- *Corresponding Author:
- H. Wang
Department of Neurology,
Nankai University Affiliated Nankai Hospital,
Nankai,
Tianjin 300100,
China
E-mail: hengwang6677@gmail.com
| This article was originally published in a special issue, “New Advancements in Biomedical and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(2) Spl Issue “268-274” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Accumulation of amyloid beta and its toxicity are the common features in Alzheimer’s disease. Autophagy plays an important role in development and progression of Alzheimer’s disease by causing selfdegradation of accumulated amyloid beta from neurons. Glycitin has been found as major phytochemical constituent of soybean, which shows neuroprotective function in Alzheimer’s disease. The present study aims to explore the role of glycitin in clearance of amyloid beta and reducing amyloid beta toxicity in neuronal cells. The human microglial cells 3 and neuroblastoma cell SHSY-5Y were used in the current study. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay was used to access cell viability and neuroprotection. Western blotting was used to determine expression levels of different proteins. Autophagosome marker (light chain 3) was studied using confocal microscopy. Clearance of amyloid beta was studied using confocal microscopy and enzyme-linked immunosorbent assay. We reported that glycitin induces autophagy in human microglial cells 3 cells through phosphatidylinositol 3-kinase/protein kinase B pathway. Glycitin clears amyloid beta and its associated toxicity in neuroblastoma SHSY-5 cells. Furthermore, light chain 3 knockdown inhibits glycitin associated neuroprotective effects in neuroblastoma SHSY-5 cells. We conclude that glycitin can be used as an alternate strategy for curing Alzheimer’s disease.
Keywords
Glycitin, amyloid beta, autophagy, autophagosome marker, phosphatidylinositol 3-kinase/ protein kinase B pathway
Accumulation of Amyloid Beta (Aβ) is the main hallmark in Alzheimer Disease (AD) [1]. AD is mainly classified into two forms familial (early onset) and sporadic (late onset). In former truncated Aβ plaques are formed due to mutation in one of the gene, where as in later an imbalance between the clearance of Aβ occurs, causing more accumulation of the toxic protein [2]. Autophagy plays an important role in development and progression of AD by causing selfdegradation of accumulated Aβ from neurons [3]. It has been reported that cellular integrity is preserved by autophagy and therefore prevents neurological diseases. Deficiency in autophagy is more likely to increases AD severity. Aβ accumulation, due to defective autophagy, has been reported to intensify the disease pathology [4]. Reduced levels of beclin 1 in AD mouse model hampers autophagy and therefore enhances Aβ accumulation and ultimately causes neurodegeneration. It has been reported that genetic modification of belin1 in AD mouse model significantly improves the disease pathology [5]. Growing body of evidence suggests that the pharmacological intervention of autophagy significantly clears accumulated Aβ and therefore provides neuroprotection [6-10].
Natural products impacts drug discovery and design in the modern era [11]. They have been extensively searched upon by scientists for their medicinal and biological properties [12]. Several plant-derived phytochemicals and secondary metabolites played striking roles in pharmacotherapy [13]. Some of the biologically active compounds were found effective drug against different neurological conditions [14]. Flavonoids are a significant class of natural products consisting of over 4000 phenylbenzopyrones mostly found among edible plants [15]. Flavonoids show a rich pharmacological profiles, including anticancer, anti-inflammatory and antioxidative [16]. Among flavonoids, isoflavones from soy like glycitin, daidzein, glycitin and genistein have been shown with anti-osteoporosis, cardioprotective and chemoprotective effects [17,18]. Glycitin is an isoflavone found in soy and products[19]. This molecule has been shown to exhibit remarkable biological activities like promoting wound healing, cardioprotective, antiobesity and antioxidant. Furthermore, glycitin has been found as major phytochemical constituent of soybean, which shows neuroprotective function in AD [20]. In the current study, we try to explore the possible role of glycitin in inducing autophagy to clear Aβ from neuronal cells.
