- *Corresponding Author:
- M. Rafiq
Discovery Sciences Group, Himalaya Wellness Company, Bengaluru, Karnataka 562162, India
E-mail: dr.rafiq@himalayawellness.com
| Date of Received | 10 June 2020 |
| Date of Revision | 28 October 2021 |
| Date of Acceptance | 16 August 2022 |
| Indian J Pharm Sci 2022;84(4):1063-1070 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Varicose vein is one of the common vascular diseases, in which veins become widened, bulged and twisted. Development of drug to combat varicose vein is a challenging task due to complex nature of the disease and non-availability of specific animal model system. In this context, relevant experimental models were developed and polyherbal formulations HVVL-041702 a liniment intended for topical use (200 µl/ animal) and HVVT-041701 a granule intended for oral use (250 mg/kg body weight, orally) were evaluated. In vitro elastase inhibition assay was carried out using the human leukocyte elastase enzyme. Ex vivo venotonic activity was performed in isolated rat vena cava. In vivo anti-inflammatory, analgesic and anti-edemagenic activity were carried out in Wistar rats. Along with the aforementioned activities, HVVT-041701 was also evaluated for chronic venous insufficiency. Both the formulations showed elastase inhibition, venotonic, anti-inflammatory, analgesic and anti-edemagenic activities. HVVT-041701 was also effective in ameliorating chronic venous insufficiency by decreasing the venous pressure. Both the formulations showed beneficial effects in the management of varicose veins by improving the tonicity, venous insufficiency and vascular hyper permeability which are the pathologic hallmark of varicose veins.
Keywords
Varicose vein, chronic venous insufficiency, venotonic, polyherbal formulation, elastase inhibition
Varicose veins are visible, enlarged, elongated and tortuous veins which includes vein from great/small saphenous, perforators or small venules[1]. Varicose veins and venous insufficiency of the lower extremity are among the most common disease entities affecting approximately 1/3rd of the adult population resulting in significant social and economic burden[2]. Symptoms of chronic venous disorders may result from primary venous insufficiency associated with spectrum of skin changes with persistent venous hypertension which leads to deep vein thrombosis[3]. Other symptoms include wide clinical spectrum, ranging from asymptomatic but cosmetic problems to severe symptoms including pain, itching, limb heaviness, cramps and decreased elasticity of the vein wall, allowing pooling of blood within the veins[4]. Several options recommended by physicians for varicose vein treatment are conservative treatment, foam sclerotherapy, thermal procedures and surgical procedures. But these treatments have their own draw backs and varicose veins have a recurrence rate of 26 % to 60 % following surgical treatment[5], thereby underscoring the importance of alternative methods like herbal drugs to halt and delay progression and maintenance of the disease. Consequently, the polyherbal drug offers a promising alternative for the treatment and management of varicose vein.
In this context two polyherbal formulations were selected, one is liniment for topical application and another one in the tablet for the oral dosage form. Varicose vein liniment was coded as HVVL-041702 which contains Sesamum indicum (S. indicum) (sesame oil), Zingiber officinale (Z. officinale) (ginger oil)andMentha piperita (M. piperita)(Peppermint oil)and extracts of Centella asiatica (C. asiatica) whole plantand Rubia cordifolia (R. cordifolia) stem. Varicose vein tablet was coded as HVVT-041701, which containblend of Allium sativum (A. sativum) bulb,C. asiatica whole plant, Curcuma longa (C. longa) rhizome,Vitis vinifera (V. vinifera) seedandEmblica officinalis (E. officinalis) fruit. These formulations were prepared based on Ayurvedic wisdom and evidences available in the modern literature on the role of individual herbs in the management of varicose veins. C. asiatica is reported for venotonic, elastase inhibition and anti-inflammatory activities[6–8]. R. cordifolia is known to possess anti-inflammatory and anti-oxidant activities[9,10]. Z. officinale (ginger oil) has analgesic, anti-inflammatory and anti-oxidant activities[11,12]. M. piperita (peppermint) is reported to possess anesthetic/cooling activity, which help numb the pain associated with varicose vein and it also exhibit anti-oxidant activity[13,14]. S. indicum (sesame oil) is reported to improve collagen content, which help in strengthening of the vein and also it has anti-oxidant activity[15,16]. A. sativum (garlic) was reported to have analgesic, anti-inflammatory and thrombolytic activities[17–19]. C. longa (turmeric/haridra) exhibits anti-oxidant, anti-inflammatory activity and also down-regulates the Matrix Metalloproteinases (MMPs) which play a key role in pathogenesis of varicose veins[20–22]. V. vinifera (grapes) possess venotonic and anti-oxidant properties[23–25] and amla (E. officinalis) is reported to have anti-collagenase and anti-elastase activities and it also exhibits good anti-inflammatory action, in both acute and chronic models[26–28].
Above mentioned criteria were the rationale behind selecting the herbs and finally, these herbs were mixed to form a blend and formulations, which were evaluated for the efficacy in experimental models of varicose veins. As such, there is no specific animal model for varicose veins in this study various models are selected based on the signs and symptoms of varicose veins which collectively gives an insight on the overall effect of the polyherbal formulations on the management of varicose.
