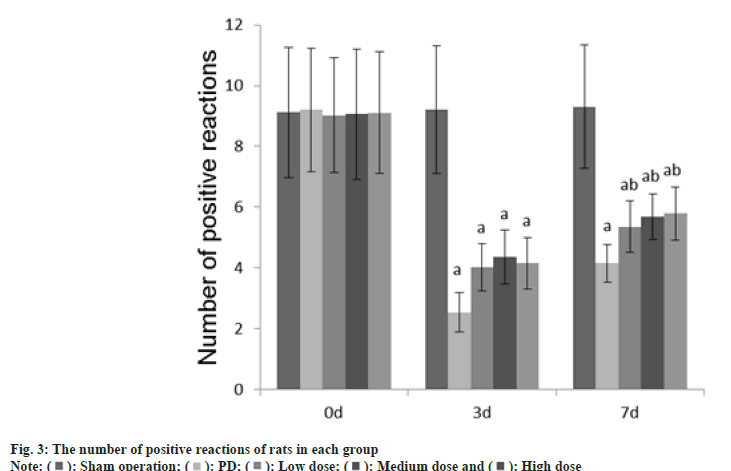
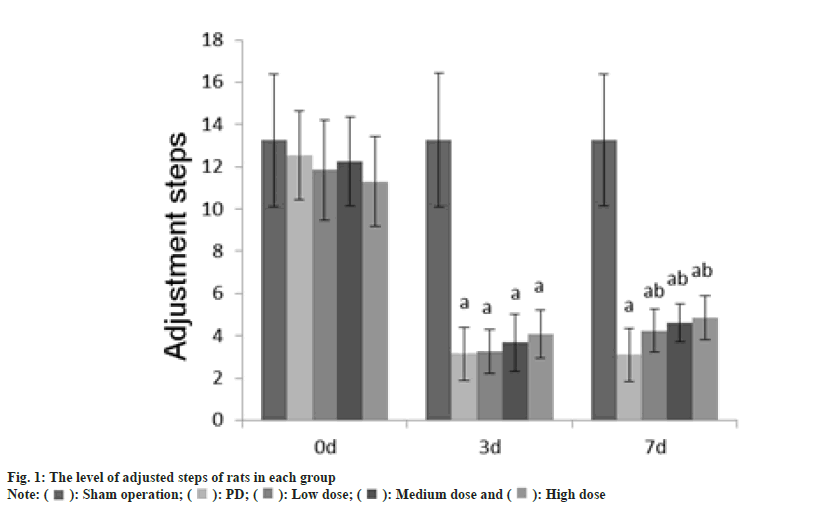
Animal experiment tests such as adjusting the number of steps, the number of forepaw movements, and the number of positive reactions were performed at 0 d, 3 d and 7 d after GLB injection.
The rat was placed on the experimental table, the tail of the rat was pulled to make the front paw touch the table, and the rat was dragged backward about 90 cm at a speed of 1 m/min, and adjusted number of steps were counted on the front paw of the rats during the dragging process.
Number of front paw movements: One of the front paws of the rat was trapped, the tail of the rat was pulled to vacate the two hind legs of the rat, and the rat’s beard was touched the table top 10 times. The number of forepaw movements caused by the beard touch was recorded; the other side used the same method to measure.
Number of positive reactions: The rat was put into an empty cylinder and the number of climbing movements of the rat's limbs within 5 min was observed. As long as the rat's forelimb touched the wall, it was considered as a positive reaction and counted once.
Real-time PCR method
The tissue samples were placed in 1.5 Eppendorf (EP) tubes, 1 ml of Reticulocyte Lysate (RL) tissue lysate was added to dissolve each sample, and the EP tubes were placed at 25° for 5 min. Afterwards, 200 μl of chloroform were added, the EP tube was placed at room temperature for 3 min and centrifuged for 10 min at 12 000 rpm at 4°. The water phase was carefully transferred to a new EP tube, 1 volume of 70 % ethanol was added and mixed well. It was centrifuged for 30 s at 4° and 12 000 rpm, 500 μl of protein-removing solution was added, and centrifuged at 4° and 12 000 rpm for 30 s. 500 μl of rinsing solution Rewritable (RW) was added, centrifuged at 12 000 rpm at 4° for 30 s, the waste solution was discarded after finishing, the RNA sample obtained was reverse transcribed, and the complementary Deoxyribonucleic Acid (cDNA) was obtained by reverse transcription of the total RNA. A fluorimeter was used for quantitative fluorescence analysis.
