- *Corresponding Author:
- D. Sokolovic
Department of Biochemistry, Faculty of Medicine, University of Niš, Niš 18000, Serbia
E-mail: dusantsokolovic@gmail.com
| Date of Submission | Received 06 June 2021 |
| Date of Revision | 28 December 2021 |
| Date of Acceptance | 01 August 2022 |
| Indian J Pharm Sci 2022;84(4):988-998 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Application of cisplatin for the treatment of various solid tumors is known to cause liver damage, through an increase in lipid peroxidation and reactive oxygen species production. Lycopene is a powerful antioxidant agent capable of preventing the cells damage, the formation of stronger intercellular bonds and faster cellular metabolism. This study aims to estimate the potential of lycopene in preventing cisplatin induced liver tissue damage by studying the levels of several biochemical parameters (arginase, aminotransferases, alkaline phosphatase, gamma-glutamyl transpeptidase activity, total protein and albumin concentration) reflecting liver function and a panel of liver tissue biomarkers (xanthine oxidase, reduced glutathione, malondialdehyde, protein carbonylated concentration and diamino oxidase activity). These parameters would be studied in male Wistar rats treated with either cisplatin alone or with cisplatin and lycopene. Additionally, microscopic analysis of liver tissue would be conducted as well. Application of the combination of lycopene and cisplatin significantly prevented the disturbance in all here-studied biomarkers of liver tissue damage. Morphological liver tissue alterations followed the changes in hepatic biochemical status. Our results suggest that lycopene could act as a protective agent in cisplatin-induced liver damage in rats.
Keywords
Cisplatin, liver damage, lycopene, reactive oxygen species, antioxidant defense
The liver is the major organ that detoxifies various exogenous and endogenous substances within the body. These substances may, directly or through their metabolic products, be the cause of the liver's damage. Mostly, those harmful factors are alcohol, environmental pollutants[1] and drugs, among which the most common ones include anti-inflammatory, anti-depressant and anticancer medications[2,3]. They usually induce damage by generating free oxygen/nitrogen radicals, thus producing oxidative/nitrosative stress[4]. Free radicals are highly unstable atoms and molecules, hence, they interact with the complex cell building molecules, including lipids, proteins and Deoxyribonucleic Acid (DNA) or with the other free radicals[2,3]. The resulting state of these interactions leads to a downfall in cells’ antioxidant defenses and causing a state of oxidative/nitrosative stress[2,3].
Cisplatin (CP) is the platinum-based antineoplastic drug, widely used in the treatment of various solid tumors such as ovarian, cervical, non-small cell lung, cancer of head and neck, relapsed lymphoma and colorectal cancer and testicular cancer[5]. It is one of the most effective drugs used in chemotherapy and one of the first described in the history of cancer treatment[6]. Administration of CP includes intravenous injection, which is soon followed by a high concentration in several vital organs, especially in the liver and the kidneys[7]. The mechanism of its activity is by interference with a purine base of DNA and the formation of DNA adducts, which leads to apoptosis. However, despite the significant chemotherapeutic efficacy, CP can produce serious side effects. The most affected organs are liver and kidneys since the drug's metabolism is mainly performed through them. Further complications of CP therapy include neurotoxicity, cardiotoxicity, ototoxicity, ovarian insufficiency and infertility[8,9]. Nephrotoxicity is acknowledged as the main dose-limiting factor of CP's application and hepatotoxicity is usually induced by higher doses of this drug[10]. Research indicates that the liver damage is represented by damaged parenchyma, significant increases of serum Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT) and a deficiency in liver function[7]. The exact mechanism of CP induced liver damage is not completely clarified; however, it is assumed that oxidative stress and antioxidant defense impairment are the main causes. Besides, studies indicated a strong relation between tissue toxicity and an increase in lipid peroxidation[9]. Unfortunately, the majority of chemoprotective antioxidant substances (e.g., ascorbic acid, beta-carotene, niacin, coenzyme Q, zinc, selenium) are not efficient enough and some of them might even interfere with the anti-cancer activity of CP, although this evidence is still a subject of ongoing debate[10-12].
Lycopene (LYC) is a powerful antioxidant agent that belongs to the carotenoid family of plant pigments[13]. The major source of LYC are tomatoes and its products; the remainder could be obtained from watermelon, papaya, pink grapefruit, apricots, rosehips and pink guava[9,13]. LYC is a non-provitamin A carotenoid[13], with a high antioxidant activity deriving from conjugated double bonds[14], thus distinguishing it as more efficient than the other carotenoids[15]. Previous studies have shown that this activity is high both in vitro[16] and in vivo[17] and especially efficient in quenching singlet oxygen[18]. Except for preventing the cells damage induced by free radicals, LYC is also responsible for the formation of stronger intercellular bonds and faster cellular metabolism[13].
Since there is a real necessity for the discovery of new antioxidants that would prevent liver tissue damage arising from CP treatment, this study aimed to determine the potential efficacy of LYC in preventing CP induced liver damage in rats. This was to be done through a panel of serum and tissue biochemical parameters that reflect liver cell state and function. Also, the involvement of reduced Glutathione (GSH) and the enzymes involved in its metabolism would be studied as a potential mechanism through which LYC might exert its effects. To confirm the changes occurring in liver tissue, the histopathological analysis will be conducted.
Materials and Methods
Chemicals:
CP (50 mg/100 ml) was purchased from TEVA (Actavis D.O.O., Serbia) and LYC from Sigma Aldrich (United States of America (USA)) and applied to animals intraperitoneally (i.p.). CP was applied in a single dose of 8 mg/kg[19] and LYC in a dose of 6 mg/kg[17]. The solutions of LYC were prepared daily and applied immediately after preparation. Ketamine was used as a general anesthetic and was purchased from Richter Pharma AG (Wels, Austria). All other chemicals were purchased either from Sigma Aldrich (USA) or Carl Roth (Germany).
Animals and housing:
Healthy, 7 w old male Wistar rats (weighing from 200 to 250 g, in total 24 animals) were obtained from the Vivarium of the Institute of Biomedical Research, Faculty of Medicine, Niš, Serbia. The animals were kept under standard laboratory conditions, in plexiglass cages and a temperature-controlled room (22°±2°) with a 12 h lightdark cycle, standard rodent feed and tap water provided ad libitum. The commencement of the experiment was preceded by a 7 d acclimatization period of rats to the laboratory environment. The study was conducted in accordance with the Basic and Clinical Pharmacology and Toxicology Policy for experimental and clinical studies[20]. The protocol of this study was approved by the local ethics committee and all experimental procedures were conducted following ethical regulations of the European Union (EU) (Directive of 2010; 2010/63/EU) and ones given by laws of the Republic of Serbia.