Materials and Methods
Chemicals, cell culture and conditions:
The glycitin molecule (≥98 purity by High Performance Liquid Chromatography (HPLC)) was obtained from Sigma-Aldrich. Aβ (1-42) was purchased from Eurogentec (Belgium). 3-(4,5-Dimethylthiazol- 2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT), 4’,6-Diamidino-2-Phenylindole Dihydrochloride (DAPI), Dimethylsulfoxide (DMSO) and phosphate buffer saline were obtained from Sigma. Antibiotics, Dulbecco’s Modified Eagle Medium (DMEM) and Fetal Bovine Serum (FBS) were procured from Gibco™ (United States). The Human Microglial Cells 3 (HMC3) and neuroblastoma cells (SHSY-5Y) were procured from American Type Culture Collection (ATCC) (Rockville, MD, United States). Cells were cultured in 10 % FBS/antibiotics/DMEM under standard 5 %/air, 37° conditions.
Cell transfection:
The coding sequence of Light Chain 3 (LC3) was amplified by Polymerase Chain Reaction (PCR) from HMC3 cells and cloned into the Promega Monster Green™ Fluorescent Protein (phMGFP) expression vector (Invitrogen, USA), which was referred as phMGFP-LC3. The empty phMGFP plasmid was used as Negative Control (NC). Cell transfection was performed on cells cultured on cover slip using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s protocol. 48 h post transfection, HMC3 cells were fixed with paraformaldehyde (5 %) for 25 min and analyzed through confocal microscopy. Transfection efficiency was by quantitative real time PCR.
For LC3 knockdown, cells were grown in a six-well plates and transfected with 50 nm small interfering Ribonucleic Acid (RNA) targeting LC3 with Lipofectamine™. For successful silencing of LC3, three small interfering RNA (siRNA) sequences were designed from LC3 sequence (GenBank: accession No: NM032514.3), as shown in Table 1.
| Name | Sequences (5'-3') | Position |
|---|---|---|
| siLC3-1 | Sense: GCGAGUUGGUCAAGAUCAUTAATACGACTCACTATAGGGAGA | 352 |
| Antisense: AUGAUCUUGACCAACUCGCTAATACGACTCACTATAGGGAGA | ||
| siLC3-2 | Sense: GCUUCCUCUAUAUGGUCUATAATACGACTCACTATAGGGAGA | 490 |
| Antisense: UAGACCAUAUAGAGGAAGCTAATACGACTCACTATAGGGAGA | ||
| siLC3-3 | Sense: GUAAGGAGGUACAGCAGAUTAATACGACTCACTATAGGGAGA | 220 |
| Antisense: AUCUGCUGUACCUCCUUACTAATACGACTCACTATAGGGAGA |
Table 1: Sequences of Three siLC3
Western blot:
The expression levels of different genes in glycitin treated cells were evaluated by Western blotting. In brief, the cells were seeded in 24-well plates containing different glycitin concentrations (0, 30, 60 and 120 μm) and incubated for 24 h then harvested upon attaining 80 % of confluence. Post glycitin treatment, cells were lysed using Radio Immunoprecipitation Assay (RIPA) lysis buffer. Proteins were quantified using bicinchoninic acid assay and identical amounts (40 μg) were separated on sodium dodecyl-sulfate polyacrylamide gel electrophoresis (12 %) and transferred onto polyvinylidene difluoride membranes. Blocking of membranes was done with bovine serum albumin (5 %) followed by incubation overnight at 4° with corresponding primary antibodies like LC3, ubiquitin-binding protein p62, phosphorylated protein kinase B (p-AKT), total AKT (t-AKT), phosphorylated mammalian Target of Rapamycin (p-mTOR), mTOR, Beclin 1, Autophagy related protein 7 (ATG7), Autophagy related protein 5 (ATG5), Autophagy related protein 12 (ATG12) and Glyceraldehyde- 3-Phosphate Dehydrogenase (GAPDH) (1:1000 dilutions, Santa Cruz, CA, United States). Post incubation period, washing with Tris-Buffered Saline Tween-20 (TBST) was carried out for 25 min. After that, secondary antibodies were introduced with 1:3000 dilutions (Thermo Scientific) and membranes were further incubated for 1 h in the dark. Post incubation, the secondary antibodies treated membranes were rewashed three times again in TBST for 15 min. Finally, the protein bands were visualized with a Bio- Rad Enhanced chemiluminescence clarity max kit over X-ray film.