Detection of ROS level in midbrain tissue:
20 times the volume of Phosphate Buffer Saline (PBS) was added to the tissue and centrifuged at 10005 xg for 10 min. A micropipette was used to add 0, 1, 2, 4, 8, 12, 16, 20 μl of Bovine Serum Albumin (BSA) protein standard solution to each well of the microtiter plate. A micropipette was used to add 19 μl of PBS to the EP tube containing 1 μl of protein extract of the sample to be tested and mix well. According to the instructions for the Bicinchoninic acid (BCA) working solution, solution A and solution B were prepared, and 200 μl of the prepared A and B mixture was added to each well of a 96-well culture plate. After placing the 96-well plate at 37° for 20 min, the plate was detected at 570 nm using a microplate reader. A standard curve was drawing based on the standard protein concentration and the corresponding absorbance value. And the concentration of sample protein in each well was calculated by regression equation. A dichloro-dihydrofluorescein diacetate method was used to measure ROS levels in the supernatant.
Detection of SOD activity in midbrain tissue:
9-fold volume of PBS was added to the tissue, centrifuged at 10005 xg for 10 min, and the supernatant was transferred to a new tube for subsequent operations. BSA protein standard solution was added to each well of the microtiter plate using a micropipette. A micropipette was used to add 19 μl of PBS to the EP tube containing 1 μl of protein extract of the sample to be tested and mix well. 200 μl of the prepared A and B mixture was added to each well of the 96-well culture plate. After placing the 96-well plate at 37° for 20 min, the plate was detected at 570 nm using a microplate reader. A standard curve was drew based on the standard protein concentration and the corresponding absorbance value. And the concentration of sample protein in each well was calculated by regression equation. The liquid was mixed thoroughly with a micropipette, and the sample was placed at 37° to react for 20 min. The Optical Density (OD) value of each well at 450 nm was detected using a microplate reader.
Determination of MPO activity in midbrain tissue:
In a 37° water bath for 15 min, 0.5 ml of 10 % tissue homogenate was mixed with 0.9 ml of 5 % tissue homogenate and 0.1 ml of reagent three solution, and it was taken out to be tested. The liquid was mixed, and the water was bathed at 60° for 10 min. The absorbance of samples in each group was measured at 460 nm using an ultraviolet spectrophotometer, 1 cm light path, and zero adjustment was performed with double distilled water. Calculate the MPO content (U/g)=(the absorbance of the fixed tube-the absorbance of the control tube)/(11.3×sampling volume).
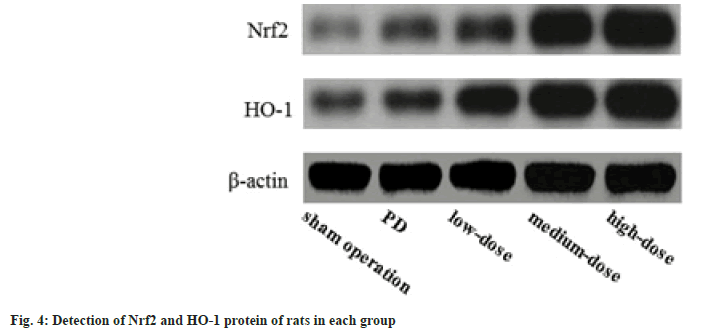
Nrf2 and HO-1 level detected using Western blot method:
1 % volume of Phenylmethylsulfonyl Fluoride (PMSF) was added to the lysate and mixed well for later use. Using RIPA lysis buffer, total protein was extracted from tissues or cells, and nuclear or cytoplasmic proteins were isolated. An appropriate volume of lysis buffer was added to lyse the tissue samples, and each group of samples with the added lysis buffer were placed on ice for 5 min. It was centrifuged for 10 min at 4° and 12 000 rpm, and the supernatant obtained was the protein extract in each tissue sample. BSA protein standard solution was added to each microtiter plate well using a micropipette.
A micropipette was used to add 19 μl of PBS to the EP tube containing 1 μl of protein extract of the sample to be tested and mix well. After placing the 96-well plate at 37° for 20 min, the plate was detected at 570 nm using a microplate reader. A standard curve was drew based on the standard protein concentration and the corresponding absorbance value. And the concentration of sample protein in each well was calculated by regression equation. Sodium Dodecyl Sulphate- Polyacrylamide Gel Electrophoresis (SDS-PAGE), transfer, block, incubate primary antibody, incubate secondary antibody, Enhanced Chemiluminescence (ECL) substrate luminescence, antibody stripping, block, incubate internal control antibody, incubate secondary antibody, substrate luminescence and analyze the result.
Statistical methods:
A Statistical Package for the Social Sciences (SPSS) 20.0 software package was used to analyze the data, and all measurement data were compared with (x̄ ±s). For comparison between the groups, t-tests were used; the enumeration data were expressed as percentages, and Chi-square (χ2) tests were used for comparison between the groups. p<0.05 seen as statistically significant, a means ap<0.05 compared with the sham operation group, and b means bP<0.05 compared with the PD group.