Experimental design:
The treatment protocol included 4 identical groups of 6 animals, treated daily for 5 d by an i.p. injection (in the same side of the abdomen), as follows:
Vehicle treated group (group I): The animals were given a daily injection of 0.1 % ethanol in 0.9 % Sodium chloride (NaCl) 1 ml/kg.
LYC treated group (group II): The animals were given a daily injection of LYC (6 mg/kg) for 5 d.
CP treated group (group III): The animals were given a single CP injection (8 mg/kg) on the 3rd d of the experiment.
CP-LYC treated group (group IV): The animals were given LYC for 5 d (6 mg/kg) and a single injection of CP (8 mg/kg) on the 3rd d.
All the animals were sacrificed by ketamine overdose (80 mg/kg) 24 h after the completion of the experiment. Blood was withdrawn from animals using a Vacutainer system (BD Vacutainer®) and the tubes containing blood were left to clot at room temperature. The liver tissue was removed for evaluation of tissue biochemical parameters (frozen and stored at -80°) and histopathological analyses (fixated in 10 % buffered formalin).
Serum biochemical analysis:
To obtain the serum, the tubes with the clotted blood were centrifuged at 1500 rpm for 10 min at 4°. The separated serum was further used for the determination of total, direct, and indirect bilirubin, albumin and Total Protein (TP) concentrations, as well as ALT, AST, Gamma- Glutamyltransferase (γ-GT) and Alkaline Phosphatase (ALP) activities using automated biochemical analyzer (BTS-370; BioSystems).
Tissue isolation and homogenate preparation:
Liver tissue specimens were homogenized (10 % w/v) in ice-cold distilled water. Tissue biochemical parameters were determined by using clear supernatant obtained after centrifugation (12 000 rpm, 10 min, 4°). Protein content in liver tissue was measured and determined by the method of Stojanović et al.[21], using the standard bovine serum albumin curve.
Tissue biochemical analyses:
Determination of Total Oxidative Status (TOS): TOS was determined spectrophotometrically with an assay based on the oxidation of ferrous to ferric ion in acidic medium. The oxidation was enhanced by glycerol molecules. The ferric ions form a colored complex with xylenol orange and the color is measured spectrophotometrically. The assay is calibrated with Hydrogen peroxide (H2O2) and the results are expressed in μmol H2O2 equivalent/l.
Determination of Malondialdehyde (MDA) concentrations: MDA concentrations, as an index of lipid peroxidation intensity in the liver tissue, were evaluated spectrophotometrically. The method is based on the reaction between Thiobarbituric Acid (TBA) and MDA, according to the method by Stojanović et al.[22]. Absorbance was read at the wavelength of 532 nm and values were expressed as nmol MDA/mg proteins, including the molecular absorbance coefficient of MDA (1.56×10-5 mol/cm).
Determination of Glutathione S-Transferase (GST) activity: The activity of GST was determined using the method of Habig et al.[23]. The reaction mixture consisted of reduced GSH, 4-nitrobenzyl chloride and phosphate buffers (pH=6.5). The activity was evaluated spectrophotometrically at 310 nm and expressed as nM/ mg of protein.
Determination of Glutathione-Reductase (GR) activity: The activity of GR in the liver tissue was determined spectrophotometrically, according to the method described by Popovic et al.[24]. The method was based on the catalyzing reduced nicotinamide adenine dinucleotide phosphate-dependent reduction of Glutathione Disulfide (GSSG) to GSH. The results were determined at 412 nm and expressed in nM/min/mg of proteins.
Determination of reduced GSH concentration: The concentration of reduced GSH in the liver tissue was determined using the method of Ellman et al.[25]. The reaction mixture comprised supernatants of protein removal and Ellman’s reagent (DTNB), which were shortly incubated. Thereafter, the absorbance of the mixture was measured by a standard spectrophotometric technique at the wavelength of 412 nm and evaluated using a standard curve. The concentration of GSH was expressed in μmol/mg of proteins.
Determination of Xanthine Oxidase (XO) activity: The activity of XO was evaluated by measuring the amount of produced uric acid for a fixed time interval. The reaction mixture included 0.1 ml liver homogenate and 0.1 M Tris Hydrochloride (Tris/HCl) buffer, pH 7.4. The volume of 2.5 ml was preincubated at 37° for 15 min. The reaction was started by adding xanthine. The reaction mixture was incubated afterward, for 30 min at 37°. The increase of the uric acid demonstrated the activity of XO. After incubation, it was stopped by adding perchloric acid. The XO activity was determined by measuring the absorption spectrophotometrically, at 293 nm. The enzyme activity was expressed as U/g tissue protein of liver homogenate, using a molar coefficient of 7.6×10-3 and expressed as U/mg proteins[26].
Determination of Diamine Oxidase (DAO) activity: The activity of DAO was determined by evaluating the amount of amino aldehyde formed in the reaction of DAO putrescine dihydrochloride as its substrate in the Tris-HCl buffer pH 7.7. In the reaction mixture were added 0.4 % 3-methyl-2-benzothiazolone hydrazone and 0.2 % Ferric chloride (FeCl3), which is responsible for coloring. The activity was measured at 660 nm. One unit of the enzyme activity was the amount capable of increasing the optical density by 0,100[27]. The activity of DAO was expressed in mU/mg protein.
Determination of Catalase (CAT) activity: The activity of CAT was determined spectrophotometrically at the wavelength of 405 nm, according to the method that previously described Goth et al.[28]. The enzyme activity was evaluated quantifying the formation of a yellow complex between ammonium molybdate and H2O2. The activity of CAT was expressed in μM/mg proteins.
Histopathological findings:
The liver tissue samples separated for histopathological examination were fixed in formaldehyde solution (10 %, w/v), dehydrated with ethanol solutions of differing concentration (50-100 %, v/v), embedded in paraffin molds, cut into 4-5 μm thick sections and stained routinely with Hematoxylin and Eosin (HE) for further examination with a light microscope (Olympus BH2). The following parameters were scored: cell degeneration, cell/tissue edema, the extent of tissue necrosis, and blood stasis. Observed histopathological changes in tissue morphology were evaluated based on HE stained tissue sections and graded by the scoring system as follows; absent (−), mild (+), moderate (++) and severe (+++)[1].
Statistical analysis:
The obtained data were expressed as the mean values±Standard Deviation (SD). Statistically significant differences were determined by one-way Analysis of Variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons (GraphPad Prism version 5.03, San Diego, CA, USA). Probability values (p)≤0.05 were considered to be statistically significant.
