- *Corresponding Author:
- Xiuda Peng
Department of General Surgery, The Second Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China
E-mail: xiudapengusc@126.com
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “56-65” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of this research is to investigate the prognostic factors and enhance the outlook for patients with colorectal cancer. The possible molecular mechanism affecting the prognosis of colorectal cancer was explored by conducting Kyoto encyclopedia of genes and genomes, gene ontology, and gene set enrichment analysis using glutamate pyruvate transaminase 2 positive and negative associated genes, as well as examining the high and low expression of glutamate pyruvate transaminase 2 in colorectal cancer, according to the Kyoto encyclopedia of genes and genomes. To investigate the potential association between glutamate pyruvate transaminase 2 and epithelial-mesenchymal transition and mesenchymal-epithelial transition, a correlation analysis was conducted utilizing the tumor immune estimation resource database and immunohistochemistry to analyze the relationship between glutamate pyruvate transaminase 2 and epithelial-mesenchymal transition transcription factors. The level of glutamate pyruvate transaminase 2 expressions in colorectal cancer tissues was markedly greater than in non-tumor tissues, and it exhibited a positive correlation with unfavorable prognosis. Furthermore, it was found to be associated with pathological stage, tumor stage and tumor grade. Experimental investigations have demonstrated that increased glutamate pyruvate transaminase 2 expression results in reduced cellular adhesion, alterations in components of the extracellular matrix, and enhancement in both the Wnt/beta catenin signaling pathway and the Notch signaling pathway. In the meantime, the strong association between glutamate pyruvate transaminase 2 and E-cadherin provides justification for the notion that glutamate pyruvate transaminase 2 facilitates the spread of tumors by means of epithelial-mesenchymal transition and mesenchymal-epithelial transition. Glutamate pyruvate transaminase 2 is strongly associated with the unfavorable outcome of colorectal cancer. It controls epithelial-mesenchymal transition to enhance metastasis via the Wnt/beta catenin and Notch pathways, while also facilitating mesenchymal-epithelial transition to support tumor metastasis colonization. These findings establish glutamate pyruvate transaminase 2 as a reliable prognostic biomarker and a promising therapeutic target for metastatic colorectal cancer.
Keywords
Glutamate pyruvate transaminase 2, colorectal cancer, epithelial-mesenchymal transition, mesenchymal-epithelial transition, metastasis
The prevalence of Colorectal Cancer (CRC) is high globally and it is estimated that the global incidence of CRC will rise by 60 % by the year 2030[1]. CRC is on the rise and has now emerged as the 3rd most prevalent form of cancer in China[2]. The study of the mechanism of CRC has become more important due to the growing occurrence and fatality rates of this disease. Metastasis, recurrence, and incomplete surgical resection are among the various factors that impact the prognosis of cancer, with metastasis being a significant contributor[3]. CRC has seen significant progress in its diagnostic and therapeutic abilities due to the ongoing advancements in medical technology. Nonetheless, the precise molecular process underlying the formation and advancement of CRC remains uncertain, a crucial factor in addressing CRC[4].
Glutamate Pyruvate Transaminase 2 (GPT2), a mitochondrial glutamate-dependent transaminase, serves as a pivot between glycolysis and glutaminolysis to produce alanine and Alpha-Ketoglutaric acid (α-KG)[5]. Research has indicated that GPT2 has the ability to alter glutamine metabolism and trigger both Lactate Dehydrogenase-A (LDHA) and Lactate Dehydrogenase-B (LDHB), two forms of LDH. These enzymes play a crucial role in promoting tumor formation and are essential for the growth of tumors[6,7]. Deficiencies in mitochondrial glutamate aminotransferase result in the generation of reactive oxygen species, making it a crucial controller of cellular Reduction-Oxidation (REDOX) balance[8,9]. The research has demonstrated that GPT2 plays a significant part in the proliferation of cancer cells and the development of cancer[7]. Invasive breast cancer exhibits a significant increase in the expression of GPT2[5]. CRC may be curable if it is detected before it metastasizes. Nevertheless, during later phases, individuals diagnosed with primary colorectal carcinoma may experience the formation of secondary tumors, particularly in the liver, thereby significantly complicating the treatment process[10]. To enhance the success rate of treating CRC, our efforts focused on identifying a specific gene associated with the spread of CRC, aiming to enhance the effectiveness of treatment. Nevertheless, the impact of GPT2 on the progression of colorectal carcinoma remains unclear, prompting a comprehensive investigation in this scholarly piece.
Epithelial Mesenchymal transition (EMT) is crucial for both the advancement and recovery of CRC. EMT is additionally engaged in the early stages of colorectal formation and facilitates CRC in obtaining the capacity to migrate and infiltrate[11]. During embryonic development, it has a significant impact on the promotion of gastrulation, neurospinal stratification, and the formation of various cellular and tissue types. To achieve a metastatic phenotype, cancer cells employ EMT to detach from the original tumor and disseminate through the bloodstream. This process also enables them to gain stem cell-like properties and increase their resistance to different therapeutic interventions[12,13]. EMT is a significant process where epithelial cells undergo a metamorphosis, adopting the traits of mesenchymal cells. This transformation results in the morphological changes of mesenchymal cells and contributes to the advancement of tumors[14,15]. The role of EMT in tumor development and metastasis is crucial and cannot be overlooked, as it is a significant biological process[16-19]. It is a cellular process that transiently transforms epithelial cells into a quasi-mesenchymal state. This state can be reversed back to an epithelial state through Mesenchymal Epithelial Transition (MET), which takes place in both normal development and cancer progression[20].