Confocal microscope:
HMC3 cells were grown on coverslips and treated with HiLyte™ Aβ1-42 555 for 24 h. It was followed by treatment with glycitin for 12 h. DAPI (1 μg/ml) treatment was performed for 15 min. HMC3 cells were fixed with paraformaldehyde (5 %) for 25 min and analyzed through confocal microscopy.
Enzyme-Linked Immunosorbent Assay (ELISA):
HMC3 cells were grown in 12 well plates and treated with HiLyte™ Aβ1-42 555 for 24 h. It was followed by treatment with glycitin for 12 h. Cells were washed thrice, followed by lysis and analysis was carried out in according to protocol.
MTT assay:
The proliferation of SHSY-5Y cells was monitored by MTT assay. In brief, SHSY-5Y cells (1×104 per well) were grown for 24 h in 96-well microplates to 80 % of confluence. Cells were treated as: Aβ1-42 (10 μM) for 24 h followed by treatment with glycitin for different concentrations like 0, 30, 60 and 120 μm, for 12 h. It was followed by treatment with different glycitin concentrations viz. 0, 30, 60, and 120 μm, for 24 h respectively. After test drug treatment, 25 μl of 5 mg/ml MTT solution was left to incubate for 2.5 h.
The formazan crystals formed by the addition of MTT were dissolved in DMSO (100 μl) and the solution was placed in the dark for 20 min. Finally, the absorbance was measured at 570 nm with a multimode plate reader (PerkinElmer, Boston, United States).
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) software was used to carry out statistical analysis. Experimental values were given as mean±standard deviation. Statistical significance was measured with Analysis of Variance (ANOVA) and for comparisons t-test was used (p<0.05 as statistically significant).
Results and Discussion
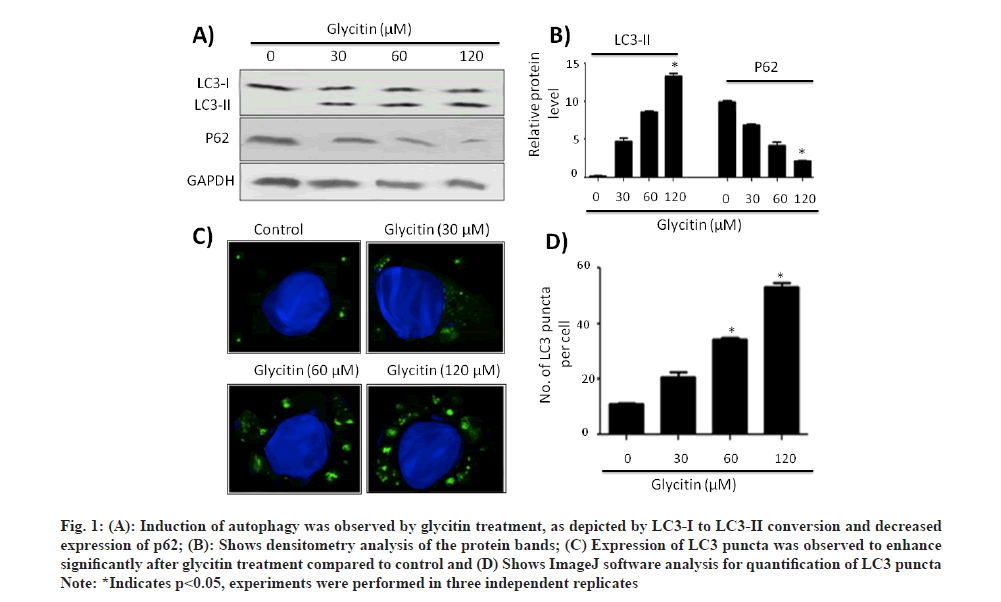
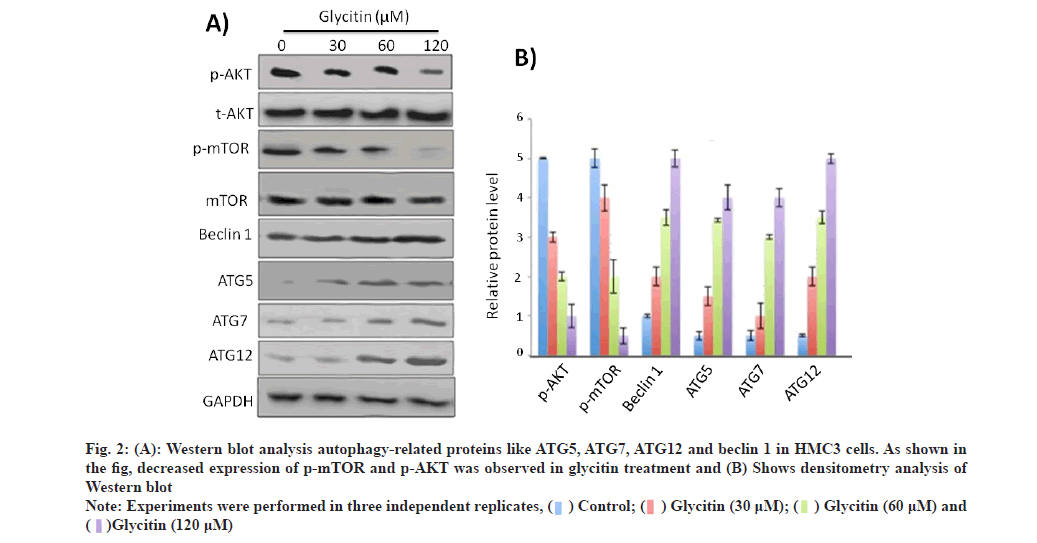
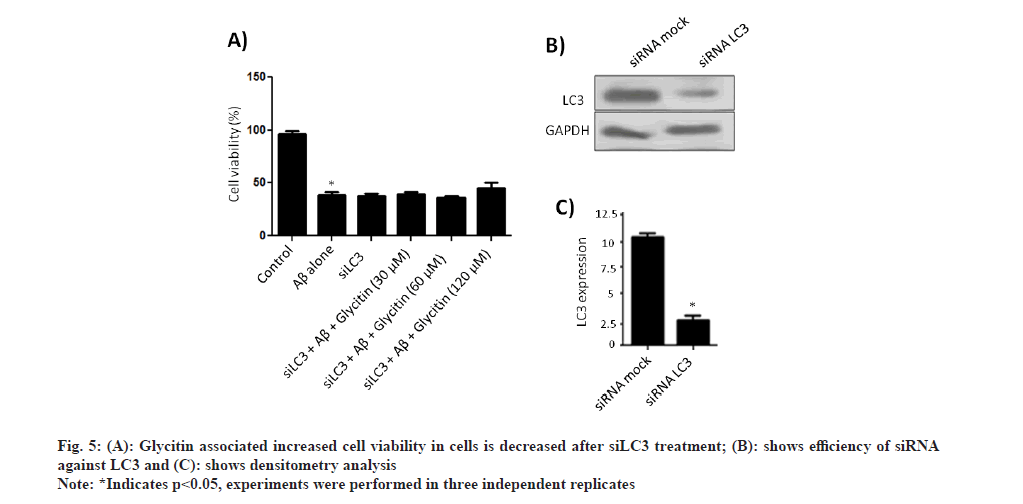
To explore the possible role of glycitin in inducing autophagy, HMC3 cells treated with different consecration of glycitin (1, 5 and 10 μm) for 12 h. Induction of autophagy was observed by glycitin treatment, as depicted by LC3-I to LC3-II conversion and decreased expression of p62 (fig. 1A). Fig. 1B shows densitometry analysis of the protein bands. Expression of LC3 puncta was observed to enhance significantly after glycitin treatment compared to control (fig. 1C). Glycitin was found to increase expression levels of autophagy-related genes like ATG5, ATG7, ATG12 and beclin 1 in HMC3 cells (fig. 2A).