Results and Discussion
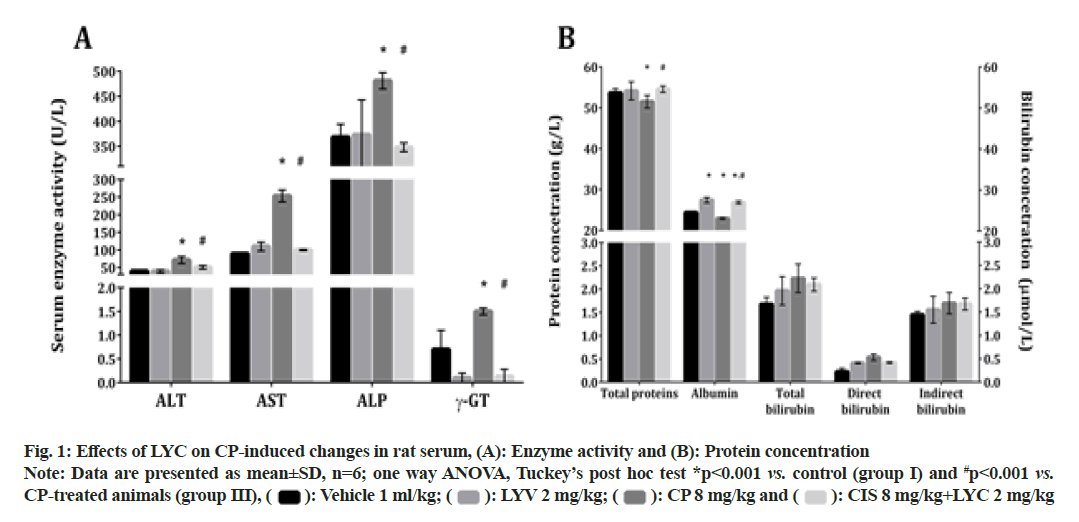
A single injection of CP induced a significant elevation in the activity of the liver-damage associated serum enzymes: ALT, AST, ALP and γ-GT (fig. 1A). This significant disturbance was reduced by the application of LYC; hence, the activities of the mentioned enzymes were significantly decreased in the group IV (CP+LYC), compared to the animals from the group III (fig. 1A). The values of the evaluated serum enzymes in rats treated only with LYC (group II) did not differ from those obtained in the control group (fig. 1A).
The application of CP also caused a significant decrease in serum concentrations of TP and albumin in group III, compared to the vehicle-treated (group I) (fig. 1B). The measured parameters were significantly increased in the CP+LYC treated (IV) group when compared to the ones from the CP only treated rats (fig. 1B). The concentration of albumin in the LYC-treated group (II) and CP+LYC treated group (IV) were both significantly increased, compared to the group I (fig. 1B). In the groups III and IV, the concentrations of total, direct and indirect bilirubin were not significantly altered in comparison with the untreated group of rats (fig. 1B).
In the liver tissue obtained from rats treated with CP (group III), the activity of XO and the concentrations of MDA and Protein Carbonyl Content (PCC) were all found to be significantly increased, compared to the control group (Table 1). Additionally, a significant decrease in the tissue arginase activity was determined in livers belonging to rats from the CP-treated group (group III) compared to the control group (Table 1). When the combination of CP and LYC was applied to animals (group IV), the activity of XO, as well as the content of MDA and PCC, were significantly decreased compared to the CP-treated group (group III). The activity of arginase in the same group was significantly increased compared to the CP treated animals, yet decreased compared to the control group (Table 1).
| Parameter/group | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| (vehicle) | (LYC 6 mg/kg) | (CP 8 mg/kg) | (CP 8 mg/kg+LYC 6 mg/kg) | |
| Tissue damage parameters | ||||
| Arginase (mU/mg of protein) | 85.1±1.1 | 81.4±3.8 | 34.2±8.7* | 47.8±9.3*,# |
| TOS (nmol/mg of protein) | 15.8±4.3 | 9.9±0.8*** | 35.7±1.6* | 19.4±4.5# |
| XO (mU/mg of protein) | 0.41±0.09 | 0.43±0.08 | 2.22±0.17* | 0.48±0.02# |
| MDA (µmol/mg of protein) | 5.6±0.6 | 6.2±0.7 | 29.1±3.4* | 5.9±0.2# |
| PCC (µmol/mg of protein) | 34.1±8.3 | 32.3±5.6 | 194.9±19.8* | 45.8±5.4# |
| GSH and GSH metabolizing enzymes | ||||
| GSH (nmol/g of protein) | 30.9±5.4 | 28±4.1 | 6.9±2.8* | 7.8±1.3* |
| GST (nmol/g of protein) | 315.7±12.4 | 332.8±16.9 | 187.7±31.8* | 308±50.4# |
| GR (nmol/g of protein) | 27.7±1.8 | 25.9±0.4 | 10.0±1.9* | 20.4±0.8*,# |
Note: Data are presented as mean±SD, n=6; ANOVA, Tuckey's post hoc test *p<0.001; **p<0.01; ***p<0.05 vs. control group (vehicle animals) and #p<0.001 vs. CP treated group
Table 1: Liver Tissue damage parameters obtained from different experimental groups
In the groups of rats treated with CP (group III) and combination of CP and LYC (group IV), tissue oxidative status, TOS was significantly increased compared to the untreated rats (Table 1). In the group of rats that received LYC only (group II), TOS was found to be significantly decreased when the values were compared to the ones obtained from the untreated animals (Table 1).