Through transcriptome sequencing results, we initially identified GPT2 as the target gene and observed its significant expression in tumor tissues. Subsequently, by conducting functional enrichment analysis and clinical prognosis analysis, we discovered that GPT2 could potentially result in unfavorable prognosis of CRC via EMT. Furthermore, the outcomes of Gene Set Enrichment Analysis (GSEA) indicated a significant enrichment of GPT2 in the Wnt/beta (β) catenin signaling pathway and the Notch signaling pathway. To summarize, GPT2 has the potential to trigger EMT via the Wnt/β catenin signaling pathway, thereby facilitating the metastasis of CRC.
Materials and Methods
Tumor specimens:
From the First Affiliated Hospital of the University of South China, we gathered a total of 144 CRC specimens that were embedded in paraffin. The specimens were staged for tumors based on the American Joint Committee on Cancer's staging criteria. The Overall Survival (OS) was determined by the time between the initial surgery and either death or the confirmed reappearance or spread of the disease. Approval for the study was granted by the Ethical Research Committee at the University Institute in Southern China, with written consent acquired from every patient.
Immunohistochemical analysis:
Immunohistochemical staining analysis of CRC tissues was performed using specimen arrays that were formalin-fixed and paraffin-embedded. These tissues were obtained from patients who underwent CRC resection at our hospital between January 2019 and January 2020. The immunohistochemical staining was conducted in a similar manner, employing specific antibodies including GPT2, E-cadherin, and N-cadherin (diluted at a ratio of 1:100, Abcam, USA). The expression of GPT2 was assessed based on the staining intensity (graded as 0, 1+, 2+ or 3+) and the percentage of positive cells (graded as 0, 1 %-25 %, 26 %-50 %, 51 %-75 %, or 76 %-100 %). To calculate the Staining Index (SI), multiply the score of intensity 1 by the score of positive staining 2.
Data source:
The genomic data and corresponding clinical data of COADREAD were downloaded from the The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). A total of 698 samples (647 tumor tissues and 51 paracancerous tissues) were collected after excluding samples from different batches of the same patient. Data analysis was performed using the R programming language version 4.2.1. Use tidyr, dplyr, rtracklayer R packages for data cleaning and ID conversion.
Differentially Expressed Genes (DEGs) screening:
DEGs screening was performed using the Limma R package. On the basis of Bayesian calculation of T value, F value and log, eligible DEGs were selected according to the criteria of |log2(FC)|>1 and p<0.01. All data were obtained by using R software.
Statistical analysis:
Data were presented as mean±standard deviation, and differences between groups were analyzed by t-test, Chi-square (χ²) test, or Fisher exact test. The Kaplan-Meier method and log-rank test were used to estimate survival rates. COX proportional hazards model was used to calculate univariate and multivariate hazard ratios of study variables. Statistical analyses were performed with IBM Statistical Package for Social Sciences (SPSS) Statistics 26 software and p<0.05 were considered statistically significant. GSEA analysis was the result of R software (R 4.2.1) and GSEA software (GSEA_4.2.3).
Results and Discussion
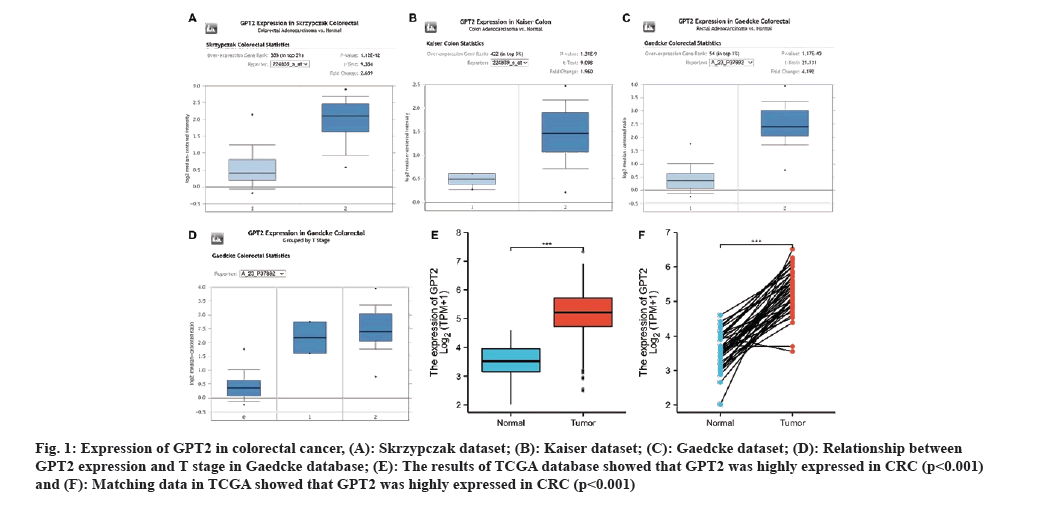
In order to investigate the manifestation of GPT2 in CRC, an analysis was conducted using public databases. Analysis of the Oncomine database showed that GPT2 exhibited significant expression in the tumor tissues of the Skyzrpczak CRC dataset (fig. 1A). In the Kaiser colon cancer dataset (fig. 1B), GPT2 exhibited significant expression in tumor tissues. In the Geadcke rectal cancer dataset, the level of GPT2 expression was markedly elevated compared to normal colon tissues (fig. 1C). Simultaneously, we observed a correlation between GPT2 expression and tumor T stage in the Gaedkce dataset (fig. 1D). The TCGA database also shows that CRC has a high expression of GPT2, as seen in fig. 1E and fig. 1F. This implies that GPT2 plays a crucial role in the progression of CRC.
