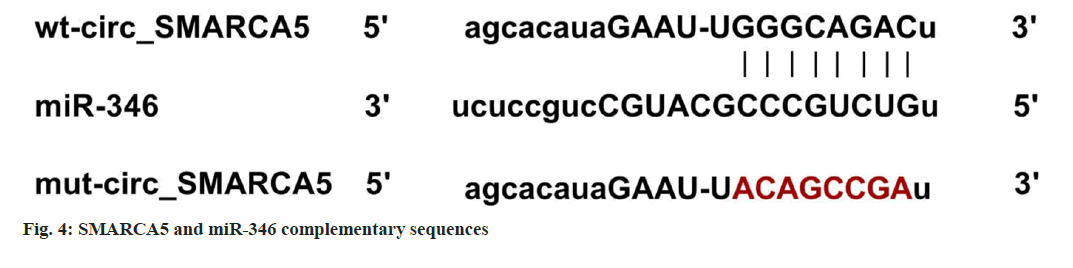- *Corresponding Author:
- Fuyu Qiao
Department of Otolaryngology-Head and Neck surgery, The Second Affiliated Hospital of The Army Medical University, Fengdu, Chongqing 400084, China
E-mail: Qiaofuyu6226@163.com
| Date of Received | 20 August 2022 |
| Date of Revision | 12 June 2023 |
| Date of Acceptance | 06 March 2024 |
| Indian J Pharm Sci 2024;86(2):448-457 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To explore the effect of houttuyn berberine on the biological behavior of nasopharyngeal carcinoma cells by regulating circ-SMARCA5/microRNA-346 pathway. Nasopharyngeal carcinoma cells C666-1 were divided into control group, houttuyn berberine (1, 2, 4 μg/ml) group, plasmid cloning deoxyribonucleic acid group-circSMARCA5 group, houttuyn berberine+si-circ-SMARCA5 group, and houttuyn berberine+microRNA-346 group. Real-time quantitative polymerase chain reaction detected the expression levels of circ-SMARCA5 and microRNA-346. Cell counting kit, plate cloning experiment, flow cytometry, wound healing assays and Transwell assays were performed to assess the cell viability, clone formation, apoptosis, migration and invasion ability of C666-1. The targeting relationship between circ-SMARCA5 and microRNA-346 was verified with the aid of dual luciferase experiment. In comparison with the control group, the cell viability of C666-1 cells, microRNA-346 expression, wound healing rate, clone formation numbers, invasive numbers in the houttuyn berberine (1, 2, 4 μg/ml) group were increased, and the expression of circ-SMARCA5 and apoptosis rate were increased (p<0.05). Compared with plasmid cloning deoxyribonucleic acid group, the cell viability of C666-1 cells, wound healing rate, microRNA-346 expression in the plasmid cloning deoxyribonucleic acidcirc-SMARCA5 group were reduced, the apoptosis rate was increased, invasive numbers and clone formation numbers were reduced (p<0.05). Circ-SMARCA5 directly bound to microRNA-346. Compared with the houttuyn berberine group, the cell viability of C666-1 cells, clone formation numbers and invasive numbers were increased in the houttuyn berberine+si-circ-SMARCA5 group and houttuyn berberine+microRNA-346 group, the apoptotic rate was decreased, and the wound healing rate was increased (p<0.05). Houttuyn berberine could inhibit nasopharyngeal carcinoma cells’ malignant proliferation, migration and invasion, and induce cell apoptosis. The mechanism is associated with the up-regulation of circ-SMARCA5/microRNA-346 pathway.