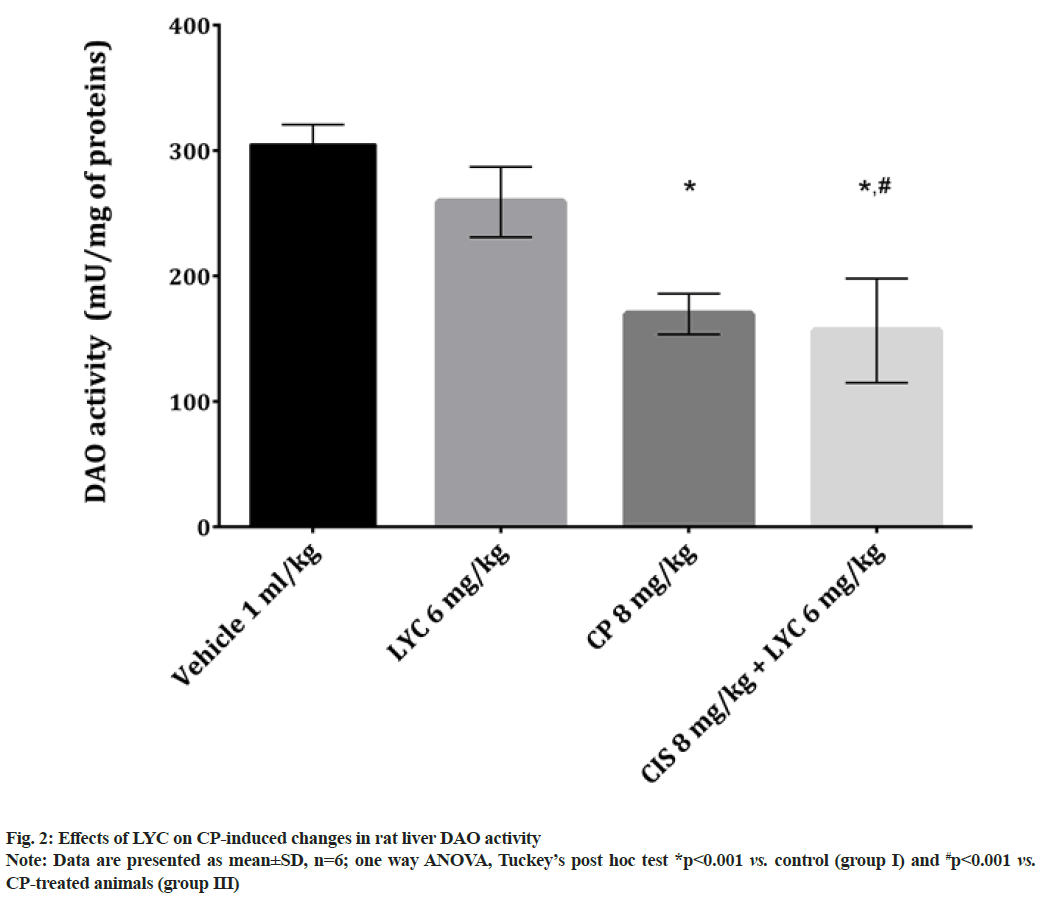
In the liver tissue of animals from both CP and CP+LYC groups, the concentrations of GSH and the activities of GSH related enzymes (GST and GR) were found to be statistically significant, disturbed in comparison to the healthy animals (Table 1). The results of GSH concentration as well as GST and GR activities were significantly decreased in the CP treated group (group III) compared to the control, while the same values were higher in group IV compared to group III (Table 1). Out of the three studied parameters related to GSH in the group treated with CP+LYC (group IV), only the activity of GR was significantly lower compared to the control group (Table 1). The activity of DAO in the group of rats that received CP (group III) was significantly decreased and even more decreased in the group treated with CP+LYC (group IV), compared to the control group (fig. 2). Also, DAO activity in group IV was significantly lower than in the livers of rats belonging to the group III (fig. 2).
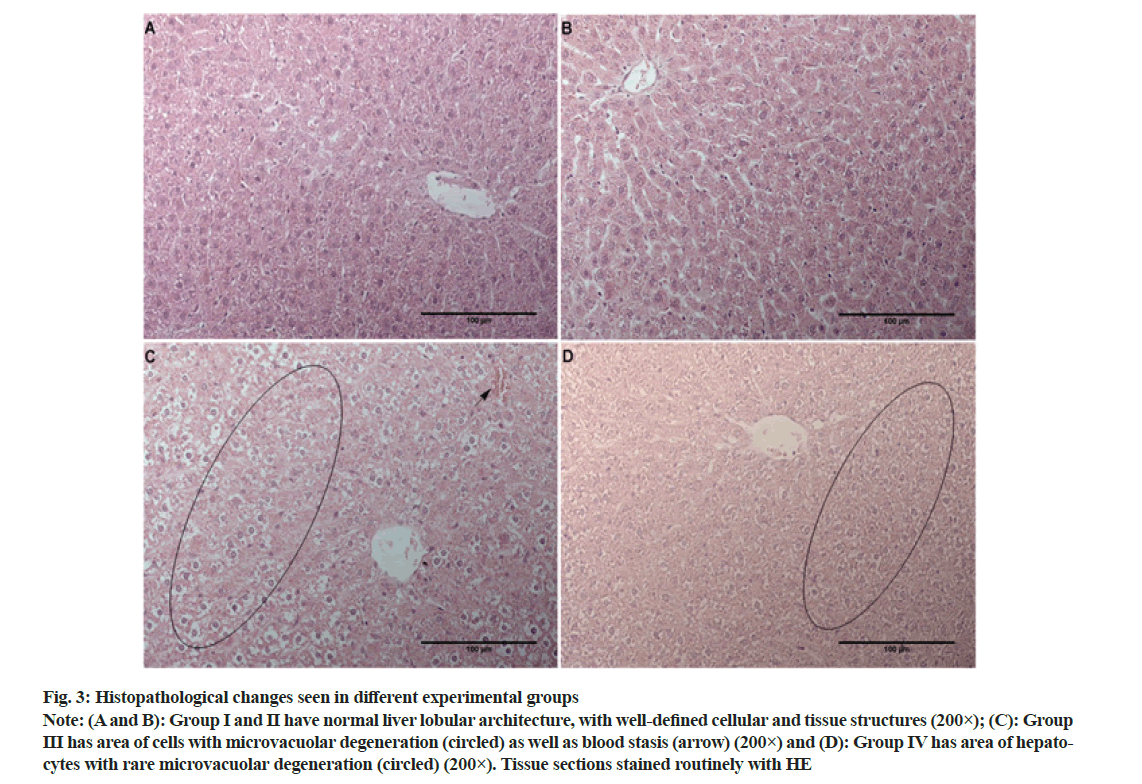
The liver tissue sections stained with HE obtained from the group I and II, control and LYC treated rats, displayed characteristic lobular architecture, with obvious sinusoids, clear central vein and portal spaces (fig. 3A and fig. 3B). Hepatocytes appeared as large polygonal cells with prominent round nuclei with eosinophilic cytoplasm and were without any pathological substrate (Table 2). In animals receiving CP most notable change was the disturbance of hepatic cords, which is mainly due to cell/tissue edema (fig. 3C). Additionally, numerous microvacuoles within hepatocytes were noted and in some cases, rarely an entire area of cells had this type of degeneration (fig. 3C, circled). Occasionally, mild (+) blood stasis was noted (fig. 3C, arrow). In animals treated with LYC during the experiment (group IV) similar changes to the ones seen in group III were noted, however, these changes were much less frequent (Table 2 and fig. 3D).