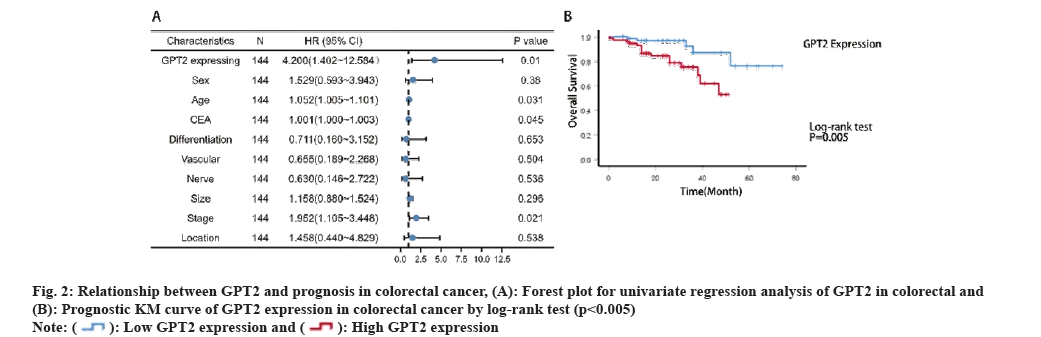
Fig. 1: Expression of GPT2 in colorectal cancer, (A): Skrzypczak dataset; (B): Kaiser dataset; (C): Gaedcke dataset; (D): Relationship between GPT2 expression and T stage in Gaedcke database; (E): The results of TCGA database showed that GPT2 was highly expressed in CRC (p<0.001) and (F): Matching data in TCGA showed that GPT2 was highly expressed in CRC (p<0.001).
To further explore the relationship between GPT2 and clinical prognosis, we analyzed the clinical data of 144 CRC patients in the First Affiliated Hospital of University of South China. The relationship between GPT2 expression and clinicopathological features of CRC showed that GPT2 expression was significantly correlated with clinicopathological stage (p=0.003) and overall survival status (p=0.020) (Table 1).
| Clinicopathological data | n | High expression of GPT2 | Low expression of GPT2 | χ² value | p value |
|---|---|---|---|---|---|
| Sex | 144 | 0.355 | 0.336 | ||
| Male | 89 | 44 | 45 | ||
| Female | 55 | 30 | 25 | ||
| The degree of differentiation | 144 | 0.206 | 0.433 | ||
| High/medium differentiation | 130 | 66 | 64 | ||
| Poorly differentiated | 14 | 8 | 6 | ||
| Vascular invasion | 144 | 1.033 | 0.232 | ||
| Yes | 14 | 9 | 5 | ||
| No | 130 | 5 | 65 | ||
| Nerve invasion | 144 | 0.897 | 0.275 | ||
| Yes | 9 | 6 | 3 | ||
| No | 135 | 68 | 67 | ||
| Preoperative HBV infection | 144 | 2.812 | 0.073 | ||
| Yes | 22 | 15 | 7 | ||
| No | 121 | 59 | 62 | ||
| Clinicopathological staging | 144 | 13.78 | 0.003 | ||
| I | 32 | 9 | 23 | ||
| II | 47 | 22 | 25 | ||
| III | 59 | 38 | 21 | ||
| IV | 6 | 5 | 1 | ||
| Overall survival state | 144 | 5.183 | 0.02 | ||
| Survival | 124 | 59 | 65 | ||
| Death | 20 | 15 | 5 |
Table 1: Relationship between GPT2 expression and clinicopathological features of CRC.
By examining the clinicopathological characteristics, it becomes evident that the clinical prognosis and clinicopathological stage are associated with the expression of GPT2. To further investigate the impact of GPT2 on the prognosis of CRC, we conducted a forest plot and KM prognosis curve analysis. From fig. 2A, it is evident that GPT2 expression, age, Carcinoembryonic Antigen (CEA), and tumor stage were identified as risk factors for CRC in the forest map. We monitored 144 individuals and graphed KM diagrams. According to the findings, patients with high GPT2 expression had a considerably poorer prognosis compared to those with low GPT2 expression (p=0.005, as determined by the log-rank test) (fig. 2B). Out of the 144 individuals, 100 managed to survive for a period of 3 y, resulting in a survival rate of 70 %. Additionally, 86 individuals out of the initial 144 were able to survive for 5 y, leading to a survival rate of 60 %. The results indicate that increased GPT2 expression in CRC is associated with an unfavorable prognosis.
Univariate COX regression analysis showed that GPT2 expression, age, CEA and tumor stage were prognostic factors for CRC (Table 2). Multivariate COX regression analysis showed that age and stage IV were independent prognostic factors for CRC (Table 3).
| Variate | Mean | Standard deviation | p value | HR (95 % CI) |
|---|---|---|---|---|
| GPT2 expression | 1.435 | 0.56 | 0.01 | 4.200 (1.402~12.584) |
| Sex | 0.425 | 0.483 | 0.38 | 1.529 (0.593~3.943) |
| Age | 0.05 | 0.023 | 0.031 | 1.052 (1.005~1.101) |
| CEA | 0.001 | 0.001 | 0.045 | 1.001 (1.000~1.003) |
| Differentiation | -0.341 | 0.76 | 0.653 | 0.711 (0.160~3.152) |
| Vascular | 0.423 | 0.634 | 0.504 | 0.655 (0.189~2.268) |
| Nerve | -0.462 | 0.747 | 0.536 | 0.630 (0.146~2.722) |
| Size | 0.146 | 0.14 | 0.296 | 1.158 (0.880~1.524) |
| Stage | 0.669 | 0.29 | 0.021 | 1.952 (1.105~3.448) |
| Location | 0.377 | 0.611 | 0.538 | 1.458 (0.440~4.829) |
Note: HR: Hazard Ratio
Table 2: Univariate COX regression analysis of colorectal cancer patients.