Keywords
Houttuyn berberine, nasopharyngeal carcinoma, circ-SMARCA5, microRNA-346, cell proliferation, apoptosis, migration, invasion
Originating from the nasopharynx, Nasopharyngeal Carcinoma (NPC) is a common tumor occurring in the head and neck. Genetic, environmental, dietary factors and Epstein Barr virus infection are closely associated with the occurrence of NPC. At present, chemotherapy combined with radiotherapy is the most commonly used therapy for NPC, which can improve the survival rate of patients, but there are still problems such as serious side effects, drug resistance, tumor recurrence, as well as metastasis[1]. Consequently, it is an emergency to find a more effective and safer treatment. Houttuyn berberine (α-Hydroxyl-β-Berberine decanoylethyl sulfonate) is an ion pair compound of berberine houttuynin, which was synthesized by Jia Benzhen of Hunan Academy of Chinese Medicine. Studies over the past decade have shown that houttuyn berberine exhibits effective anti-tumor activity in breast cancer and oral epithelial cancer by regulating cell proliferation, differentiation and apoptosis[2,3]. However, the regulatory mechanism and effect of houttuyn berberine on the biological behavior of NPC cells remain to be explored. Noncoding RNAs including circular Ribonucleic Acid (circRNA) and microRNAs (miRNA) have been confirmed to be dysregulated in tumor expression. CircRNAs have covalently closed loop structures, which indirectly regulate target messenger RNA (mRNA) expression and function by specifically binding miRNAs, and then participate in multiple tumor progression processes. Studies have reported that low expression of circRNA SMARCA5 (circ- SMARCA5) is related to poor overall survival in gastric cancer patients[4]. Overexpression of circ-SMARCA5 decreased non-small lung cancer cells’ migration and proliferation abilities[5]. Bioinformatics software predicted that circ- SMARCA5 and miR-346 had complementary binding sites. Based on previous studies, we can see that the expression of miR-346 is upregulated in NPC tissues, and the high expression of miR- 346 promotes both the migration and invasion of NPC cells, and promotes the progression of NPC[6]. However, whether circ-SMARCA5 targets miR-346 to participate in the progression of NPC remains unclear. Based on the circ-SMARCA5/ miR-346 pathway, this paper discusses the mechanism of houttuyn berberine affecting NPC cells’ apoptosis, proliferation, migration as well as invasion.
Materials and Methods
Experimental materials:
C666-1, human NPC cell, was purchased from Wuxi Xinrun Biology Science and Technology Co., Ltd., houttuyn berberine (purity 98 %) was purchased from Hunan Academy of Chinese Medicine; the one-step miRNA reverse transcription kit and Cell Counting Kit-8 (CCK-8) were purchased from Beijing Biolabs Technology Co., Ltd., luciferase reporter vector, plasmid empty vector (plasmid cloning Deoxyribonucleic Acid (pcDNA)), high expression plasmid of circ-SMARCA5 (pcDNAcirc- SMARCA5), small interfering RNA of circ- SMARCA5 (si-circ-SMARCA5), negative control of miRNA mimics (miR-NC), miR-346 mimics were purchased from Sangon Biotech (Shanghai) Co., Ltd., Propidium Iodide (PI) apoptosis detection kit/ annexin V-Fluorescein Isothiocyanate (FITC) was purchased from Beyotime Biotech Inc., Shanghai; Lipofectamine 2000 and TRIzol reagent were purchased from Invitrogen, USA; SYBR® Premix ExTaq and PrimeScript reverse transcription kit were purchased from Takara Biology, Dalian; Transwell chamber was purchased from BD, United States of America (USA); rabbit polyclonal antibody against cleaved caspase-3, goat antirabbit Immunoglobulin G (IgG) secondary antibody, as well as rabbit polyclonal antibody against Glyceraldehyde Phosphate Dehydrogenase (GAPDH) were purchased from Abcam, USA.
Methods:
Cell culture, transfection and grouping: C666- 1 cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium at 37° in a 5 % Carbon dioxide (CO2) incubator. In the 3rd generation, C666-1 cells were seeded into 6-well plates (2×105/well), pcDNA-circ-SMARCA5, sicirc- SMARCA5 and pcDNA were transfected into 50 % of the fusion cells using Lipofectamine 2000, and after 48 h of culture. The transfection effect was detected by Reverse Transcription-quantitative Polymerase Chain Reaction (RT-qPCR). To study the effect of houttuyn berberine on C666-1 cells’ biological behavior, C666-1 cells were incubated with medium containing 0, 1, 2 and 4 μg/ml of houttuyn berberine, respectively, for 24 h, which were recorded as the control group, houttuyn berberine 1 μg/ml group, houttuyn berberine 2 μg/ml group, houttuyn berberine 4 μg/ml group, and the concentration of houttuyn berberine with better anti-tumor effect (4 μg/ml) was chosen for experiment. To explore the effect of circ-SMARCA5 on C666-1 cells’ biological behavior, pcDNAcirc- SMARCA5 and pcDNA were transfected into C666-1 cells, respectively, and were recorded as pcDNA-circ-SMARCA5 group and pcDNA group; to explore whether houttuyn berberine regulates the circ-SMARCA5/miR-346 pathway and affects C666-1 cells’ biological behavior, C666-1 cells transfected with si-circ-SMARCA5 and miR- 346 mimics were incubated with culture medium containing 4 μg/ml of houttuyn berberine for 24 h and were recorded as houttuyn berberine group, houttuyn berberine+si-circ-SMARCA5 group and houttuyn berberine+miR-346 group, respectively.