| Parameter/Group | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| (Vehicle) | (LYC 6 mg/kg) | (CP 8 mg/kg) | (CP 8 mg/kg+LYC 6 mg/kg) | |
| Cell degeneration | - | - | ++ | + |
| Edema | - | - | ++ | + |
| Blood stasis | - | - | + | - |
| Necrotic/apoptotic areas | - | - | + | - |
Note: Changes were graded by the scoring system as follows; absent (−), mild (+), moderate (++) and severe (+++)
Table 2: Liver Tissue Histopathological parameters obtained from different experimental groups
Fig. 3: Histopathological changes seen in different experimental groups
Note: (A and B): Group I and II have normal liver lobular architecture, with well-defined cellular and tissue structures (200×); (C): Group III has area of cells with microvacuolar degeneration (circled) as well as blood stasis (arrow) (200×) and (D): Group IV has area of hepatocytes with rare microvacuolar degeneration (circled) (200×). Tissue sections stained routinely with HE
A disturbance in levels of serum enzymes AST, ALT, ALP and γ-GT are known to follow liver parenchyma damage[29]. Also arginase, an enzyme responsible for converting arginine into urea and ornithine[30] mainly found in the liver tissue, is known to be useful for monitoring hepatic damage[31] and liver function[32]. It has been proposed that high doses of CP induce hepatotoxicity due to the accumulation of this drug in the liver[33]. The present study demonstrated the significant increase of these biomarkers in the serum of CP treated experimental group, compared to the vehicle-treated group. Also, in the same experimental group liver tissue arginase activity was found to be decreased (Table 1). Previous studies investigating CP induced hepatotoxicity in rats also demonstrated significantly elevated liver damage-associated-enzymes[7,8]. The application of LYC prevented such a significant disturbance in the mentioned biomarkers (fig. 1A and Table 1). The study of Jiang et al.[18] demonstrated the same effects of LYC on levels of AST and ALT in a high-fat diet-induced non-alcoholic rat fatty liver disease. Additionally, LYC administered to animals with hepatitis induced by d-galactosamine and Lipopolysaccharide (d-GalN/LPS) prevented an increase in serum levels of AST, ALT, ALP and γ-GT[15]. In a model of liver damage induced by bisphenol A LYC also showed its ability to prevent an increase in AST, ALT and γ-GT activities and a reduction of TP and albumin concentration[34]. To the best of our knowledge there are no data showing the impact of LYC on arginase activity, thus herein we present for the first time that LYC applied on its own does not modify liver arginase activity. Another carotenoid, beta carotene, when applied in fish fed was also found to not affect liver arginase activity[35].
The results related to the albumin concentration in LYC only treated group of rats in that study were not significantly different from the ones in the control group. These results are in contrast to the findings of the present study that showed a significantly increased concentration of albumins in rats belonging to group II. The reason for this discordance may be related to the route of LYC administration (parenteral/oral) or animals’ gender (male/female) that are different in our and the previously conducted study. The finding that LYC increases albumin concentrations might be of vital importance since the CP application is known to cause a decrease in its concentrations (fig. 1A)[36].
TOS is an indicator of tissue pro-oxidant molecules presence and in this case, TOS is mostly defined by the levels of MDA, PCC and XO products i.e. O2 -. In the liver tissue of animals treated with CP only, TOS was significantly increased, compared to untreated and LYC treated animals (Table 1), which is in accordance with the previous studies[37]. The treatment with LYC (group II) leads to a significant reduction in TOS in comparison with the untreated animals (Table 1). This can be attributed to the free radical scavenging activity of LYC, more precisely to the presence of conjugated double bonds[14] and its singlet oxygen quenching capacities[18].
One of the tissue oxidative parameters determined in this study is XO activity. This enzyme is responsible for the majority of Reactive Oxygen Species (ROS) production and its products are known to cause the disturbance in hepatic antioxidative capacities[26]. Hence, it would be advantageous to have a drug with the ability to inhibit XO, apart from acting on some other targets. The results of the present study demonstrated the reduction of levels of XO in animals treated with CP+LYC compared to the levels in animals treated with CP only (Table 1). These findings are especially important since XO is included in various pathophysiological processes[38,39], but not in livers of rats treated with a 7 mg/kg of CP[40]. Interestingly enough the kidney XO was found to be increased by CP application[41].
In the current study, CP application led to a decrease in the content of GSH (Table 1), which has the main role in maintaining Xanthine Dehydrogenase (XDH) in reduced form[42]. The previous study on oxidative stress in rats revealed the significant increase of GSH after applying LYC[43], which may be the explanation of the decrease in XO activity (Table 1).
It is known that MDA is associated with a toxicity of many xenobiotics, including CP[44]. The treatment of rats with CP only caused a significant elevation in MDA content (Table 1), which is in accordance with the findings of the previous studies[45,46]. Treatment with LYC, in combination with CP, prevented such a significant increase in MDA content and maintained it to near-normal levels (Table 1). The mechanism underlying LYC action is the formation of carboncentered radicals with peroxy radicals, thus forming a new chain carrying peroxyl radicals. This chain is a more stable form than free ROS, thus it enables LYC to inhibit the initiation of the lipid peroxidation process[47]. Antioxidant effects of LYC are especially manifested in the protection of cell/organelle membranes damage and the reason for this effectiveness is attributed to its lipophilic nature[48]. PCC is a parameter that represents stable and irreversible proteins produced in the early stage of oxidative stress[49]. In contrast to products of lipid peroxidations, which removal is within minutes, PCC are stable for hours or even days[50]. Thus, PCC is considered as an indicator of overall protein oxidation and oxidative stress and also protein dysfunction[51]. In this study, the application of CP produced enhanced levels of oxidative stress and therefore, increased PCC (Table 1). Treatment of rats with the combination of CP and LYC diminished the increase of created oxidative stress. Histopathological findings usually collaborate with the results of biochemical and tissue parameters of CP toxicity[52] and in the present study, microvacuolar degeneration and hepatic cell necrosis are in accordance with the CP hepatotoxic potential (Table 2 and fig. 3C) [53]. On the other hand, in the group treated with CP and LYC, rare microvacuolar degeneration were still present, however to a much lesser extent (Table 2 and fig. 3D).
As mentioned in the introduction of the present study, the main mechanism of CP toxicity is through lipid peroxidation[9]. Studies on GSH demonstrated its importance for cells’ protection against lipid peroxidation and ROS damage in general[54]. One of the mechanisms of CP toxicity involves the interaction between CP and GSH resulting in augmented ROS production, followed by GSH depletion[55]. The reason for the decreased concentrations of GSH is due to the direct binding of GSH to the platinum molecule[56]. Application of LYC prevents such a decrease through restoring GSH system and free radical scavenging[57], modulation of ROSproducing enzymes and inhibition of pro-inflammatory cascade[58]. The activity of GSH metabolizing enzyme GST was significantly decreased in rats that received CP only (Table 1), which is in agreement with previous studies[59]. It has been reported that GSH, GST and GR activities are reduced because of the increased radicals’ scavenging and therefore utilization of these antioxidants or due to their damage (inactivation/inhibition) induced by ROS[60]. Although previous studies demonstrated the elevation of levels of GSH and GSH metabolizing enzymes after LYC application[61], in the present study, there was no significant increase in these parameters.