| Variate | Mean | Standard deviation | p value | HR (95 % CI) |
|---|---|---|---|---|
| GPT2 expression | 1.114 | 0.598 | 0.063 | 3.048 (0.943~9.844) |
| Sex | -0.374 | 0.564 | 0.511 | 0.690 (0.229~2.085) |
| Age | 0.061 | 0.564 | 0.038 | 1.063 (1.004~1.127) |
| CEA | 0.001 | 0.001 | 0.216 | 1.001 (0.999~1.003) |
| Differentiation | -0.425 | 1.083 | 0.695 | 0.654 (0.078~5.461) |
| Vascular | 0.656 | 0.715 | 0.359 | 1.927 (0.474~7.833) |
| Nerve | 0.089 | 1.02 | 0.93 | 1.094 (0.148~8.076) |
| AJCC I | 0.085 | 0.173 | 0.624 | 1.089 (0.775~1.528) |
| AJCC II | 0.963 | 0.848 | 0.256 | 2.619 (0.497~13.811) |
| AJCC III | 1.347 | 0.818 | 0.1 | 3.845 (0.773~19.126) |
| AJCCIV | 2.824 | 1.086 | 0.009 | 16.847 (2.006~141.5) |
Table 3: Multivariate COX regression analysis of colorectal cancer patients.
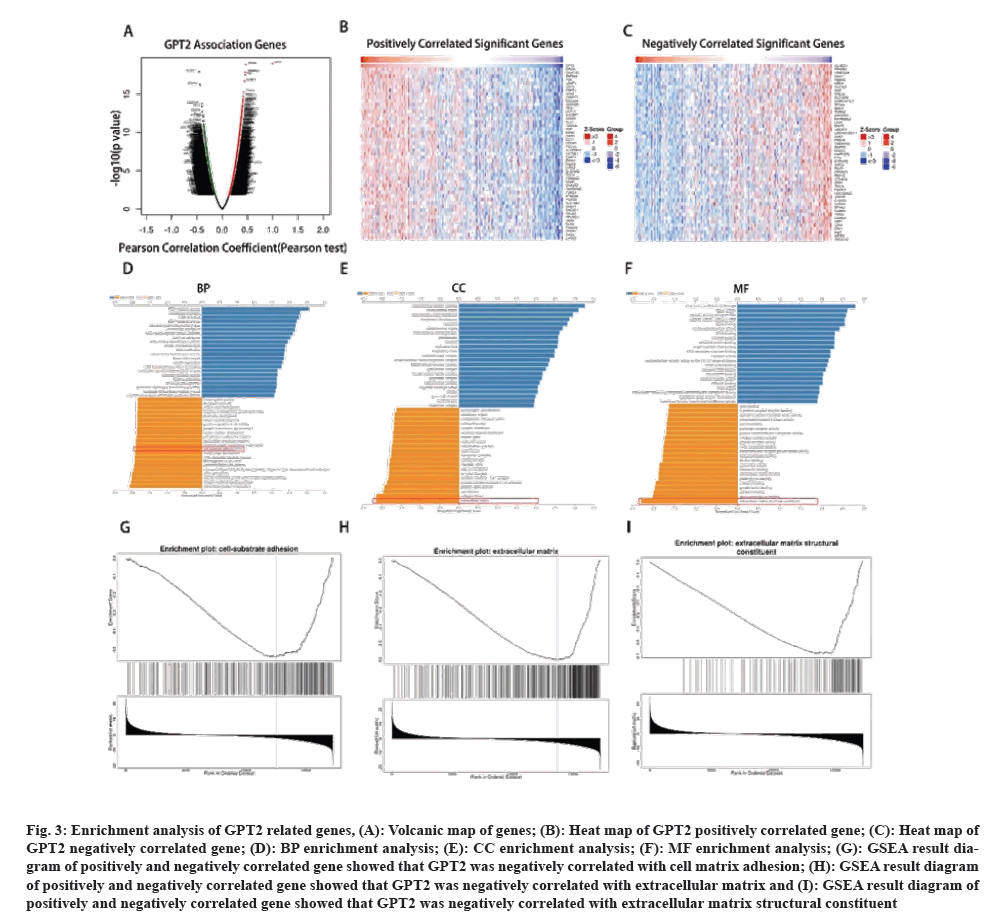
The presence of GPT2 in CRC is a predictive factor, although it is not considered a standalone predictive factor. This suggests that the presence of GPT2 has a specific impact on the progression of CRC. However, the precise mechanism by which it impacts CRC remains uncertain. To further investigate the potential mechanism by which GPT2 enhances the progression of CRC, we conducted an enrichment analysis on its associated genes. Using the online tool LinkedOmics (fig. 3A), we examined the genes that showed a strong correlation with GPT2 gene expression in the TCGA database. Subsequently, a heat map was generated to visualize the genes that exhibited positive and negative correlations with GPT2 (fig. 3B and fig. 3C). The analysis of genes with positive and negative correlation to GPT2 was conducted using Gene Ontology (GO). The cell-substrate adhesion pathway showed significant results in Biological Process (BP) functional enrichment (fig. 3D). Significant enrichment in the extracellular matrix was observed in the results of Cellular Component (CC) functional enrichment (fig. 3E). Significant enrichment in changes to the extracellular matrix was observed in the results of Molecular Function (MF) analysis (fig. 3F). The line chart representing the result of GO enrichment analysis is shown as follows (fig. 3G, fig. 3H, and fig. 3I). EMT may exert its effects through both adherence and modification of the extracellular matrix. The findings indicate a possible link between GPT2 and EMT during the progression of CRC.