Results and Discussion
The number of adjustment steps, the number of forepaw movements, and the number of positive reactions in each group at 0 d were no difference (p>0.05); on the 3rd d, these in the PD group, dose group were reduced than those in the sham operation group (p<0.05); on the 7th d, these in the PD group, dose groups were reduced than those of the sham operation group (p<0.05) as shown in fig. 1-fig. 3.
The IL-1β, TNF-α and IL-6 in the PD group, dose groups were raised than those in the sham operation group, and these in the dose groups were reduced than those in the PD group (p<0.05) as shown in Table 1.
| Group | Case | TNF-α | IL-1β | IL-6 |
|---|---|---|---|---|
| Sham operation | 30 | 1.23±0.08 | 0.89±0.08 | 1.02±0.08 |
| PD | 30 | 5.61±1.21a | 4.39±0.75a | 3.15±1.02a |
| Low dose | 30 | 4.12±1.08ab | 3.48±0.35ab | 2.56±0.745ab |
| Medium dose | 30 | 3.62±0.88ab | 2.26±0.22ab | 2.04±0.56ab |
| High dose | 30 | 3.01±0.48ab | 1.89±0.12ab | 1.75±0.34ab |
Note: Compared with the sham group, ap<0.05 and compared with the PD group, bp<0.05
Table 1: Tnf-Α, Il-1β And Il-6 Levels in Each Group Rats (X̄±S)
The ROS concentration and MPO activity of the PD group, low, medium and high dose group were raised than those of the sham operation group, while these in the low, medium and high dose group were reduced than the PD group; SOD activity level was reduced than the sham operation group, and it was raised than that of the sham operation group (p<0.05) as shown in Table 2.
| Group | Case | ROS concentration (%) | SOD activity (U/mg) | MPO activity (U/g) |
|---|---|---|---|---|
| Sham operation | 30 | 100.00±10.00 | 15.23±5.16 | 1.75±0.15 |
| PD | 30 | 201.68±42.03a | 7.53±3.15a | 4.15±1.25a |
| Low dose | 30 | 176.25±33.15ab | 9.45±4.15ab | 3.52±1.02ab |
| Medium dose | 30 | 162.10±23.56ab | 9.86±4.20ab | 2.28±0.85ab |
| High dose | 30 | 139.65±25.37ab | 11.35±4.52ab | 2.01±0.68ab |
Note: Compared with the sham group, ap<0.05 and compared with the PD group, bp<0.05
Table 2: Ros Concentration, Sod Activity and Mpo Activity Level of Rats in Each Group (X̄±S)
The Nrf2 and HO-1 in the PD, dose groups were raised than those in the sham operation group, and these in the dose groups were raised than those in the PD group; further, these in the dose groups increased with the increase of dose (p<0.05) as shown in fig. 4.
Parkinson’s often occurs in the elderly, and its incidence increases with age, which affects the quality of life in patients[9]. In this experiment, on the 7th d, the number of adjustment steps, the number of fore-paw movements and the number of positive reactions in the PD group, dose groups were reduced than those of the sham operation group, while these in the dose groups were raised than in the PD group, suggesting that GLB can obviously alleviate the dyskinesia of PD model rats, indicating that GLB may have anti-PD effects. Microglia is a cell type that exists in the central nervous system and has a certain phagocytic function after stimulation and activation. Microglia can be activated by factors including inflammation, degenerative diseases and have certain chemotactic reactions[10,11]. Over-activated microglia release large amounts of oxygen free radicals and cellular inflammatory factors IL- 1β and TNF-α to damage neurons. Studies have shown that the activation of microglia is a sign of neuroinflammatory response in the brain[12]. Recent studies have found that oxygen free radicals play a critical role in the pathogenesis of PD. Free radicals in the body mainly refer to ROS, which is a by-product of molecular oxygen after mitochondrial metabolism. Free radicals such as SOD and MPO also participate in OS reactions[13]. In this experiment, the IL-1β, TNF-α, and IL-6 in the PD group, and dose groups were raised than those in the sham operation group, and these in the dose groups were reduced than those in the PD group. The ROS concentration and MPO activity of the PD group, and the dose group were raised than those of the sham operation group, while these in the dose group were reduced than the PD group, SOD activity level was reduced than the sham operation group, while it was raised than that of the sham operation group, showing that GLB can obviously alleviate the neuroinflammatory reaction and OS level in the brain caused by PD.