Fig. 1: (A): Induction of autophagy was observed by glycitin treatment, as depicted by LC3-I to LC3-II conversion and decreased
expression of p62; (B): Shows densitometry analysis of the protein bands; (C) Expression of LC3 puncta was observed to enhance
significantly after glycitin treatment compared to control and (D) Shows ImageJ software analysis for quantification of LC3 puncta
Note: *Indicates p<0.05, experiments were performed in three independent replicates
Fig. 2: (A): Western blot analysis autophagy-related proteins like ATG5, ATG7, ATG12 and beclin 1 in HMC3 cells. As shown in the fig, decreased expression of p-mTOR and p-AKT was observed in glycitin treatment and (B) Shows densitometry analysis of Western blot
Note: Experiments were performed in three independent replicates
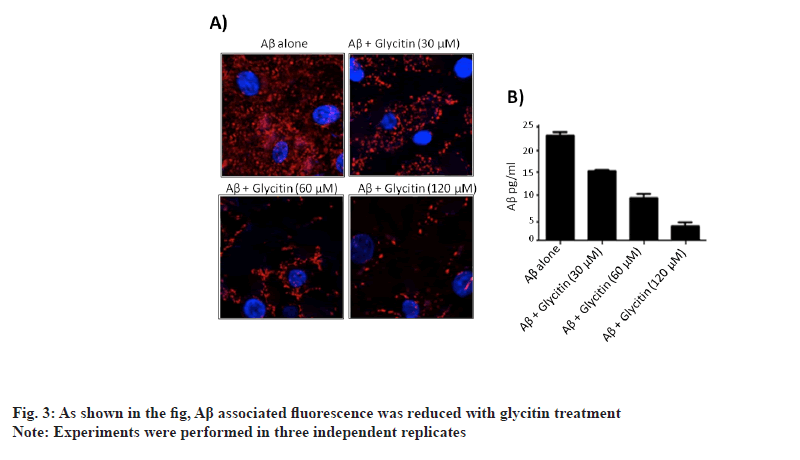
Furthermore, glycitin treatment decreases expression of p-mTOR and p-AKT in dose dependent manner. Our results suggested that glycitin induced autophagy in cells through Adenosine Monophosphate-Activated Protein Kinase (AMPK) pathway. Accumulation of Aβ in neuronal cells, triggers toxicity and ultimately death of neuron. To explore the possible role of glycitin in clearing Aβ from HMC3 cells, transfection studies were carried out. Glycitin treatment significantly reduced Aβ accumulation in cells in dependent manner (fig. 3). As shown in the figure, Aβ associated fluorescence was reduced with glycitin treatment. Thus, our results indicates that glycitin significantly clear Aβ in HMC3 cells. Accumulation of Aβ in AD occurs mainly due to defect in its clearance mechanisms [21].
Autophagy has emerged as essential process, by which clearance of Aβ takes place in the brain [22]. Defective autophagy has been reported to cause both intracellular and extracellular accumulation of Aβ in the brain [23]. Pharmacological intervention on autophagy significantly clears accumulated Aβ and therefore provides neuroprotection. We therefore, try to find out the possible role of glycitin in autophagy associated Aβ clearance in microglial HMC3 cells. Microglial cells possess greater phagocytosis capacity and therefore uptakes Aβ at higher rate to clear its build up [24]. Such specialized cells possess Aβ uptake receptors like Myeloid specific human CD33 and Triggering Receptor Expressed on Myeloid (TREM) cells [25]. We reported induction of autophagy by glycitin treatment, as depicted by LC3-I to LC3-II conversion and decreased expression of p62.