Evaluation of cell viability by CCK-8 method: The 5×103 C666-1 cells transfected with pcDNA,pcDNA-circ-SMARCA5, si-circ-SMARCA5 or C666-1 cells untransfected were seeded into 96-well plates. The cells were incubated with corresponding doses of houttuyn berberine according to the experimental groups for 24 h. Then 10 μl CCK-8 was added to each well. Moreover, cells were incubated for another 2 h, and the optical density at 450 nm was measured with a spectrophotometer.
Evaluation of cell cloning ability by plate cloning experiment: The transfected NPC cells were cultured for 24 h and made into cell suspension. Later, cell suspension was inoculated into 6-well plates at an appropriate amount for further culture. The culture was terminated when visible clones appeared on the culture plate at room temperature. Cells were fixed with paraformaldehyde for 10 min and then stained with crystal violet (0.4 %) for 20 min. Cells were counted and photographed under a microscope.
Flow cytometry analysis of apoptosis: At 500 μl binding buffer, resuspended C666-1 cells in each group of cell culture, and use 5 μl Annexin V/FITC and PI dark staining for 15 min. Apoptosis was determined by flow cytometry.
Determination of cleaved caspase-3 protein expression by Western blot: Applying Radio- Immunoprecipitation Assay (RIPA) lysis buffer, C666-1 cells were incubated to extract total protein. RIPA lysis buffer contains protease inhibitors. After denaturation, 30 μg of each protein sample were taken. Proteins were separated by polyacrylamide gel electrophoresis for 100 min. Next, the starter was transferred to the polyvinylidene difluoride membrane by the wet transfer method and placed overnight at 20 mA. It was treated with 5 % skim milk at room temperature for 2 h. Polyvinylidene difluoride membranes were probed for 2 h at room temperature with a primary antibody (rabbit anticleaved caspase-3), and GAPDH as an internal control and then treated with secondary antibody for 1 h at room temperature. The target protein was visualized by chemiluminescence, photographed by gel imaging system, and the relative expression of cleaved caspase-3 protein was analyzed by Quantity One® software.
Measurement of cell migration by wound healing assay: C666-1 cells were trypsinized and dispersed in RPMI-1640 (10 % fetal bovine serum) (1×104 cells). The cell suspension was seeded in 6-well plates at a concentration of 1 ml/well. A straight line scratch was made on the cell surface with a sterile 1 ml pipette tip after incubation at 37° with 5 % CO2 for 24 h. Then the liquid in each well was discarded and the initial scratch distance was measured. Fresh RPMI-1640 (10 % fetal bovine serum) was added to each well, and the cells were incubated at 37° and 5 % CO2 for 24 h. After discarding the liquid, measure the final scratch distance.
Final scratch distance=(Initial scratch distancefinal scratch distance)/initial scratch distance×100 %
Measurement of cell invasion by Transwell assay: C666-1 cells were trypsinized and dispersed in serum-free RPMI-1640 (1×105 cells/ml). Transwell insert containing 500 μl cell suspension (pore size 8 μm, pre-coated with Matrigel) was inserted into 24-well plates. 600 μl RPMI-1640 containing 10 % fetal bovine serum was added to the bottom of the 24-well plate. Cells were stored at 37° and 5 % CO2 for 24 h. After that, the Transwell insert was removed. Non-invading cells were scraped off with cotton swabs. The cells were fixed with formaldehyde for 20 min and then stained in 0.1 % crystal violet for 10 min. In virtue of microscopy, invading cells were observed and counted. Migration was similar to invasion experiments, except that the Transwell inserts used were not pre-coated with matrigel.
Real-time quantitative PCR detection of circ- SMARCA5 and miR-346 expression: C666-1 cell RNA was isolated by TRIzol reagent based on the operating instructions. Complementary DNA (cDNA) was synthesized by employing one-step miRNA reverse transcription kit and PrimeScript reverse transcription kit. Using SYBR® Premix ExTaq quantifies mRNA expression. U6 was used as the internal reference of miRNA, GAPDH was used as the internal reference of circRNA, and the relative expression levels of circ-SMARCA5 and miR-346 were calculated by 2-ΔΔCt method. Circ-SMARCA5 (186 bp) upstream 5'-AGATGGG CGAAAGTTCACTTAGAAA-3', downstream 5'-TCTTGCCTGCTGA GTTGAGTATATC-3'; GAPDH (262 bp), upstream 5'-C AGGAGGCATTGCTGATGAT-3', downstream 5'- GAAGGCTGGGGCTCATTT-3'; miR-346 (106 bp), upstream 5'-ACACTCCAG CTGGGTGTCTG CCTGAGTGCCT-3', downstream 5'-CTCAAC TGGTGTCGT GGAGTCGG CAATTCAGTTG AGAGAGGCAG-3'; U6 (156 bp), upstream 5'-CTCGCTTCGGCAGCACA-3' and downstream 5'-AACGCTTCACGAATTTGCGT-3'.