Fig. 3: Enrichment analysis of GPT2 related genes, (A): Volcanic map of genes; (B): Heat map of GPT2 positively correlated gene; (C): Heat map of GPT2 negatively correlated gene; (D): BP enrichment analysis; (E): CC enrichment analysis; (F): MF enrichment analysis; (G): GSEA result diagram of positively and negatively correlated gene showed that GPT2 was negatively correlated with cell matrix adhesion; (H): GSEA result diagram of positively and negatively correlated gene showed that GPT2 was negatively correlated with extracellular matrix and (I): GSEA result diagram of positively and negatively correlated gene showed that GPT2 was negatively correlated with extracellular matrix structural constituent.
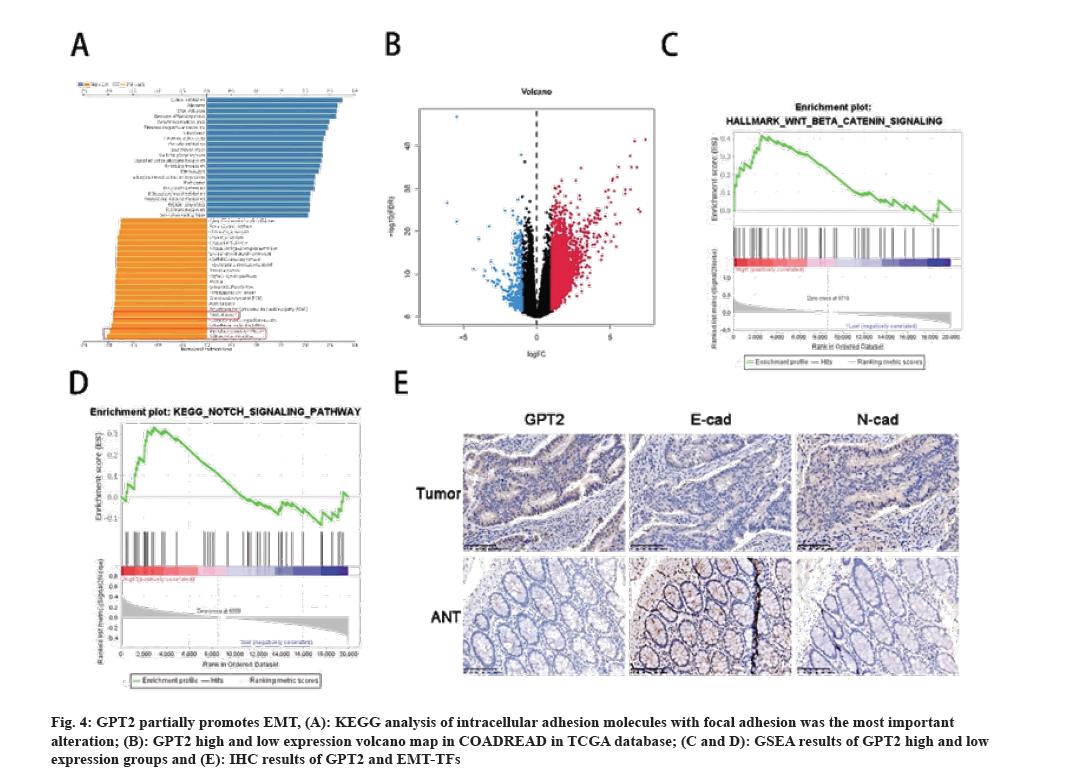
In order to delve deeper into the process by which GPT2 facilitates the progression of CRC, additional investigations were carried out. The earlier findings indicate a possible link between GPT2 and EMT in the progression of CRC. To elucidate the correlation between GPT2 and EMT, we carried out the subsequent investigations. According to the findings from KEGG, the primary changes observed were in cell adhesion molecules and focal adhesion (fig. 4A). To elucidate the functioning of GPT2 and EMT/MET, the TCGA-COADREAD samples were categorized into a high expression group and a low expression group based on the median GPT2 level (fig. 4B). The results of GSEA enrichment analysis indicated that the group with high expression of GPT2 exhibited enrichment in the Wnt/β catenin signaling pathway (Normalized Enrichment Score (NES)=1.403, p=0.115) and the Notch signaling pathway (NES=1.079, p=0.385) (fig. 4C and fig. 4D). The occurrence of EMT is heavily influenced by these two factors. Despite the lack of statistical significance, the high expression group exhibits an enriched overall trend. Subsequently, we confirmed through Immunohistochemistry (IHC) tests that GPT2 and N-cadherin exhibited positive expression in tumor tissues, whereas E-cadherin displayed positive expression in adjacent paracancerous tissues, aligning with the earlier bioinformatics findings (fig. 4E) (Table 4).