DAO is an enzyme involved in the terminal catabolism of polyamines including putrescine, spermidine, spermine and their monoacetyl derivatives[62] and histamine[63]. This enzyme is unevenly distributed in the vertebrate organism, mainly in the small intestine[62], placenta[63], but also can be found in the liver, lungs and kidneys[64]. The studies also showed that growing tissues, growthpromoting stimuli, partial hepatectomy and even some drugs significantly increase the activity of DAO, which is associated with an elevated concentration of putrescine and other polyamines[63,65]. The study of Miyoshi et al.[66] demonstrated that after the intravenous application of chemotherapeutic CP in humans, DAO activity reduction in plasma was a good indicator of the side effects severity of following anticancer drug administration. The decrease in liver tissue activity of DAO might reflect the lower activity in plasma which is known to occur after a dose-dependent chemotherapy treatment and is shown in the previous study[67]. Another explanation of lower DAO activity is the reduced amount of substrate putrescine and other polyamines that are known to be a regulatory factor for the expression of DAO[63]. Studies have shown that liver regeneration after partial hepatectomy increased levels of putrescine and also DAO activity[68]. After protein synthesis inhibition, in the liver as the most important organ for protein synthesis[69], a decrease in DAO activity was also observed[63]. These results, as well as the ones obtained for serum protein levels (fig. 1) from rats treated with CP only, might also explain a decrease in DAO activity (fig. 2). Platinum drugs are known to reduce polyamine cell levels and therefore directly affect DAO activity[70]. As it was mentioned, growth-promoting stimuli[63] are important for enhancing DAO activity, however, both CP and LYC can inactivate them[48,70]. These findings might also explain the significant decrease of DAO activity in rats treated with a combination of CP and LYC.
In conclusion, our results suggest that LYC could act as a protective agent in CP induced liver damage in rats. This can be attributed to its free radical scavenging activity. These findings are supported by the results arriving from the biochemical and pathohistological analysis. Future research should include the evaluation of LYC in some clinical studies in order to minimize the toxic effects that might arrive from platinum-based chemotherapeutic drugs application.
Acknowledgements:
This research was supported by the scientific funding agency of the Republic of Serbia (grant No. 7750154, Acronym NPATPETTMPCB).
Funding:
This research was funded by the scientific funding agency of the Republic of Serbia (grant No. 7750154, Acronym NPATPETTMPCB).
Conflict of interest:
The authors declare that there is no conflict of interest.
References
- Radulović NS, Randjelović PJ, Stojanović NM, Ilić IR, Miltojević AB. Influence of methyl and isopropyl n-methyl antranilates on carbon tetrachloride-induced changes in rat liver morphology and function. FU Phys Chem Tech 2013;11(1):67-73.
- Wu D, Cederbaum AI. Alcohol, oxidative stress and free radical damage. Alcohol Res Health 2003;27(4):277.
[Google Scholar] [PubMed]
- Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 2015;16(11):26087-124.
[Crossref] [Google Scholar] [PubMed]
- Ničković VP, Novaković T, Lazarević S, Šulović L, Živković Z, Živković J, et al. Pre-vs. post-treatment with melatonin in CCl4-induced liver damage: Oxidative stress inferred from biochemical and pathohistological studies. Life Sci 2018;202:28-34.
[Crossref] [Google Scholar] [PubMed]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 2007;33(1):9-23.
[Crossref] [Google Scholar] [PubMed]
- Singh N, Magotra R, Sharma AK, Ahmed M, Khajuria V. Effect of cisplatin on liver of male albino rats. J Evol Med Dent Sci 2015;4:8993-8.
- Palipoch S, Punsawad C. Biochemical and histological study of rat liver and kidney injury induced by cisplatin. J Toxicol Pathol 2013;26(3):293-9.
[Crossref] [Google Scholar] [PubMed]
- Mir M, Arab MR, Shahraki MR, Mashhadi MA, Shahraki SM, Sargolzaei AF, et al. Toxic effects of cisplatin on hepatocytes and liver enzymes of rats. Anat Sci J 2015;12(4):171-5.
- Kulhan N, Kulhan M, Türkler C, Ata N, Kiremitli T, Kiremitli S, et al. Effect of lycopene on oxidative ovary-damage induced by cisplatin in rats. Gen Physiol Biophys 2019;38(3):253-8.
[Crossref] [Google Scholar] [PubMed]
- Avci A, Cetin R, Ergüder İB, Devrim E, Kiliçoğlu B, Candir O, et al. Cisplatin causes oxidation in rat liver tissues: Possible protective effects of antioxidant food supplementation. Tur J Med Sci 2008;38(2):117-20.
- D'Andrea GM. Use of antioxidants during chemotherapy and radiotherapy should be avoided. CA Cancer J Clin 2005;55(5):319-21.
[Crossref] [Google Scholar] [PubMed]
- Lesperance ML, Olivotto IA, Forde N, Zhao Y, Speers C, Foster H, et al. Mega-dose vitamins and minerals in the treatment of non-metastatic breast cancer: An historical cohort study. Breast Cancer Res Treat 2002;76(2):137-43.
[Crossref] [Google Scholar] [PubMed]
- Story EN, Kopec RE, Schwartz SJ, Harris GK. An update on the health effects of tomato lycopene. Ann Rev Food Sci Technol 2010;1:189-210.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Song Y, Zhang L. Effect of lycopene supplementation on oxidative stress: An exploratory systematic review and meta-analysis of randomized controlled trials. J Med Food 2013;16(5):361-74.
[Crossref] [Google Scholar] [PubMed]
- Sheriff SA, Devaki T. Lycopene stabilizes liver function during D-galactosamine/lipopolysaccharide induced hepatitis in rats. Asian Pac J Trop Biomed 2012;2(12):975-80.
[Crossref] [Google Scholar] [PubMed]
- Stojiljković N, Ilić S, Stojanović N, Stojanović S, Stoiljković M. Lycopene improves methotrexate-induced functional alterations of the Madin–Darby kidney cells in a concentration-dependent manner. Can J Physiol Pharmacol 2020;98(2):111-6.