Luciferase reporter assay confirms the relationship between miR-346 and circ- SMARCA5: Form StarBase prediction, a binding site was between circ-SMARCA5 and miR- 346. The Wild-Type (WT) sequence containing miR-346 binding site circ-SMARCA5 or Mutant (MUT) sequence without miR-346 binding site was inserted into pmirGlo™ vector and named as wt-circ-SMARCA5, mut-circ-SMARCA5. C666-1 cells were co-transfected with or miR-NC, miR- 346 mimics and WT-circ-SMARCA5, MUT-circ- SMARCA5. After 48 h, the cells were lysed, and using dual luciferase reporter assay kit, the luciferase activity of different transfection groups was detected.
Statistical methods:
The experimental results that conform to the normal distribution are expressed as x±s. Statistical Package for the Social Sciences (SPSS) 18.0 software was applied for statistical analysis. The t-test was used for two group comparisons, while one-way Analysis of Variance (ANOVA) and Student–Newman–Keuls (SNK)-q test were used for multiple group comparisons. p<0.05 indicates statistically significant difference.
Results and Discussion
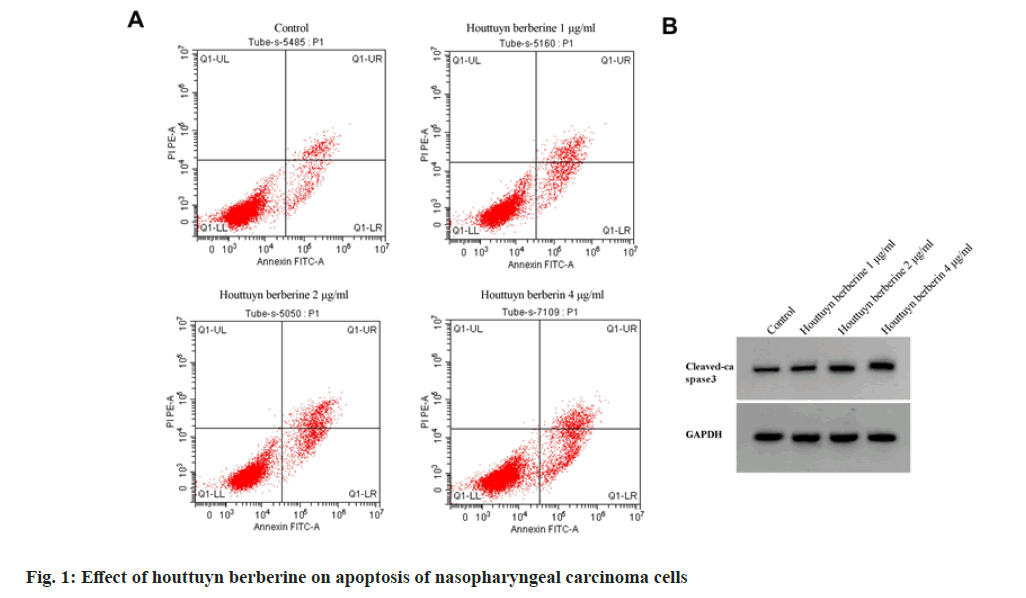
In comparison with the control group, the cell viability decreased, the number of clone formation decreased, the apoptosis rate and the expression of cleaved caspase-3 protein increased (p<0.05) in houttuyn berberine (1, 2, 4 μg/ml) group. The inhibitory effect of houttuyn berberine on the viability and clone formation of C666-1 cells and the promoting effect on the expression of cleaved caspase-3 protein and apoptosis were enhanced with the increase of concentration as shown in fig. 1 and Table 1.