Materials and Methods
Experimental animals:
Inbred Wistar rats of either sex of body weight range 200-300 g were procured from central animal house facility, Himalaya Wellness Company. The animals were housed in standard conditions of temperature (22°±3°), relative humidity (55 %±5 %) and light (12 h light/dark cycle) before and during the study. They were fed ad libitum with standard rat pellet feed manufactured by VRK Nutritional Solutions, Pune, India. Deep bore-well water passed through Aquaguard Reviva reverse osmosis plant manufactured by Eureka Forbes Ltd., Mumbai, India was provided ad libitum. The experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) of Himalaya Wellness Company, Bangalore, (Protocol no. 186/18) and the animals received human care as per the guidelines prescribed by the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA), The Ministry of Environment and Forests, Government of India.
Procedure:
In vitro elastase assay: In a 150 µl reaction mix, the final concentrations of buffer 100 mM Tris Hydrochloric acid (HCl) (pH=8.0 at 25°), a substrate (N-succinyl-Ala-Ala-Ala-p-nitroanilide) and human Leukocyte Elastase (LE) enzyme (Sigma Aldrich, India) was 75 mM, 0.44 mM, and 0.025 units respectively. The test product final concentration was 200 µg/ml. The reaction mixture was mixed by inversion and equilibrated to 25° for 15 min. Then the substrate (N-succinyl-Ala-Ala-Ala-p-nitroanilide) was added. The reaction was immediately mixed by inversion and the increase in absorbance at 410 nm was recorded for approximately 30 min in Enzyme-Linked Immunoassay (ELISA) reader (Biotek Synergy HT)[29].
Ex vivo venotonic activity: The experiment was carried out based on Raffertto et al. with modifications. Briefly Inferior Vena Cava (IVC) was rapidly excised from euthanized rat, placed in oxygenated Krebs solution, and carefully dissected and cleaned of connective tissue. The vena cava was divided into 3 mm rings in preparation for isometric contraction experiments. IVC were suspended between two fabricated stainless-steel hooks, in which one hook was fixed at the bottom of a tissue bath and the other hook was connected to a force displacement transducer (distributed by AD Instruments as MLT 0201/RAD, NSW, AUS) which in turn connected to data acquisition system (Power Lab, AD Instruments) and data recording software lab Chart 7 (AD Instruments Pty Ltd., Castle Hill, Australia) for the measurement of isometric tension). Krebs solution was bubbled with 95 % Oxygen (O2) and 5 % Carbon dioxide (CO2) for 30-45 min, at an adjusted pH 7.4 with 4 washouts in the stabilization period. Different concentration of coded variants was tested in functional IVC segments in a cumulative manner for assessing the venotonic activity. The polyherbal formulations were prepared either in Kreb’s solution or in Dimethyl Sulfoxide (DMSO) (Sigma Aldrich, India) depending on their solubility. The contraction of the vena cava was measured by evaluating the increase in tension which was analyzed by force displacement transducer. The response of the tissue was measured as mg-response, as the tension applied to the tissue for stabilization/calibration was 500 mg. The response of the tissue is directly proportional to the increase in tension/contraction and the results were expressed as mg-response[30].
Analgesic and anti-inflammatory activity: Anti-inflammatory and analgesic activity was investigated by measuring carrageenan-induced paw edema in randomized design. Female rats were randomly divided into three groups of six each. Group 1 control animals received distilled water (10 ml/kg body weight (b. wt.) p. o.), group 2 and 3 animals received polyherbal formulation HVVL-041702 (200 µl/animal topical application on both paws) and HVVT-041701 at a dose of (250 mg/kg b. wt. p. o.) respectively. The therapeutic dose was selected based on the Lethal Dose (LD)-50. The limit dose was found to be 2500 mg/kg b. wt. p. o. 1/10th of this was considered as therapeutic dose. The treatment continued for 7 d and initial paw volume was measured by using a digital Plethysmometer (IITC Life science, United States America (USA)) to obtain basal reading. Carrageenan (1 %) (Himedia laboratories, India) 0.1 ml was administered in the right paw of each animal through sub-plantar route and the inflammation was evaluated by measuring the paw volume at 90, 180 and 300 min respectively. Similarly the pain threshold was measured at 150 min after carrageenan injection. The force threshold was measured by keeping the probe between the 3rd and 4th tarsal of injected rat paw using Randal selitto meter (IITC Life science, USA)[31,32].
Anti-edematogenic activity-histamine-induced vascular permeability: Male Wistar rats were randomly divided into three groups of six each. Grouping was as similar in anti-inflammatory studies. Once after the grouping, the hairs of the animal were clipped over an area of 2×2 cm on the left dorsal side of each animal. Group 2 animals were applied topically with the coded variant HVVL-041702 (200 µl), whereas group 3 animals received the coded variant HVVT-041701 at 250 mg/kg per oral daily once for 14 d. On the last day of the study the histamine-induced vascular permeability was carried out according to the procedure mentioned in Garcia-mesa MT et al. and Matsuda et al.[33,34].