[Crossref] [Google Scholar] [PubMed]
- Stojiljkovic N, Ilic S, Jakovljevic V, Stojanovic N, Stojnev S, Kocic H, et al. The encapsulation of lycopene in nanoliposomes enhances its protective potential in methotrexate-induced kidney injury model. Oxid Med Cell Longev 2018;2018:2627917.
- Jiang W, Guo MH, Hai X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J Gastroenterol 2016;22(46):10180.
[Crossref] [Google Scholar] [PubMed]
- Bakır S, Yazgan ÜC, İbiloğlu İ, Elbey B, Kızıl M, Kelle M. The protective effect of pomegranate extract against cisplatin toxicity in rat liver and kidney tissue. Arch Physiol Biochem 2015;121(4):152-6.
[Crossref] [Google Scholar] [PubMed]
- Tveden‐Nyborg P, Bergmann TK, Lykkesfeldt J. Basic & clinical pharmacology & toxicology policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol 2018;123(3):233-5.
[Crossref] [Google Scholar] [PubMed]
- Stojanović NM, Stevanović M, Randjelović P, Mitić K, Petrović V, Sokolović D, et al. Low dose of carvacrol prevents rat pancreas tissue damage after L-arginine application, while higher doses cause pancreatic tissue impairment. Food Chem Toxicol 2019;128:280-5.
[Crossref] [Google Scholar] [PubMed]
- Stojanović NM, Randjelović PJ, Mladenović MZ, Ilić IR, Petrović V, Stojiljković N, et al. Toxic essential oils, part VI: Acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem Toxicol 2019;133:110794.
[Crossref] [Google Scholar] [PubMed]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249(22):7130-9.
[Crossref] [Google Scholar] [PubMed]
- Popović D, Kocić G, Katić V, Jović Z, Zarubica A, Veličković LJ, et al. Protective effects of anthocyanins from bilberry extract in rats exposed to nephrotoxic effects of carbon tetrachloride. Chem Biol Interact 2019;304:61-72.
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82(1):70-7.
[Crossref] [Google Scholar] [PubMed]
- Brzački V, Mladenović B, Dimić D, Jeremić L, Živanović D, Djukić D, et al. Comparison between the effects of selenomethionine and S-adenosylmethionine in preventing cholestasis-induced rat liver damage. Amino Acids 2019;51(5):795-803.
- Quash G, Gresland L, Delain E, Huppert J. Anti polyamine antibodies and cell lysis: The inhibitory effect of putrescine. Exp Cell Res 1972;75(2):363-8.
[Crossref] [Google Scholar] [PubMed]
- Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 1991;196(2-3):143-51.
[Crossref] [Google Scholar] [PubMed]
- Albrahim T, Binobead MA. Roles of Moringa oleifera leaf extract in improving the impact of high dietary intake of monosodium glutamate-induced liver toxicity, oxidative stress, genotoxicity, DNA damage and PCNA alterations in male rats. Oxid Med Cell Longev 2018;2018:4501097.
[Crossref] [Google Scholar] [PubMed]
- Nikolic J, Stojanovic I, Pavlovic R, Sokolovic D, Bjelakovic G, Beninati S. The role of L-arginine in toxic liver failure: Interrelation of arginase, polyamine catabolic enzymes and nitric oxide synthase. Amino Acids 2007;32(1):127-31.
[Crossref] [Google Scholar] [PubMed]
- Cho YE, Singh TS, Lee HC, Moon PG, Lee JE, Lee MH, et al. In-depth identification of pathways related to cisplatin-induced hepatotoxicity through an integrative method based on an informatics-assisted label-free protein quantitation and microarray gene expression approach. Mol Cell Proteomics 2012;11(1):10884.
[Crossref] [Google Scholar] [PubMed]
- Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008;245(3):194-205.
[Crossref] [Google Scholar] [PubMed]
- Fenoglio C, Boncompagni E, Chiavarina B, Cafaggi S, Cilli M, Viale M. Morphological and histochemical evidence of the protective effect of procainamide hydrochloride on tissue damage induced by repeated administration of low doses of cisplatin. Anticancer Res 2005;25(6B):4123-8.
[Google Scholar] [PubMed]
- Abdel-Daim MM, Abo-EL-Sooud K, Aleya L, Bungǎu SG, Najda A, Saluja R. Alleviation of drugs and chemicals toxicity: Biomedical value of antioxidants. Oxid Med Cell Longev 2018;2018:6276438.
[Crossref] [Google Scholar] [PubMed]
- Benzer F, Özçelik M, Yildirim NC. The effects of dietary antioxidants on the arginase activity and nitric oxide level of narrow-clawed Turkish crayfish (Astacus leptodactylus, Esch. 1823) in moulting period. Turkish J Fish Aquat Sci 2016;16(2):283-8.
- Mansour HH, Hafez HF, Fahmy NM. Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. BMB Rep 2006;39(6):656-61.
- Cerig S, Geyikoglu F, Bakir M, Colak S, Sonmez M, Koc K. Hepatoprotective effect of oleuropein against cisplatin-induced liver damage in rat. World Acad Sci Eng Technol Int J Med Health Biomed Bioeng Pharm Eng 2016;10:260-7.
- Topham RW, Walker MC, Calisch MP. Liver xanthine dehydrogenase and iron mobilization. Biochem Biophys Res Commun 1982;109(4):1240-6.
[Crossref] [Google Scholar] [PubMed]
- Kuppusamy P, Zweier JL. Characterization of free radical generation by xanthine oxidase: Evidence for hydroxyl radical generation. J Biol Chem 1989;264(17):9880-4.
[Crossref] [Google Scholar] [PubMed]
- Yilmaz HR, Sogut S, Ozyurt B, Ozugurlu F, Sahin S, Isik B, et al. The activities of liver adenosine deaminase, xanthine oxidase, catalase, superoxide dismutase enzymes and the levels of malondialdehyde and nitric oxide after cisplatin toxicity in rats: Protective effect of caffeic acid phenethyl ester. Toxicol Ind Health 2005;21(1-2):67-73.
[Crossref] [Google Scholar] [PubMed]
- Söğüt S, Kotuk M, Yılmaz HR, Ulu R, Özyurt H, Yıldırım Z. In vivo evidence suggesting a role for purine‐catabolizing enzymes in the pathogenesis of cisplatin‐induced nephrotoxicity in rats and effect of erdosteine against this toxicity. Cell Biochem Funct 2004;22(3):157-62.
[Crossref] [Google Scholar] [PubMed].
- Kooij A, Schiller HJ, Schijns M, van Noorden CJ, Frederiks WM. Conversion of xanthine dehydrogenase into xanthine oxidase in rat liver and plasma at the onset of reperfusion after ischemia. Hepatology 1994;19(6):1488-95.