| Group | OD value | Clone formation numbers | Apoptosis rate % | Cleaved caspase-3 |
|---|---|---|---|---|
| Control | 1.15±0.07 | 128.67±3.30 | 8.03±0.29 | 0.16±0.01 |
| Houttuyn berberine 1 μg/ml | 0.94±0.04a | 105.67±3.09a | 13.66±0.44a | 0.34±0.02a |
| Houttuyn berberine 2 μg/ml | 0.72±0.03ab | 86.00±1.63ab | 17.57±0.57ab | 0.51±0.04ab |
| Houttuyn berberine 4 μg/ml | 0.48±0.02abc | 64.00±0.82abc | 21.59±0.70abc | 0.68±0.05abc |
| F | 127.628 | 384.528 | 366.937 | 130.152 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared with the control group, ap<0.05; compared with 1 μg/ml houttuyn berberine group, bp<0.05 and compared with 2 μg/ml houttuyn berberine group, cp<0.05
Table 1: Houttuyn Berberine Inhibits Proliferation and Induces Apoptosis of Nasopharyngeal Carcinoma Cells (x±s, n=3)
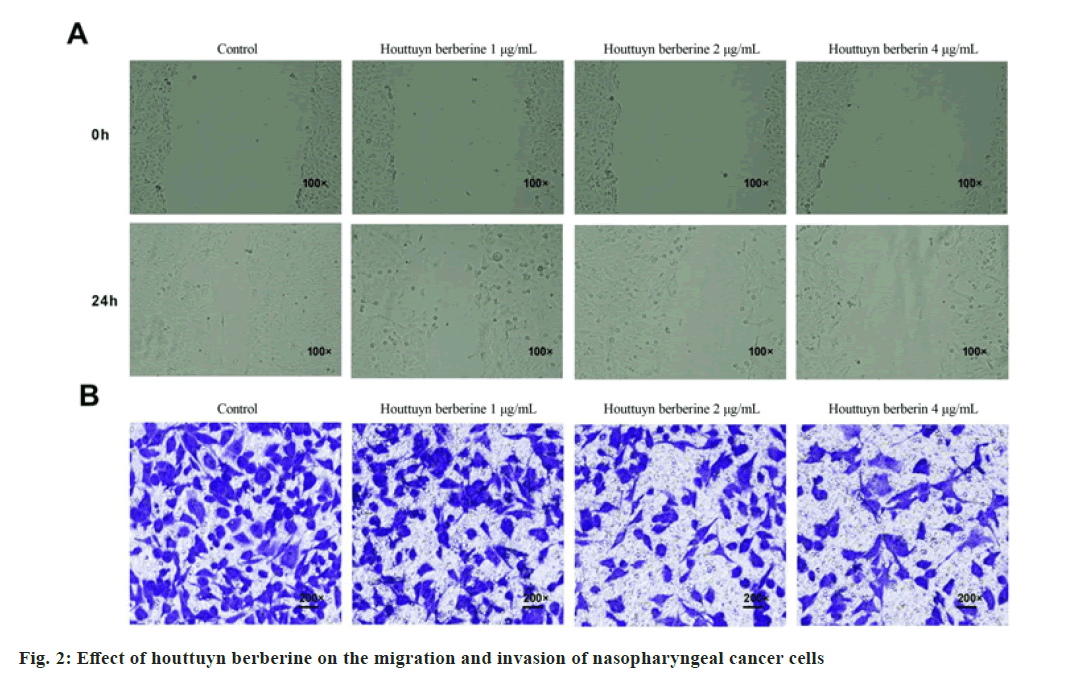
In comparison with the control group, the wound healing rate of C666-1 cells and invasion numbers decreased in the houttuyn berberine (1, 2, 4 μg/ ml) group (p<0.05). With the increase of the concentration of houttuyn berberine, its inhibitory effect on C666-1 cells’ migration and invasion gradually enhanced as shown in fig. 2 and Table 2.
| Group | Wound healing rate % | Invasive numbers |
|---|---|---|
| Control | 61.70±2.30 | 167.33±4.78 |
| Houttuyn berberine 1 μg/ml | 48.77±1.11a | 138.33±2.87a |
| Houttuyn berberine 2 μg/ml | 35.21±1.07ab | 98.00±2.45ab |
| Houttuyn berberine 4 μg/ml | 25.42±0.80abc | 72.67±1.25abc |
| F | 362.355 | 548.185 |
| p | 0.000 | 0.000 |
Note: Compared with the control group, ap<0.05; compared with 1 μg/ml houttuyn berberine group, bp<0.05 and compared with 2 μg/ml houttuyn berberine group, cp<0.05
Table 2: Houttuyn Berberine Inhibits The Migration and Invasion of Nasopharyngeal Carcinoma Cells
The expression of miR-346 decreased and the expression of circSMARCA5 of C666-1 increased in the houttuyn berberine (1, 2, 4 μg/ml) group (p<0.05) when comparing with the control group. The effect of houttuyn berberine on the expression of circSMARCA5 and miR-346 in C666-1 cells was enhanced with the increase of concentration as shown in Table 3.