Chronic Venous Insufficiency (CVI) of lower limbs modeling in rats:
Male Wistar rats were anaesthetized using 5 % isoflurane (Neon laboratories Limited, India) and 2 % isoflurane to maintain anesthesia. CVI of the lower limbs model was developed by partial restriction blood flow in caudal vena cava with the procedure similar to Plotnikov et al. Animals were randomized and grouped into group 1-sham control (no occlusion), group 2-path control/CVI control and group 3-HVVT-041701 (250 mg/kg b. wt. p. o.) and treatment was carried for 14 d. Pressure measurement was performed on 14th d of the experiment by using fluid filled catheter connected to pressure transducer (distributed by AD Instruments as MLT844, NSW, AUS) which in turn connected to data acquisition system (Power Lab, AD Instruments) and data recording software lab chart 7 (AD Instruments Pty Ltd., Castle Hill, Australia). Pressure in a caudal vena cava was measured by the direct method at the level of ileac vena convergence and the approach was through a femoral vein[35].
Statistical analysis:
The values are expressed as mean±Standard Deviation (SD). The results were analyzed statistically using one-way Analysis of Variance (ANOVA) followed by post Dunnet’s multiple comparison tests Prism GraphPad 6.07 (GraphPad Software Inc., San Diego, CA, USA) to find out the level of significance. A p<0.05 was considered as statistically significant.
Results and Discussion
The enzyme activity of elastase enzyme was evaluated by the nanomoles of product N-succinyl-Ala-Ala-Ala (SucAla3) released per minute from substrate (SucAla3-pNA) at the pH 8 at 25°. The enzyme activity was calculated by the slope of the graph (Y2-Y1/X2-X1) which represents rate of enzymatic reaction (units/min). The inhibition of the elastase enzyme by the test products HVVL-041702 and HVVT-041701 at 200 µg/ml was found to be 33.3 % and 65.6 % respectively over the control (enzyme, buffer and substrate) as shown in fig. 1.
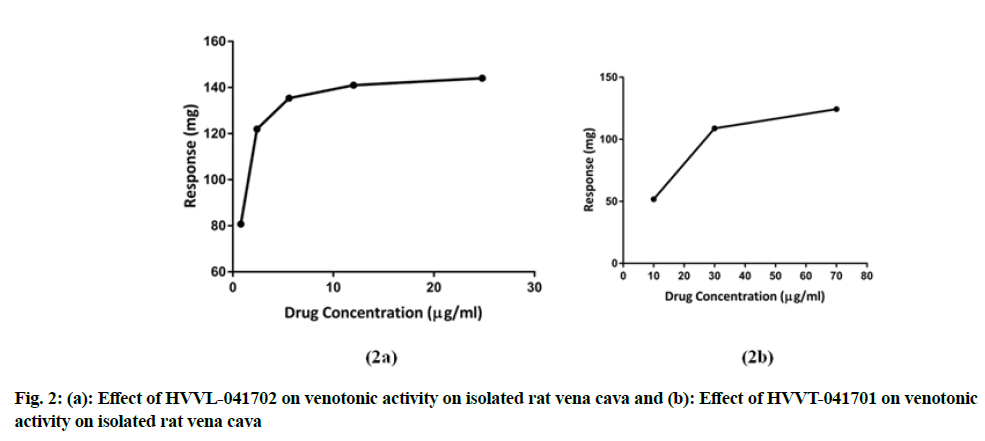
The formulations, HVVL-041702 and HVVT-041701 showed contraction of vena cava at different concentrations. HVVL-041702 showed venotonic property by contracting at 0.8 µg/ml and maximum contraction was observed at 25 µg/ml and venotonic actions remained constant after further addition of the drug, whereas HVVT-041701 exhibited venotonic activity at the concentration of 10 µg/ml and it has reached the maximum at 70 µg/ml. HVVL-041702 showed better venotonic activity compared to HVVT-041701 as shown in fig. 2a and fig. 2b.
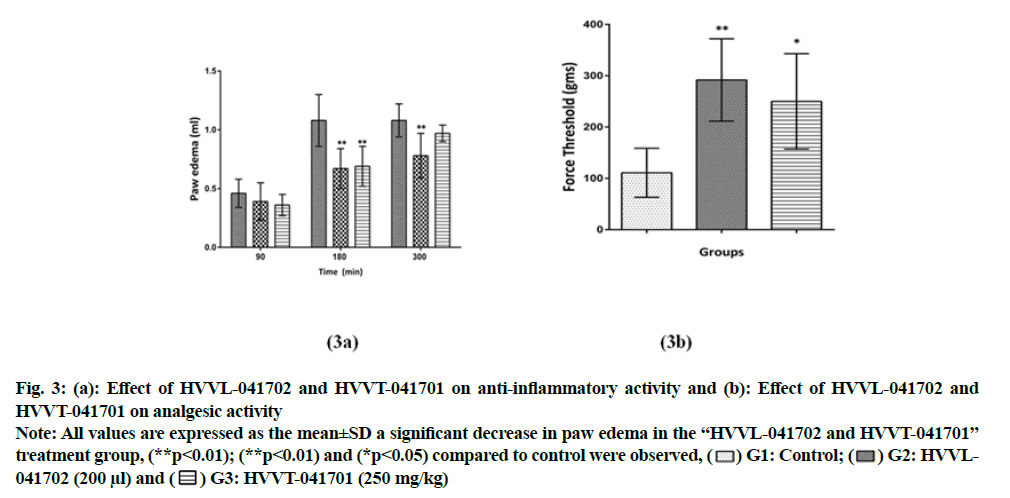
Polyherbal formulations HVVL-041702 and HVVT-041701 reduced the inflammation at 90, 180 and 300 min, and maximum reduction was observed at 180 min with a reduction of 38 % and 36 % respectively. HVVL-041702 showed significant (p<0.01) reduction at 180 and 300 min when compared to control animals, whereas HVVT-041701 showed significant (p<0.01) reduction only at 180 min compared to control group (fig. 3a). Also both formulations HVVL-041702 and HVVT-041701 showed significantly (p<0.01) and (p<0.05) increased paw withdrawal threshold compared to control group (fig. 3b), indicating both polyherbal formulations showed peripheral analgesic activity, measured at 150 min after carrageenan injection.