Fig. 4: GPT2 partially promotes EMT, (A): KEGG analysis of intracellular adhesion molecules with focal adhesion was the most important alteration; (B): GPT2 high and low expression volcano map in COADREAD in TCGA database; (C and D): GSEA results of GPT2 high and low expression groups and (E): IHC results of GPT2 and EMT-TFs.
| GPT2 | p value | R (Pearson) | ||
|---|---|---|---|---|
| High | Low | |||
| E-CAD | 26 | 22 | ||
| High | 17 | 7 | ||
| Low | 9 | 15 | 0.02 | 0.334 |
| N-CAD | 26 | 22 | ||
| High | 15 | 18 | ||
| Low | 11 | 4 | 0.075 | -0.259 |
Table 4: Correlation analysis of GPT2 expression with E-CAD and N-CAD.
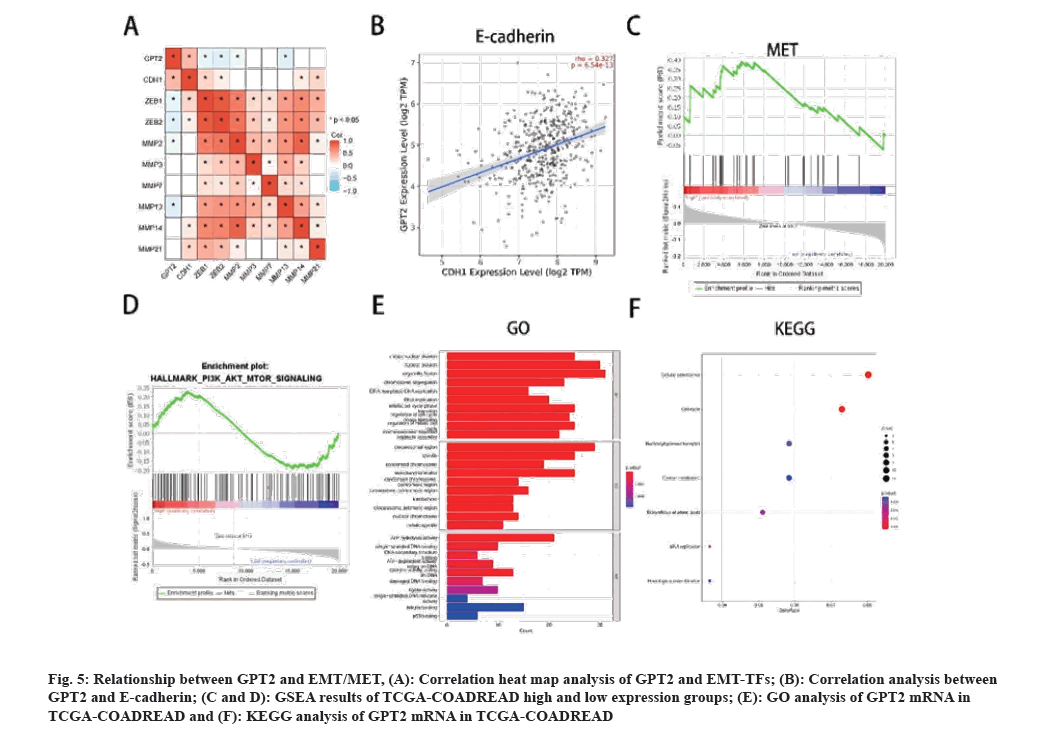
In order to elucidate the mechanism of GPT2 and EMT/MET, we analyzed the TFs of EMT and MET respectively. The heat map of GPT2 and EMT related genes shows that GPT2 is positively correlated with CDH1, but with Snail1, Snail2, Smad2, Smad3, ZEB1, ZEB2, MMP2, MMP3, MMP7, MMP13, MMP14 and MMP21 (fig. 5A). We used Tumor Immune Estimation Resource (TIMER) 2.0 online database to analyze the correlation between GPT2 and E-cadherin gene, and found that GPT2 was positively correlated with E-cadherin (fig. 5B). The expression of E-cadherin can restore the epithelial characteristics of cells, thereby promoting the colonization of tumor metastases. This correlates with the features of MET. Next, we will conduct GSEA enrichment analysis for transcription factors of MET and find that GPT2 is enriched in the high expression group (NES=1.446, p=0.038) (fig. 5C), and the hallmark results also showed that the GPT2 high expression group was enriched in the PI3K/AKT signaling pathway (NES=0.987, p=0.47) (fig. 5D). This is an important downstream pathway of MET. GO and KEGG analysis showed that cell mitosis and cell cycle were significantly enriched, and mitosis was significantly related to MET (fig. 5E and fig. 5F). Therefore, we have reason to believe that GPT2 promotes CRC tumor invasion through EMT, and promotes the colonization of tumor metastases through MET, thereby promoting cell cycle and tumor development.
Fig. 5: Relationship between GPT2 and EMT/MET, (A): Correlation heat map analysis of GPT2 and EMT-TFs; (B): Correlation analysis between GPT2 and E-cadherin; (C and D): GSEA results of TCGA-COADREAD high and low expression groups; (E): GO analysis of GPT2 mRNA in TCGA-COADREAD and (F): KEGG analysis of GPT2 mRNA in TCGA-COADREAD.