| Group | circ-SMARCA5 | miR-346 |
|---|---|---|
| Control | 1.00±0.00 | 1.00±0.00 |
| Houttuyn berberine 1 μg/ml | 1.67±0.07a | 0.69±0.04a |
| Houttuyn berberine 2 μg/ml | 2.31±0.07ab | 0.38±0.02ab |
| Houttuyn berberine 4 μg/ml | 3.66±0.08abc | 0.14±0.01abc |
| F | 952.642 | 798.238 |
| p | 0.000 | 0.000 |
Note: Compared with the control group, ap<0.05; compared with 1 μg/ml houttuyn berberine group, bp<0.05 and compared with 2 μg/ml houttuyn berberine group, cp<0.05
Table 3: Detection of Expression of circ-SMARCA5 and miR-346
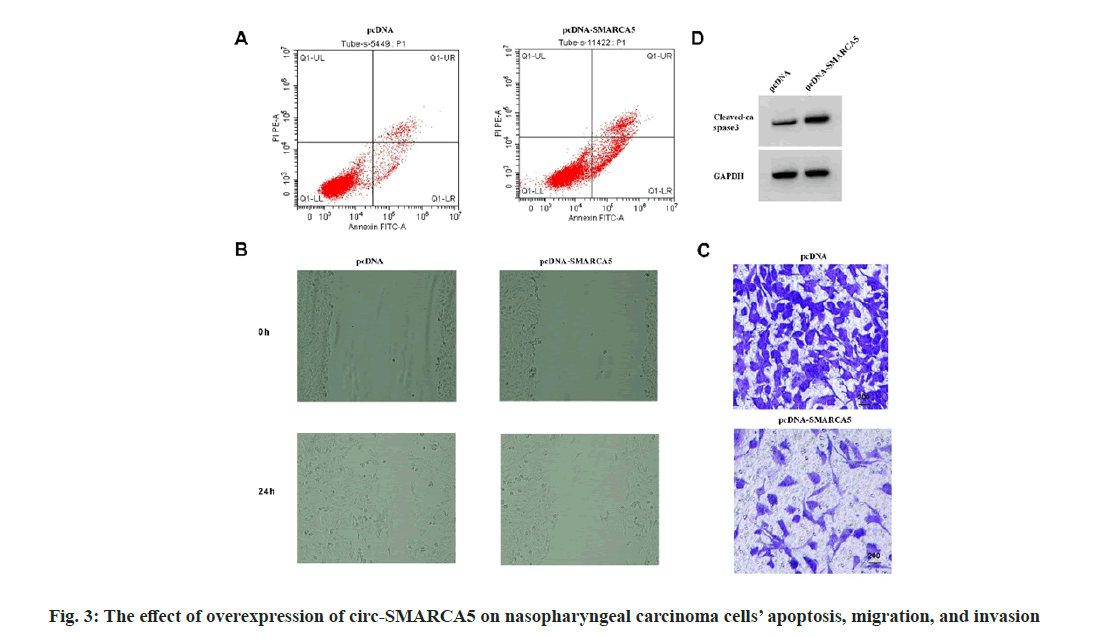
Compared with the pcDNA group, the expression level of circ-SMARCA5 in C666-1 cells increased, the cell viability decreased, the expression of cleaved caspase-3 protein and apoptosis rate increased, the wound healing rate and miR-346 expression decreased, and the invasion numbers and clone formation decreased in the pcDNA-circ- SMARCA5 group (p<0.05) as shown in fig. 3 and Table 4.