Fig. 3: (a): Effect of HVVL-041702 and HVVT-041701 on anti-inflammatory activity and (b): Effect of HVVL-041702 and
HVVT-041701 on analgesic activity.
Note: All values are expressed as the mean±SD a significant decrease in paw edema in the “HVVL-041702 and HVVT-041701” treatment group, (**p<0.01); (**p<0.01) and (*p<0.05) compared to control were observed, ( ) G1: Control; (
) G1: Control; ( ) G2: HVVL-
041702 (200 μl) and (
) G2: HVVL-
041702 (200 μl) and ( ) G3: HVVT-041701 (250 mg/kg).
) G3: HVVT-041701 (250 mg/kg).
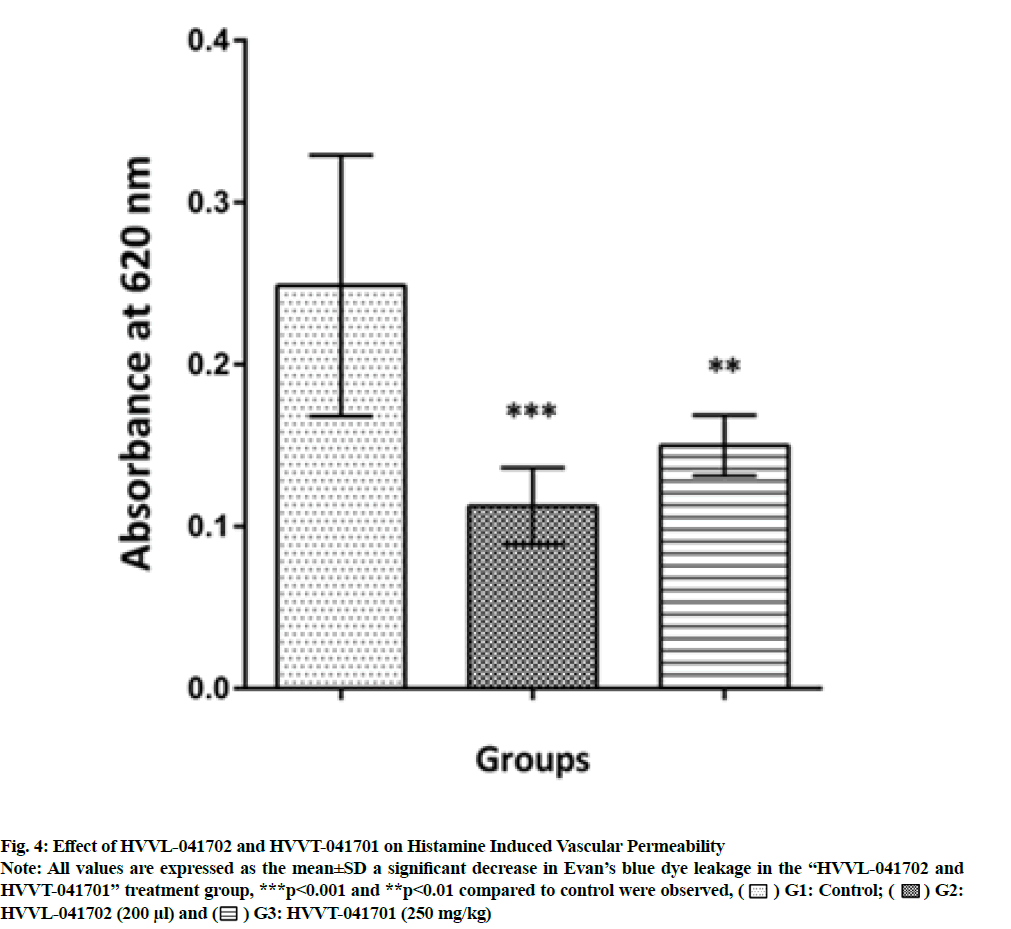
As expected there was a prominent increase in histamine-induced Evan’s blue dye leakage in the control animals. Pretreatment with polyherbal formulation HVVL-041702 and HVVT-041701 significantly (p<0.001) and (p<0.01) decreased Evan’s blue leakage during 14 d of treatment. These values were expressed as Optical Density (OD) values at 620 nm as shown in fig. 4.