[Crossref] [Google Scholar] [PubMed]
- Saada HN, Rezk RG, Eltahawy NA. Lycopene protects the structure of the small intestine against gamma‐radiation‐induced oxidative stress. Phytother Res 2010;24(S2):S204-8.
[Crossref] [Google Scholar] [PubMed]
- Koyuncu I, Kocyigit A, Gonel A, Arslan E, Durgun M. The protective effect of naringenin-oxime on cisplatin-induced toxicity in rats. Biochem Res Int 2017;2017:9478958.
- Karale S, Kamath JV. Effect of daidzein on cisplatin-induced hematotoxicity and hepatotoxicity in experimental rats. Indian J Pharmacol 2017;49(1):49-54.
[Crossref] [Google Scholar] [PubMed]
- Ahmadipour A, Sharififar F, Nakhaipour F, Samanian M, Karami-Mohajeri S. Hepatoprotective effect of Zataria multiflora Boisson cisplatin-induced oxidative stress in male rat. J Med Life 2015;8(4):275.
[Google Scholar] [PubMed]
- Aggarwal S, Singh K, Nagpal M, Kaur A, Ahluwalia P. Studies on the effect of lycored supplementation (lycopene) on lipid per-oxidation and reduced glutathione in pregnancy induced hypertensive patients. Biomed Res 2009;20(1):51-4.
- Sahin K, Gencoglu H, Bilir B, Kucuk O. Protective role of lycopene against oxidative stress in liver. Liver 2018:155-67.
- Weber D, Davies MJ, Grune T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol 2015;5:367-80.
[Crossref] [Google Scholar] [PubMed]
- Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J Biol Chem 1996;271(26):15504-9.
[Crossref] [Google Scholar] [PubMed]
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 2003;329(1-2):23-38.
[Crossref] [Google Scholar] [PubMed]
- El-Sayyad HI, Ismail MF, Shalaby FM, Abou-El-Magd RF, Gaur RL, Fernando A, et al. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int J Biol Sci 2009;5(5):466.
[Crossref] [Google Scholar] [PubMed]
- Bano N, Najam R. Histopathological and biochemical assessment of liver damage in albino Wistar rats treated with cytotoxic platinum compounds in combination with 5-fluorouracil. Arch Med Sci 2019;15(4):1092-103.
[Crossref] [Google Scholar] [PubMed]
- Tirmenstein MA, Reed DJ. Role of a partially purified glutathione S-transferase from rat liver nuclei in the inhibition of nuclear lipid peroxidation. Biochim Biophys Acta 1989;995(2):174-80.
[Crossref] [Google Scholar] [PubMed]
- Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A, et al. Systems biology of cisplatin resistance: Past, present and future. Cell Death Dis 2014;5(5):e1257.
[Crossref] [Google Scholar] [PubMed]
- Berners-Price SJ, Kuchel PW. Reaction of cis-and trans-[PtCl2(NH3)2] with reduced glutathione inside human red blood cells, studied by 1H and 15N-{1H} DEPT NMR. J Inorg Biochem 1990;38(4):327-45.
[Crossref] [Google Scholar] [PubMed]
- Choi SK, Seo JS. Lycopene supplementation suppresses oxidative stress induced by a high fat diet in gerbils. Nutr Res Pract 2013;7(1):26-33.
[Crossref] [Google Scholar] [PubMed]
- Camara M, de Cortes Sánchez-Mata M, Fernández-Ruiz V, Cámara RM, Manzoor S, Caceres JO. Lycopene: A review of chemical and biological activity related to beneficial health effects. In: Rahman A, editors. Studies in Natural Products Chemistry; 2013. p. 383-426.
- Yasuyuki S, Yoshihiko S, Yoshio T, Sadao H. Protection against cisplatin-induced nephrotoxicity in the rat by inducers and an inhibitor of glutathione S-transferase. Biochem Pharmacol 1994;48(3):453-9.
[Crossref] [Google Scholar] [PubMed]
- Velmurugan B, Bhuvaneswari V, Nagini S. Antiperoxidative effects of lycopene during N-methyl-N′-nitro-N-nitrosoguanidine-induced gastric carcinogenesis. Fitoterapia 2002;73(7-8):604-11.
[Crossref] [Google Scholar] [PubMed]
- Breinholt V, Lauridsen ST, Daneshvar B, Jakobsen J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett 2000;154(2):201-10.
[Crossref] [Google Scholar] [PubMed]
- Seiler N, Bolkenius FN, Knödgen B. The influence of catabolic reactions on polyamine excretion. Biochem J 1985;225(1):219-26.
[Crossref] [Google Scholar] [PubMed]
- Perin A, Sessa A, Desiderio MA. Response of tissue diamine oxidase activity to polyamine administration. Biochem J 1986;234(1):119-23.
[Crossref] [Google Scholar] [PubMed]
- Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, et al. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev 2019;2019:6175804.
[Crossref] [Google Scholar] [PubMed]
- Sessa A, Desiderio MA, Perin A. Stimulation of hepatic and renal diamine oxidase activity after acute ethanol administration. Biochim Biophys Acta 1984;801(2):285-9.
[Crossref] [Google Scholar] [PubMed]
- Miyoshi J, Miyamoto H, Goji T, Taniguchi T, Tomonari T, Sogabe M, et al. Serum diamine oxidase activity as a predictor of gastrointestinal toxicity and malnutrition due to anticancer drugs. J Gastroenterol Hepatol 2015;30(11):1582-90.
[Crossref] [Google Scholar] [PubMed]
- Tsujikawa T, Uda K, Ihara T, Inoue T, Andoh A, Fujiyama Y, et al. Changes in serum diamine oxidase activity during chemotherapy in patients with hematological malignancies. Cancer Lett 1999;147(1-2):195-8.
[Crossref] [Google Scholar] [PubMed]
- Sessa A, Desiderio MA, Perin A. Diamine oxidase activity induction in regenerating rat liver. Biochim Biophys Acta 1982;698(1):11-4.
[Crossref] [Google Scholar] [PubMed]
- Abdel-Misih SR, Bloomston M. Liver anatomy. Surg Clin North Am 2010;90(4):643-53.
[Crossref] [Google Scholar] [PubMed]
- Hector S, Porter CW, Kramer DL, Clark K, Prey J, Kisiel N, et al. Polyamine catabolism in platinum drug action: Interactions between oxaliplatin and the polyamine analogue N1, N11-diethylnorspermine at the level of spermidine/spermine N 1-acetyltransferase. Mol Cancer Ther 2004;3(7):813-22.
[Crossref] [Google Scholar] [PubMed]