| Group | circ-SMARCA5 | miR-346 | OD value | Clone formation numbers | Apoptosis rate % | Cleaved caspase-3 | Wound healing rate % | Invasive numbers |
|---|---|---|---|---|---|---|---|---|
| pcDNA | 1.00±0.00 | 1.00±0.00 | 1.15±0.07 | 129.33±4.50 | 8.07±0.39 | 0.15±0.01 | 61.89±2.28 | 167.67±3.68 |
| pcDNA-circ-SMARCA5 | 4.36±0.08a | 0.05±0.01a | 0.37±0.03a | 52.33±0.47a | 24.57±0.97a | 0.79±0.04a | 22.49±0.79a | 56.00±0.82a |
| t | 72.746 | 164.545 | 17.739 | 29.477 | 27.336 | 26.885 | 28.281 | 51.301 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: Compared to the pcDNA group, ap<0.05
Table 4: Overexpression of circ-SMARCA5 Induces apoptosis and Blocks Nasopharyngeal Carcinoma Cells’ Migration, Invasion, and Proliferation
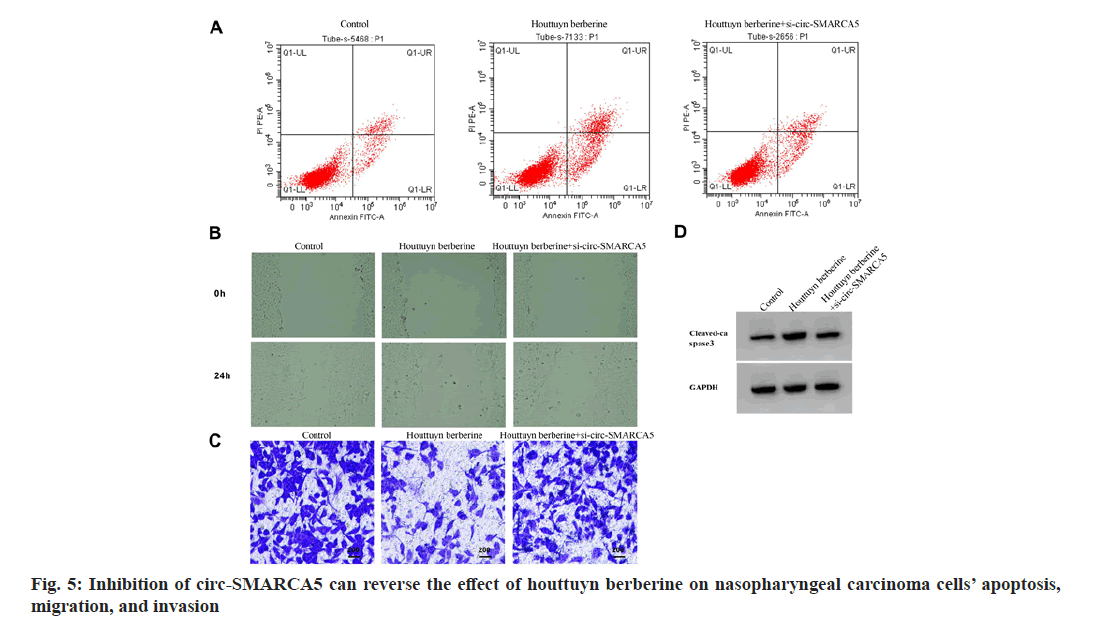
Starbase predicted that there was a targeted binding site between circ-SMARCA5 and miR-346, see fig. 4. In addition, co-transfection with miR- 346 mimics could inhibit the relative luciferase activity of C666-1 cells transfected with WT-circ- SMARCA5 (p<0.05), while the cells transfected with mut-circ-SMARCA5 had no change, as shown in Table 5.
| Group | Wt-circ-SMARCA5 | Mut-circ-SMARCA5 |
|---|---|---|
| miR-NC | 0.99±0.08 | 1.00±0.09 |
| miR-346 | 0.13±0.01a | 0.95±0.08 |
| t | 18.476 | 0.719 |
| p | 0.000 | 0.512 |
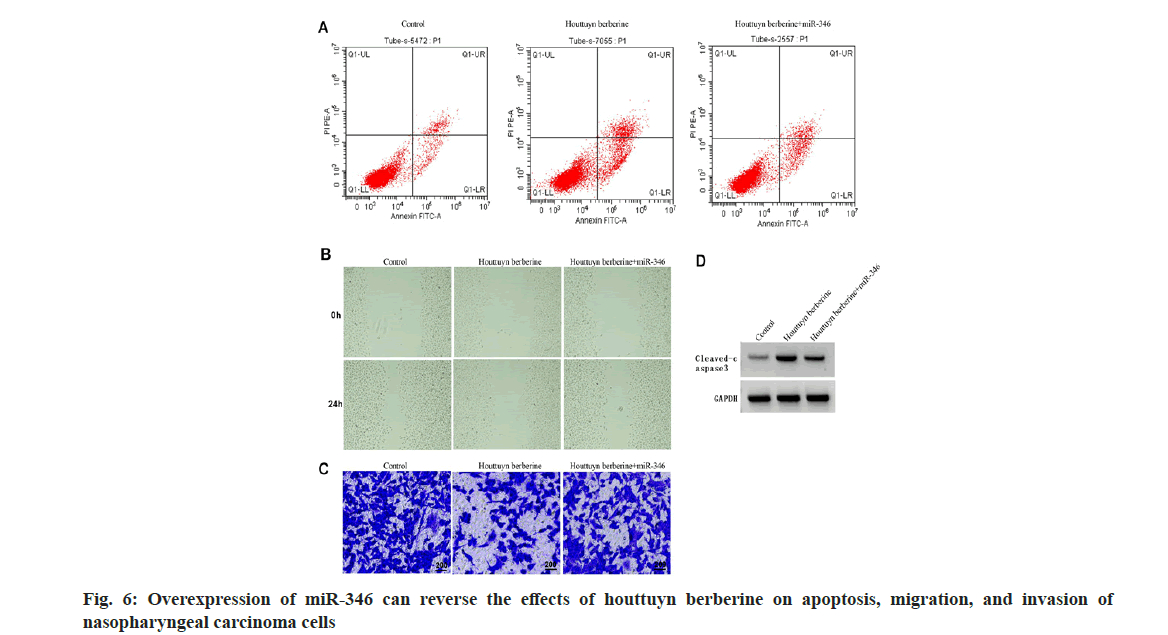
Note: Compared to the miR-NC group, ap<0.05 Table 5: Double Luciferase Report Experiment Note: Compared with the control group, ap<0.05 and compared with the houttuyn berberine group, bp<0.05 Table 6: Inhibition of circ-SMARCA5 Can Reverse the effects of Houttuyn Berberine on Nasopharyngeal Carcinoma Cells’ Proliferation, Apoptosis, Migration and Invasion By comparing with the control group, the expression of circ-SMARCA5 increased, the expression of miR-346 decreased, the cell viability decreased, the clone formation numbers and invasion numbers decreased, the expression of cleaved caspase-3 protein and apoptosis rate increased, and the wound healing rate decreased in the houttuyn berberine group (p<0.05); in comparison with the houttuyn berberine group, the expression of miR- 346 increased, the expression of circ-SMARCA5 decreased, the cell viability increased, the invasion numbers and clone formation numbers increased, the cleaved caspase-3 protein’s apoptosis rate and the expression decreased, and the wound healing rate increased in the houttuyn berberine+si-circ- SMARCA5 group (p<0.05) as shown in fig. 5 and