Fig. 4: Effect of HVVL-041702 and HVVT-041701 on Histamine Induced Vascular Permeability.
Note: All values are expressed as the mean±SD a significant decrease in Evan’s blue dye leakage in the “HVVL-041702 and
HVVT-041701” treatment group, ***p<0.001 and **p<0.01 compared to control were observed, ( ) G1: Control; (
) G1: Control; ( ) G2:
HVVL-041702 (200 μl) and (
) G2:
HVVL-041702 (200 μl) and ( ) G3: HVVT-041701 (250 mg/kg).
) G3: HVVT-041701 (250 mg/kg).
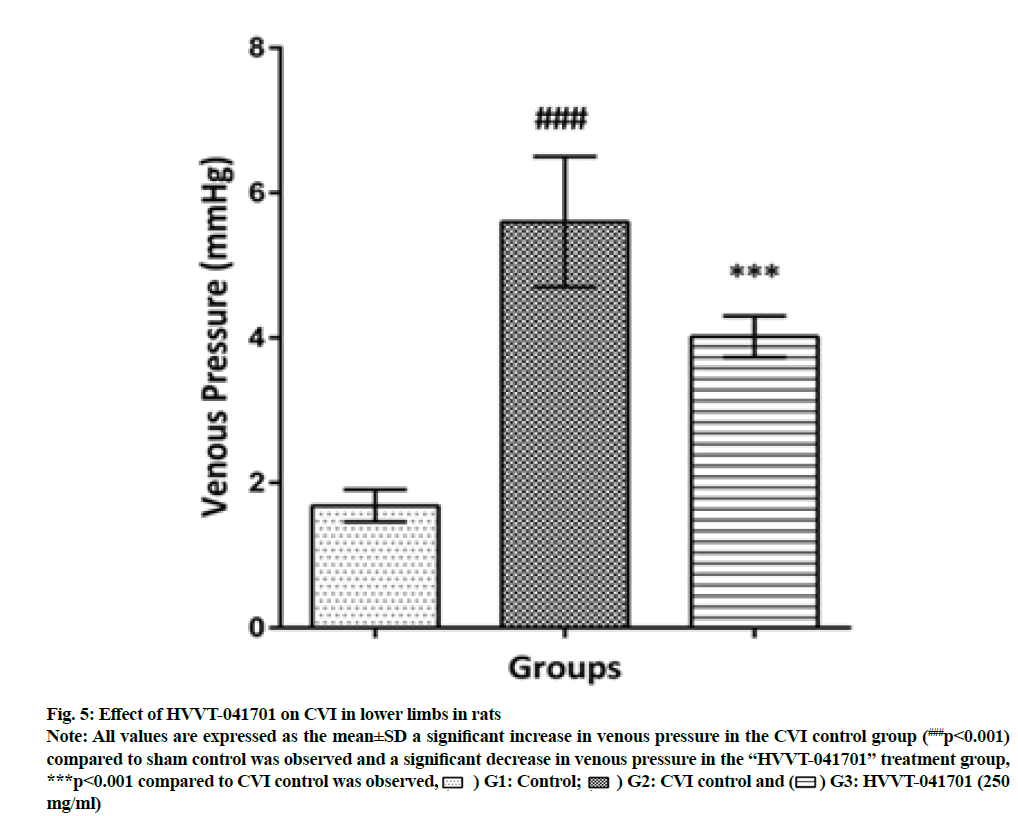
Partial occlusion of the vena cava caused persistent venous hypertension and was being maintained for 14 d which remains as pathological hallmark. On the 14th d, the venous pressure in CVI groups of animals with partial occlusion of the vena cava was greater, 3.3 times (p<0.001) as compared with the values in sham-operated animals. Administration of polyherbal formulation HVVT-041701 for 14 d in the CVI model had significantly (p<0.001) reduced the venous pressure with 24.4 % decrease in venous pressure when compared to CVI control as shown in fig. 5.
Fig. 5: Effect of HVVT-041701 on CVI in lower limbs in rats.
Note: All values are expressed as the mean±SD a significant increase in venous pressure in the CVI control group (###p<0.001)
compared to sham control was observed and a significant decrease in venous pressure in the “HVVT-041701” treatment group,
***p<0.001 compared to CVI control was observed, ( ) G1: Control; (
) G1: Control; ( ) G2: CVI control and (
) G2: CVI control and ( ) G3: HVVT-041701 (250
mg/ml).
) G3: HVVT-041701 (250
mg/ml).