In this research, it was discovered that GPT2 is considerably upregulated in colorectal carcinoma. Additionally, it was observed that GPT2 contributes to the spread of CRC by influencing EMT. The expression pattern of GPT2 in CRC aligns with the earlier discovery[21]. Numerous researches have indicated that the process of EMT plays a crucial role in facilitating the spread of cancer cells to distant sites[22-24]. The development of EMT in fibrosis and spread of certain tumors has been explained[25-27]. The spread of CRC results in a poorer outlook for individuals diagnosed with cancer[28,29]. The task of finding prognostic predictors is increasingly urgent. We are of the opinion that GPT2 has the potential to serve as a forecaster for the spread of CRC and can function as a prognostic indicator to enhance the cancer survival rate. GPT2 exhibits high levels of expression in triple-negative breast cancer, bladder cancer, and glioblastoma, contributing to the aggressive spread of cancer[5,30,31]. Abnormal tissues have a higher tendency to undergo proliferation and specialization compared to normal tissues[32,33]. Research has indicated that tyrosine and glutamine serve as metabolic indicators of initial CRC[34], GPT2 plays a crucial function in glutamylation, a significant characteristic of metabolic reprogramming in cancer. The most prevalent metabolic signature in tumors is the Warburg effect, which impacts tumorigenesis through its involvement in metabolic reprogramming. It catalyzes a reversible reaction that converts glutamine, essential for tumor growth, into alanine and α-KG[7]. The promotion of tumor development is facilitated by this crucial mechanism employed by GPT2. However, the role of GPT2 in promoting CRC may not solely rely on this mechanism, but also exhibits a strong correlation with EMT.
The single multivariate COX regression analysis revealed that several pathological characteristics, including the tumor's M stage, serve as prognostic factors. This indicates that GPT2 facilitates the spread of CRC. The independent prognostic factors of tumor staging are divided into four stages. Patients diagnosed with metastatic CRC and liver or lung metastases have a 5 y survival rate ranging from 30 % to 50 %[35]. Increased glutamine metabolism is associated with cancer cell migration, invasion and metastatic colonization in CRC[36]. In this study, we found that GPT2 was significantly correlated with cell adhesion molecules and extracellular matrix according to KEGG, GO analysis and GSEA results. These results all point to a possible link between GPT2 and EMT. Previous studies have also reported that glutamine deficiency promotes the recurrence and metastasis of CRC by promoting EMT[37]. This is consistent with our results. These evidence shows that GPT2 is significantly associated with metastasis of CRC. Interestingly, most studies believe that the downregulation of E-cadherin leads to the occurrence of EMT[22-24,38]. However, we discovered that GPT2 has the ability to enhance tumor metastasis through the promotion of E-cadherin expression. The occurrence of anoikis can be inhibited by the up-regulation of E-cadherin, as cancer cells require overcoming anoikis prior to metastasis. Restoring epithelial properties of cells by up-regulating E-cadherin enhances the colonization potential of CRC metastases, thereby promoting tumor metastasis and resulting in unfavorable prognosis for patients.
To summarize, our findings indicate that GPT2 serves as a reliable indicator of unfavorable prognosis and facilitates EMT and MET in CRC through the activation of Wnt/β catenin and Notch signaling pathways. Consequently, this mechanism enhances the migration and invasion capabilities of cells. The results not only offer reliable indicators for forecasting prognosis, but also present a hopeful objective for addressing metastatic CRC.
Conflict of interests:
The authors declared no conflict of interests.
References
- O’Connell E, Reynolds IS, McNamara DA, Burke JP, Prehn JH. Resistance to cell death in mucinous colorectal cancer-A review. Cancers 2021;13(6):1-14.
[Crossref] [Google scholar] [PubMed]
- Yusufu A, Shayimu P, Tuerdi R, Fang C, Wang F, Wang H. TFF3 and TFF1 expression levels are elevated in colorectal cancer and promote the malignant behavior of colon cancer by activating the EMT process. Int J Oncol 2019;55(4):789-804.
[Crossref] [Google scholar] [PubMed]
- Fakih MG. Metastatic colorectal cancer: Current state and future directions. J Clin Oncol 2015;33(16):1809-24.
[Crossref] [Google scholar] [PubMed]
- Punt CJ, Koopman M, Vermeulen L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat Rev Clin Oncol 2017;14(4):235-46.
[Crossref] [Google scholar] [PubMed]
- Mitra D, Vega‐Rubin‐de‐Celis S, Royla N, Bernhardt S, Wilhelm H, Tarade N, et al. Abrogating GPT2 in triple‐negative breast cancer inhibits tumor growth and promotes autophagy. Int J Cancer 2021;148(8):1993-2009.
[Crossref] [Google scholar] [PubMed]
- Hao Y, Samuels Y, Li Q, Krokowski D, Guan BJ, Wang C, et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat Commun 2016;7(1):11971.
[Crossref] [Google scholar] [PubMed]
- Smith B, Schafer XL, Ambeskovic A, Spencer CM, Land H, Munger J. Addiction to coupling of the Warburg effect with glutamine catabolism in cancer cells. Cell Rep 2016;17(3):821-36.
[Crossref] [Google scholar] [PubMed]
- Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013;496(7443):101-5.
[Crossref] [Google scholar] [PubMed]
- Yang S, Hwang S, Kim M, Seo SB, Lee JH, Jeong SM. Mitochondrial glutamine metabolism via GOT2 supports pancreatic cancer growth through senescence inhibition. Cell Death Dis 2018;9(2):1-10.
[Crossref] [Google scholar] [PubMed]
- Zucker S, Pei D, Cao J, Lopez-Otin C. Membrane Type-Matrix Metalloproteinases (MT-MMP). Curr Top Dev Biol 2003;54:1-74.
[Crossref] [Google scholar] [PubMed]
- Sipos F, Galamb O. Epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions in the colon. World J Gastroenterol 2012;18(7):601-8.
[Crossref] [Google scholar] [PubMed]
- Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell 2016;166(1):21-45.
[Crossref] [Google scholar] [PubMed]
- Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell 2019;49(3):361-74.
[Crossref] [Google scholar] [PubMed]
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000;2(2):84-9.