Studies have confirmed that the Nrf2/HO-1 pathway plays important role in the body's neuroinflammation and antioxidant response. Nrf2 exists in the cytoplasm in a resting state. Under OS conditions, Nrf2 transfers to the nucleus, combines with the DNA sequence of the antioxidant response element in the nucleus, and regulates the expression of downstream antioxidant genes. Nrf2 can induce the transcription of HO-1, which is one of the most important anti-OS mechanisms in cells[14]. HO-1 is a rate-limiting enzyme that can catalyze the degradation of heme, and can exert antioxidant effects through diversified pathways[15]. In this experiment, the levels of Nrf2 and HO-1 in the PD group, dose groups were raised than those in the sham operation group, meanwhile, these in the dose groups were raised than those in the PD group; further, these in the dose groups increased with the increase of dose, noting that the anti-PD effect of GLB may be related to the activation of Nrf2/HO-1 pathway.
In conclusion, GLB can alleviate the dyskinesia of PD model rats and reduce the inflammatory response and OS response in rats through activating the Nrf2/HO-1 pathway.
Conflict of interests:
The authors declared no conflict of interests.
References
- Tang TY, Choke EC, Walsh SR, Tiwari A, Chong TT. What now for the endovascular community after the paclitaxel mortality meta-analysis: Can sirolimus replace paclitaxel in the peripheral vasculature? J Endovasc Ther 2020;27(1):153-6.
[Crossref] [Google Scholar] [PubMed]
- Wan Y, Hu W, Gan J, Song L, Wu N, Chen Y, et al. Exploring the association between cerebral small-vessel diseases and motor symptoms in Parkinson's disease. Brain Behav 2019;9(4):e01219.
[Crossref] [Google Scholar] [PubMed]
- Chao TL. New advances in research of pharmacological effects of hesperetin and its derivatives. Chin Tradit Herbal Drugs 2018;49:3446-51.
- Sato Y, Honda Y, Kaji M, Asoh T, Hosokawa K, Kondo I, et al. Amelioration of osteoporosis by menatetrenone in elderly female Parkinson’s disease patients with vitamin D deficiency. Bone 2018;31(1):114-8.
[Crossref] [Google Scholar] [PubMed]
- Fei F, Jiang XG, Zhang J, Zheng LT, Zhen XC, Gan P. Mechanism of glaucocalyxin A that induces apoptosis of glioma cell and up-regulates GEF-H1. Acta Pharm Sin 2018;53:1825-33.
- Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology 2016;86(6):566-76.
[Crossref] [Google Scholar] [PubMed]
- Li ZY, You HJ, Sun ZH. The role of environmental and genetic factors in Parkinson's disease. J Yanan Univ 2017;15:70-2.
- Giannoccaro MP, La Morgia C, Rizzo G, Carelli V. Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson's disease. Mov Dis 2017;32(3):346-63.
[Crossref] [Google Scholar] [PubMed]
- Ma D, Ng SH, Zeng L, Zhao Y, Tan EK. Generation of a human induced pluripotent stem cell (iPSC) line carrying the Parkinson’s disease linked LRRK2 variant S1647T. Stem Cell Res 2017;18:54-6.
[Crossref] [Google Scholar] [PubMed]
- Choi EY, Choe SH, Hyeon JY, Park HR, Choi IS, Kim SJ. Josamycin suppresses Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-1β in murine macrophages. Biomed Pharmacother 2018;105:498-505.
[Crossref] [Google Scholar] [PubMed]
- Zheng L, Zhang J, Yuan X, Tang J, Qiu S, Peng Z, et al. Fluorofenidone attenuates interleukin-1 β production by interacting with NLRP3 inflammasome in unilateral ureteral obstruction. Nephrology 2018;23(6):573-84.
[Crossref] [Google Scholar] [PubMed]
- Mishra B, Chand S, Sangwan NS. ROS management is mediated by ascorbate-glutathione-α-tocopherol triad in co-ordination with secondary metabolic pathway under cadmium stress in Withania somnifera. Plant Physiol Biochem 2019;139:620-9.
[Crossref] [Google Scholar] [PubMed]
- Farooq MA, Niazi AK, Akhtar J, Farooq M, Souri Z, Karimi N, et al. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem 2019;141:353-69.
[Crossref] [Google Scholar] [PubMed]
- Wang Q, Wang Z, Bao Z, Zhang C, Wang Z, Jiang T. PABPC1 relevant bioinformatic profiling and prognostic value in gliomas. Future Oncol 2020;16(1):4279-88.
[Crossref] [Google Scholar] [PubMed]
- Borg NA, Dixit VM. Ubiquitin in cell-cycle regulation and dysregulation in cancer. Annual Rev Cancer Biol 2017;1:59-77.
 ): Sham operation; (
): Sham operation; ( ): PD; (
): PD; ( ): Low dose; (
): Low dose; ( ): Medium dose and (
): Medium dose and ( ): High dose
): High dose