Ayurveda is one of the traditional medicinal systems of India, which mainly uses single or multiple herbs (polyherbal) for treating various diseases and disorders. A combination of the multiple herbs in a specific ratio will achieve a better therapeutic effect. So in the present study, the polyherbal formulations were evaluated locally as well as systemically for the management of the varicose[36]. LE is a major proteinase responsible for inflammation and extracellular proteolysis of the vein wall that is mediated by neutrophils. The activity of LE is controlled by specific serine protease inhibitor, Alpha 1-Antitrypsin (AAT). Any imbalance between these two leads to local tissue inflammation and other pathological conditions, like cancer, inflammation in the vein[37]. It is well reported that increase in the level of neutrophil elastase was measured as a marker of neutrophil degranulation, which is well observed in varicose vein patients compared to age-matched control humans[38]. So both coded variants were screened for elastase inhibition activity and both formulations showed elastase inhibition, which is mainly attributed by the inhibitory potential of C. asiatica and E. officinalis, which are well known for anti-elastase activity[7,26].
The venoactive drugs are medicines intended to improve venous circulation, to maintain tonicity and proper function of the vein. To check the beneficial aspect the polyherbal formulations were screened for the venotonic activity in the rat saphenous vein[39]. Both polyherbal formulations were effective in maintaining the elasticity and tonicity of the venous wall which may be increased in the circulation of blood at the damaged veins. This may be attributed to the total triterpenic fraction of the C. asiatica which decreased enzyme responsible for the mucopolysaccharide metabolism, since mucopolysaccharides are one of the main components of Extracellular Matrix (ECM) which contribute for vascular integrity, which ultimately helps in maintaining the elasticity in varicose veins conditions[6]. So combination of the both herbs may have the synergistic effect which helps in maintaining the vascular integrity. Also sesame is known to improve collagen content and may help strengthen vein wall[15].
In addition to the above condition, in varicose vein pain/aching and inflammation hampers the lifestyle and quality of life of the people. As expected the local injection of the carrageenan-induced significant increase in the inflammation and pain in the rat. Pretreatment of animals with polyherbal formulation resulted in a significant inhibition of carrageenan-evoked hind paw edema and pain which is attributed by the herbs like C. asiatica, R. cordifolia and Z. officinale (ginger oil) present in the HVVL-041702[8,9,11] and also due to A. sativum, C. longa and E. officinalis present in HVVT-041701 in the herbal formulation which has been reported to possess a potent anti-inflammatory and analgesic activity[17,18,20,28].
An altered blood flow and reduced venous return in the large veins of legs result in increased pressure and forced movement of blood and fluid in smaller veins and capillaries of the skin, which results in capillary and microvasculature dilation, elongated and tortuous and also abnormal pressure in capillaries resulting in the increased permeability edema, leakage of red blood cells and leucocyte infiltration and activation. These abnormal capillary remodeling and permeability have also been linked to the high plasma levels of vascular endothelial growth factor found in patients, especially those with skin change[39]. Both polyherbal formulations HVVL-041702 and HVVT-041701 exhibited a significant decrease in vascular permeability when compared to control group, which may be attributed by the ginger oil present in the HVVL-04170211 and also due to A. sativum and E. officinalis present in HVVT-041701 which have been reported for anti-edemagenic activity[18,28].
The pathogenesis of CVI of lower limbs involves an array of actions, including venous hypertension which leads to disturbances of blood flow, venous stasis, activation and adhesion of leukocytes, endothelial dysfunction, and expression of inflammatory mediator’s that leads to microcirculatory disorder. All these factors act on the walls of veins and cause varicosities, edema and ulcers[40]. In this context, our prime aim is to decrease the venous pressure which ultimately decreases/reduces the complications of CVI. Polyherbal formulation HVVT-041701 had showed a significant decrease in the venous pressure which ameliorates the venous insufficiency thus helps in varicose veins and its management; this may be mainly attributed by active phytochemical compounds of C. asiatica as it is known to improve venous wall alterations in chronic venous hypertension and in protecting the venous endothelium. The triterpenoid fraction of C. asiatica are known to be active on connective tissue modulation, improve the synthesis of collagen and other tissue proteins by modulating the action of fibroblasts in the vein wall and stimulate collagen remodeling in and around the venous wall[41].
From the results obtained it can be inferred that both the polyherbal formulations showed a beneficial effect in the management of varicose veins. The effect may be due to the design of the formulations or due to the synergism of the herbs which are acting on various pathophysiological pathways of varicose veins. Thus the obtained results are in favor of the individually designed formulations; these can be recommended for the management of varicose veins.
Acknowledgments:
The authors acknowledge Phytochemistry, Natural Product Innovations, and Preclinical Pharmacology and Toxicology Departments, Himalaya Wellness Company, for providing support during the study. The authors also acknowledge M/S Himalaya Wellness Company, for providing the facilities, fund and support for performing this research work.
Conflict of interests:
The authors declared no conflict of interests.
References
- de Popas E, Brown M. Varicose veins and lower extremity venous insufficiency. Semin Intervent Radiol 2018;35(1):56-61.
[Crossref] [Google Scholar] [PubMed]
- Atta HM. Varicose veins: Role of mechanotransduction of venous hypertension. Int J Vasc Med 2012;2012.
- Piazza G. Varicose veins. Circulation 2014;130(7):582-7.
[Crossref] [Google Scholar] [PubMed]
- Tisi PV. Varicose veins. BMJ Clin Evid 2011;2011:0212.
[Google Scholar] [PubMed]
- Subramonia S, Lees TA. The treatment of varicose veins. Ann R Coll Surg Eng 2007;89(2):96-100.