[Crossref] [Google scholar] [PubMed]
- Wang Z, Wang L, Shi B, Sun X, Xie Y, Yang H, et al. Demethyleneberberine promotes apoptosis and suppresses TGF‐β/Smads induced EMT in the colon cancer cells HCT‐116. Cell Biochem Funct 2021;39(6):763-70.
[Crossref] [Google scholar] [PubMed]
- Tran HD, Luitel K, Kim M, Zhang K, Longmore GD, Tran DD. Transient SNAIL1 expression is necessary for metastatic competence in breast cancer. Cancer Res 2014;74(21):6330-40.
[Crossref] [Google scholar] [PubMed]
- Xu Y, Lee DK, Feng Z, Xu Y, Bu W, Li Y, et al. Breast tumor cell-specific knockout of Twist1 inhibits cancer cell plasticity, dissemination, and lung metastasis in mice. Proc Natl Acad Sci 2017;114(43):11494-9.
[Crossref] [Google scholar] [PubMed]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004;117(7):927-39.
[Crossref] [Google scholar] [PubMed]
- Krebs AM, Mitschke J, Losada ML, Schmalhofer O, Boerries M, Busch H, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol 2017;19(5):518-29.
[Crossref] [Google scholar] [PubMed]
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019;20(2):69-84.
[Crossref] [Google scholar] [PubMed]
- Wang R, Xiang W, Xu Y, Han L, Li Q, Dai W, et al. Enhanced glutamine utilization mediated by SLC1A5 and GPT2 is an essential metabolic feature of colorectal signet ring cell carcinoma with therapeutic potential. Ann Transl Med 2020;8(6):1-9.
[Crossref] [Google scholar] [PubMed]
- Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med 2019;216(5):1016-26.
[Crossref] [Google scholar] [PubMed]
- Chaffer CL, San BPJ, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev 2016;35(4):645-54.
[Crossref] [Google scholar] [PubMed]
- Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol 2019;29(3):212-26.
[Crossref] [Google scholar] [PubMed]
- Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: Parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia 2010;15(2):117-34.
[Crossref] [Google scholar] [PubMed]
- Byler S, Goldgar S, Heerboth S, Leary M, Housman G, Moulton K, et al. Genetic and epigenetic aspects of breast cancer progression and therapy. Anticancer Res 2014;34(3):1071-7.
[Google scholar] [PubMed]
- Chua KN, Sim WJ, Racine V, Lee SY, Goh BC, Thiery JP. A cell-based small molecule screening method for identifying inhibitors of epithelial-mesenchymal transition in carcinoma. PloS One 2012;7(3):1-10.
[Crossref] [Google scholar] [PubMed]
- Wang J, Cai H, Liu Q, Xia Y, Xing L, Zuo Q, et al. Cinobufacini inhibits colon cancer invasion and metastasis via suppressing Wnt/β-catenin signaling pathway and EMT. Am J Chin Med 2020;48(3):703-18.
[Crossref] [Google scholar] [PubMed]
- Kim EK, Song MJ, Jung Y, Lee WS, Jang HH. Proteomic analysis of primary colon cancer and synchronous solitary liver metastasis. Cancer Genomics Proteomics 2019;16(6):583-92.
[Crossref] [Google scholar] [PubMed]
- Zhang B, Chen Y, Bao L, Luo W. GPT2 is induced by Hypoxia-Inducible Factor (HIF)-2 and promotes glioblastoma growth. Cells 2022;11(16):1-13.
[Crossref] [Google scholar] [PubMed]
- Zhao H, Wu W, Li X, Chen W. Long noncoding RNA UCA1 promotes glutamine-driven anaplerosis of bladder cancer by interacting with hnRNP I/L to upregulate GPT2 expression. Transl Oncol 2022;17:1-13.
[Crossref] [Google scholar] [PubMed]
- Matsuno T, Goto I. Glutaminase and glutamine synthetase activities in human cirrhotic liver and hepatocellular carcinoma. Cancer Res 1992;52(5):1192-4.
[Google scholar] [PubMed]
- Martin M, Beauvoit B, Voisin PJ, Canioni P, Guerin B, Rigoulet M. Energetic and morphological plasticity of C6 glioma cells grown on 3-D support; effect of transient glutamine deprivation. J Bioenerg Biomembr 1998;30(6):565-78.
[Crossref] [Google scholar] [PubMed]
- Li J, Li J, Wang H, Qi LW, Zhu Y, Lai M. Tyrosine and glutamine-leucine are metabolic markers of early-stage colorectal cancers. Gastroenterology 2019;157(1):257-9.
[Crossref] [Google scholar] [PubMed]
- Steele Jr G, Bleday R, Mayer RJ, Lindblad A, Petrelli N, Weaver D. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: Gastrointestinal Tumor Study Group Protocol 6584. J Clin Oncol 1991;9(7):1105-12.
[Crossref] [Google scholar] [PubMed]
- Xiang L, Mou J, Shao B, Wei Y, Liang H, Takano N, et al. Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion and metastatic colonization. Cell Death Dis 2019;10(2):1-15.
[Crossref] [Google scholar] [PubMed]
- Sun H, Zhang C, Zheng Y, Liu C, Wang X, Cong X. Glutamine deficiency promotes recurrence and metastasis in colorectal cancer through enhancing epithelial-mesenchymal transition. J Transl Med 2022;20(1):1-11.
[Crossref] [Google scholar] [PubMed]
- Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K, et al. EMT and tumor metastasis. Clin Transl Med 2015;4(1);1-13.
[Crossref] [Google scholar] [PubMed]
 .
.