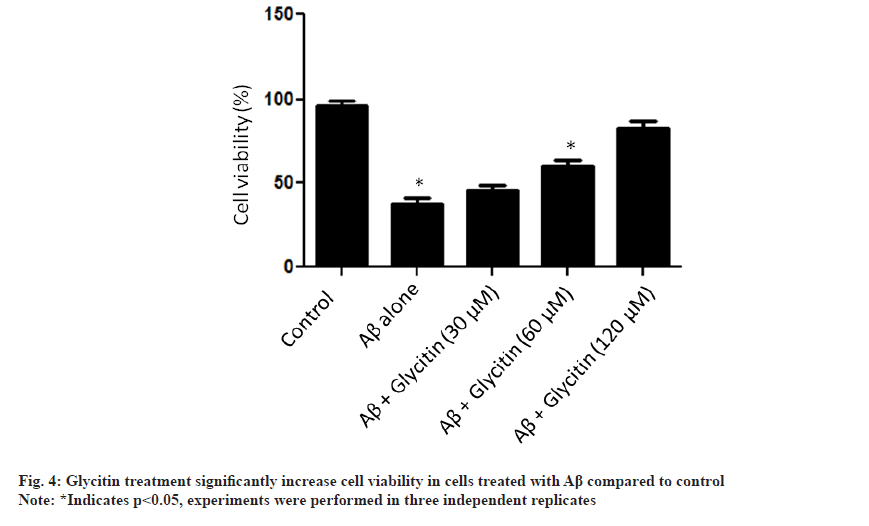
To further validate the role of glycitin in Aβ clearance, silencing of LC3 was done following by treatment with glycitin for 12 h. Interestingly, glycitin associated clearance of Aβ was inhibited in cells tranfected with si-LC3 (fig. 4A). Transfection efficiency of si-LC3 was detected by immunoblotting (fig. 4B). Accumulation of Aβ has been reported to enhance Reactive Oxygen Species (ROS) production and therefore leads to neurodegeneration. As reported above, glycitin was able to significantly clear Aβ in HMC3 cells; we therefore set experiment to look for its role in reducing neurotoxicity in SHSY-5Y cells.
We reported that glycitin treatment significantly increase cell viability in cells treated with Aβ (fig. 5). Furthermore, the process of autophagy is mainly either AMPK activation or Phosphatidylinositol 3-Kinase/ Protein Kinase B (PI3K/AKT) pathway inhibition [26]. We found that glycitin significantly causes activation of AMPK pathway via phosphorylation of p-AMPK (Thr 172) in HMC3 cells. An increased expression level of autophagy related proteins like beclin1, ATG5, ATG7, and ATG12 was found with glycitin treatment. Accumulation of Aβ in neuronal cells, leads toxicity and ultimately death of neuron. Glycitin was found to significantly reduce Aβ accumulation within in HMC3 cells in dependent manner. Glycitin associated Aβ clearance was inhibited by LC3 knockdown, therefore further highlights its role in preventing Aβ accumulation. Accumulation of Aβ has been reported to enhance ROS production and therefore leads to neurodegeneration. Glycitin effectively reduce Aβ associated toxicity SHSY-5Y cells.
In conclusion, glycitin induces autophagy cells through AMPK pathway, causes clearance of Aβ and ultimately offers neuroprotection against Aβ associated toxicity in neuronal cells.
Conflict of interests:
The authors declared no conflicts of interest.
References
- Rajmohan R, Reddy PH. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J Alzheimer's Dis 2017;57(4):975-99.
[Crossref] [Google Scholar] [PubMed]
- Dorszewska J, Prendecki M, Oczkowska A, Dezor M, Kozubski W. Molecular basis of familial and sporadic Alzheimer's disease. Curr Alzheimer Res 2016;13(9):952-63.
[Crossref] [Google Scholar] [PubMed]
- Funderburk SF, Marcellino BK, Yue Z. Cell “self‐eating”(autophagy) mechanism in Alzheimer's disease. Mt Sinai J Med 2010;77(1):59-68.
[Crossref] [Google Scholar] [PubMed]
- Tung YT, Wang BJ, Hu MK, Hsu WM, Lee H, Huang WP, et al. Autophagy: A double-edged sword in Alzheimer’s disease. J Biosci 2012;37(1):157-65.
[Crossref] [Google Scholar] [PubMed]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J Clin Invest 2008;118(6):2190-9.
[Crossref] [Google Scholar] [PubMed]
- Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, Ashford JW, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease. J Alzheimer's Dis 2010;19(4):1155-67.
[Crossref] [Google Scholar] [PubMed]
- Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z, et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 2018;69:351-63.