- Arpaia MR, Ferrone R, Amitrano M, Nappo C, Leonardo G, Del Guercio R. Effects of Centella asiatica extract on mucopolysaccharide metabolism in subjects with varicose veins. Int J Clin Pharmacol Res 1990;10(4):229-33.
[Google Scholar] [PubMed]
- Nema NK, Maity N, Sarkar BK, Mukherjee PK. Matrix metalloproteinase, hyaluronidase and elastase inhibitory potential of standardized extract of Centella asiatica. Pharm Biol 2013;51(9):1182-7.
[Crossref] [Google Scholar] [PubMed]
- Chippada SC, Volluri SS, Bammidi SR, Vangalapati M. In vitro anti-inflammatory activity of methanolic extract of Centella asiatica by HRBC membrane stabilisation. Rasayan J Chem 2011;4(2):457-60.
- Basu S, Hazra B. Evaluation of nitric oxide scavenging activity, in vitro and ex vivo of selected medicinal plants traditionally used in inflammatory diseases. Phytother Res 2006;20(10):896-900.
[Crossref] [Google Scholar] [PubMed]
- Kaur P, Singh B, Kumar S, Kaur S. In vitro evaluation of free radical scavenging activity of Rubia cordifolia L. J Chin Clin Med 2008;3(5):278-84.
- Chiba N, Aiuchi T, Suzuki T, Mori T, Shibasaki M, Kawahito Y, et al. Comparison of anti-nociceptive and anti-inflammatory/analgesic effects of essential oils in experimental animal models. Jpn J Pharm Palliat Care Sci 2014;7:63-70.
- Wei W, Lin Z, Nan L, Yuangang Z. Chemical composition and in vitro antioxidant, cytotoxicity activities of Zingiber officinale Roscoe essential oil. Afr J Biochem Res 2012;6(6):75-80.
- Galeotti N, Ghelardini C, Mannelli LD, Mazzanti G, Baghiroli L, Bartolini A. Local anaesthetic activity of (+)-and (-)-menthol. Planta Med 2001;67(2):174-6.
[Crossref] [Google Scholar] [PubMed]
- Singh R, Shushni MA, Belkheir A. Antibacterial and antioxidant activities of Mentha piperita L. Arab J Chem 2015;8(3):322-8.
- Pai SA, Gagangras SA, Kulkarni SS, Majumdar AS. Potential of ozonated sesame oil to augment wound healing in rats. Indian J Pharm Sci 2014;76(1):87-92.
[Google Scholar] [PubMed]
- Chiang JP, Hsu DZ, Jui-Chen T, Sheu HM, Liu MY. Effects of topical sesame oil on oxidative stress in rats. Altern Ther Health Med 2005;11(6):40-5.
[Google Scholar] [PubMed]
- Jayanthi MK, Dhar M, Jayanthi M. Anti-inflammatory effects of Allium sativum (garlic) in experimental rats. Biomedicine 2011;31(1):84-9.
- Jayanthi MK, Jyoti M. Experimental animal studies on analgesic and anti-nociceptive activity of Allium sativum (garlic) powder. Indian J Res Rep Med Sci 2012;2(1):1-6.
- Singh G, Chaturvedi GN. Experimental study on anticoagulant and fibrinolysis activities of single clove garlic (Allium ascalonicum). J Ayurveda Physicians Surg 2016;3(2).
- Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med 2000;28(8):1303-12.
[Crossref] [Google Scholar] [PubMed]
- Gupta B, Ghosh B. Curcuma longa inhibits TNF-α induced expression of adhesion molecules on human umbilical vein endothelial cells. Int J Immunopharmacol 1999;21(11):745-57.
[Crossref] [Google Scholar] [PubMed]
- Saja K, Babu MS, Karunagaran D, Sudhakaran PR. Anti-inflammatory effect of curcumin involves downregulation of MMP-9 in blood mononuclear cells. Int Immunopharmacol 2007;7(13):1659-67.
- Costantini A, de Bernardi T, Gotti A. Clinical and capillaroscopic evaluation of chronic uncomplicated venous insufficiency with procyanidins extracted from Vitis vinifera. Minerva Cardioangiol 1999;47(1-2):39-46.
[Google Scholar] [PubMed]
- Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem 2001;73(3):285-90.
- Mandic AI, Đilas SM, Ćetković GS, Čanadanović-Brunet JM, Tumbas VT. Polyphenolic composition and antioxidant activities of grape seed extract. Int J Food Prop 2008;11(4):713-26.
- Pientaweeratch S, Panapisal V, Tansirikongkol A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm Biol 2016;54(9):1865-72.
[Crossref] [Google Scholar] [PubMed]
- Fujii T, Wakaizumi M, Ikami T, Saito M. Amla (Emblica officinalis Gaertn.) extract promotes procollagen production and inhibits matrix metalloproteinase-1 in human skin fibroblasts. J Ethnopharmacol 2008;119(1):53-7.
[Crossref] [Google Scholar] [PubMed]
- Muthuraman A, Sood S, Singla SK. The antiinflammatory potential of phenolic compounds from Emblica officinalis L. in rat. Inflammopharmacology 2011;19(6):327-34.
[Crossref] [Google Scholar] [PubMed]
- Schorr K, Rott A, da Costa F, Merfort I. Optimisation of a human neutrophil elastase assay and investigation of the effect of sesquiterpene lactones. Biologicals 2005;33(3):175-84.
[Crossref] [Google Scholar] [PubMed]
- Raffetto JD, Khalil RA. Ca2+−dependent contraction by the saponoside escin in rat vena cava: Implications in venotonic treatment of varicose veins. J Vasc Surg 2011;54(2):489-96.
[Crossref] [Google Scholar] [PubMed]
- Sibilia V, Pagani F, Mrak E, Dieci E, Tulipano G, Ferrucci F. Pharmacological characterization of the ghrelin receptor mediating its inhibitory action on inflammatory pain in rats. Amino Acids 2012;43(4):1751-9.
[Crossref] [Google Scholar] [PubMed]
- Zambelli VO, Chioato L, Gutierrez VP, Ward RJ, Cury Y. Structural determinants of the hyperalgesic activity of myotoxic Lys49-phospholipase A2. J Venom Anim Toxins Incl Trop Dis 2017;23:7.
[Crossref] [Google Scholar] [PubMed]
- García-Mesaa MT, Alfonso-Valiente MA, Armenteros-Herrera DM. Antiedemagenic activity of hydroalcoholic extracts of citrus plant fruit peels and leaves in rat skin. Pharmacologyonline 2017;2:55-9.
- Matsuda H, Li Y, Murakami T, Ninomiya K, Araki N, Yoshikawa M, et al. Antiinflammatory effects of escins Ia, Ib, IIa and IIb from horse chestnut, the seeds of Aesculus hippocastanum L. Bioorg Med Chem Lett 1997;7(13):1611-6.
- Plotnikov M, Ivanov I, Sidehmenova A, Aliev O, Tykavkina N. Influence of antioxidant complex on the adhesion of leukocytes in chronic venous insufficiency of lower limbs in rats. Indian J Pharmacol 2012;44(6):710.
[Crossref] [Google Scholar] [PubMed]
- Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: Concept of ayurveda. Pharmacogn Rev 2014;8(16):73.
[Crossref] [Google Scholar] [PubMed]
- Budzyn-Napierala M, Iskra M, Krasinski Z, Turkiewicz W, Gryszczynska B, Kasprzak M, et al. Altered elastase—alpha1-antitrypsin balance in the blood of patients with chronic venous disease. Phlebology 2016;31(2):125-32.
[Crossref] [Google Scholar] [PubMed]
- Shields DA, Andaz SK, Sarin S, Scurr JH, Smith PC. Plasma elastase in venous disease. Br J Surg 1994;81(10):1496-9.
[Crossref] [Google Scholar] [PubMed]
- Mansilha A, Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci 2018;19(6):1669.
[Crossref] [Google Scholar] [PubMed]
- Mortimer PS, Pearson IC. Lymphatic function in severe chronic venous insufficiency. Phlebolymphology 2004;44:253-7.
- Incandela L, Cesarone MR, Cacchio M, de Sanctis MT, Santavenere C, D'Auro MG, et al. Total triterpenic fraction of Centella asiatica in chronic venous insufficiency and in high-perfusion microangiopathy. Angiology 2001;52(2):S9-13.
[Crossref] [Google Scholar] [PubMed]
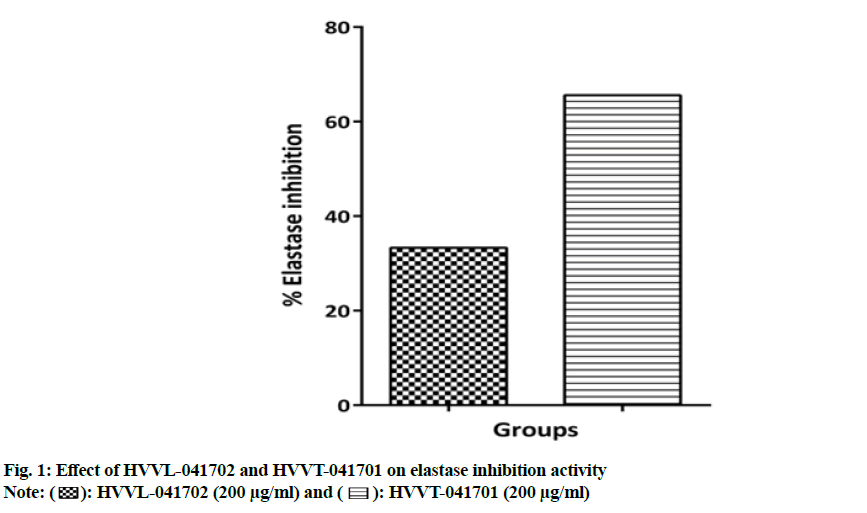
 ): HVVL-041702 (200 μg/ml) and (
): HVVL-041702 (200 μg/ml) and ( ): HVVT-041701 (200 μg/ml) .
): HVVT-041701 (200 μg/ml) .