[Crossref] [Google Scholar] [PubMed]
- Tian Y, Bustos V, Flajolet M, Greengard P. A small‐molecule enhancer of autophagy decreases levels of Aβ and APP‐CTF via Atg5‐dependent autophagy pathway. FASEB J 2011;25(6):1934-42.
[Crossref] [Google Scholar] [PubMed]
- Chu C, Zhang X, Ma W, Li L, Wang W, Shang L, et al. Induction of autophagy by a novel small molecule improves Aβ pathology and ameliorates cognitive deficits. PLoS One 2013;8(6):e65367.
[Crossref] [Google Scholar] [PubMed]
- Wani A, Gupta M, Ahmad M, Shah AM, Ahsan AU, Qazi PH, et al. Alborixin clears amyloid-β by inducing autophagy through PTEN-mediated inhibition of the AKT pathway. Autophagy 2019;15(10):1810-28.
[Crossref] [Google Scholar] [PubMed]
- Thomford NE, Senthebane DA, Rowe A, Munro D, Seele P, Maroyi A, et al. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int J Mol Sci 2018;19(6):1578.
[Crossref] [Google Scholar] [PubMed]
- Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites 2012;2(2):303-36.
[Crossref] [Google Scholar] [PubMed]
- Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018;5(3):93.
[Crossref] [Google Scholar] [PubMed]
- Serpeloni JM, Specian AF, Ribeiro DL, Tuttis K, Vilegas W, Martínez-López W, et al. Antimutagenicity and induction of antioxidant defense by flavonoid rich extract of Myrcia bella Cambess. in normal and tumor gastric cells. J Ethnopharmacol 2015;176:345-55.
[Crossref] [Google Scholar] [PubMed]
- Ginwala R, Bhavsar R, Chigbu DG, Jain P, Khan ZK. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019;8(2):35.
[Crossref] [Google Scholar] [PubMed]
- Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. J Nutr Sci 2016;5:e47.
[Crossref] [Google Scholar] [PubMed]
- Pan W, Quarles LD, Song LH, Yu YH, Jiao C, Tang HB, et al. Genistein stimulates the osteoblastic differentiation via NO/cGMP in bone marrow culture. J Cell Biochem 2005;94(2):307-16.
[Crossref] [Google Scholar] [PubMed]
- Seo GY, Lim Y, Koh D, Huh JS, Hyun C, Kim YM, et al. TMF and glycitin act synergistically on keratinocytes and fibroblasts to promote wound healing and anti-scarring activity. Exp Mol Med 2017;49(3):e302.
[Crossref] [Google Scholar] [PubMed]
- Song TT, Hendrich S, Murphy PA. Estrogenic activity of glycitein, a soy isoflavone. J Agric Food Chem 1999;47(4):1607-10.
[Crossref] [Google Scholar] [PubMed]
- Bhatt PC, Pathak S, Kumar V, Panda BP. Attenuation of neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer’s disease by fermented soybean nanonutraceutical. Inflammopharmacology 2018;26(1):105-18.
[Crossref] [Google Scholar] [PubMed]
- Yoon SS, Jo SA. Mechanisms of amyloid-β peptide clearance: Potential therapeutic targets for Alzheimer’s disease. Biomol Ther 2012;20(3):245.
[Crossref] [Google Scholar] [PubMed]
- Baranello R, L Bharani K, Padmaraju V, Chopra N, K Lahiri D, H Greig N, et al. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr Alzheimer Res 2015;12(1):32-46.
- van Weering JR, Scheper W. Endolysosome and autolysosome dysfunction in Alzheimer’s disease: Where intracellular and extracellular meet. CNS Drugs 2019;33(7):639-48.
[Crossref] [Google Scholar] [PubMed]
- Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer’s disease. J Cell Biol 2018;217(2):459-72.
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, et al. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron 2013;78(4):631-43.
[Crossref] [Google Scholar] [PubMed]
- Sridharan S, Jain K, Basu A. Regulation of autophagy by kinases. Cancers 2011;3(2):2630-54.
[Crossref] [Google Scholar] [PubMed]