Table 6. Compared with the houttuyn berberine group, the cell viability and wound healing rate increased (p<0.05), the apoptosis rate and cleaved caspase-3 protein level decreased (p<0.05) and the clone formation numbers and invasive cells increased (p<0.05) in the houttuyn berberine+miR-346 group, as shown in fig. 6 and Table 7. Note: Compared with the control group, ap<0.05 and compared with the houttuyn berberine group, bp<0.05 Table 7: Over expression of Mir-346 can reverse the Effects of Houttuyn Berberine on Nasopharyngeal Carcinoma Cells’ Proliferation, Apoptosis, Migration and Invasion This study first explored the effect of houttuyn berberine on NPC cells’ biological behavior. As per the results showed, houttuyn berberine increased C666-1 cells’ apoptosis rate in a dose-dependent manner, reduced cell viability and clone formation ability, which was consistent with the houttuyn berberine’s anti-tumor effect on liver cancer, gastric cancer and colon cancer cells reported by Peng[7]. Many factors are involved in the process of apoptosis, among which caspase-3 is considered to be the executive factor of apoptosis. Caspase-3 is activated under the stimulation of intracellular and extracellular signals to induce apoptosis. Studies have reported that silencing UBE4B can induce apoptosis of NPC cells by activating caspase-3[8]. According to this study, houttuyn berberine upregulated the expression level of cleaved caspase-3 in C666-1 cells, which was consistent with the pro-apoptotic effect of houttuyn berberine on C666-1 cells. In addition, houttuyn berberine could also inhibit the migration and invasion ability of C666-1 cells. These data indicate that houttuyn berberine can effectively inhibit the proliferation, migration as well as invasion of NPC cell C666- 1. However, the specific molecular mechanism remains to be studied. CircRNAs and miRNAs are considered as potential anti-tumor targets of Traditional Chinese Medicine (TCM) compounds, and Chi et al.[9] reported that matrine could induce autophagy and apoptosis in glioma cells by down-regulating circ_104075 expression. Sun et al.[10] reported that the proliferation can be inhibited and apoptosis of gastric cancer cells can be induced by curcumin, and its mechanism is associated with the upregulation of intracellular miR-34a expression. This study found that houttuyn berberine decreased the expression level of miR-670-5p in C666-1 cells but increased the expression level of circ-SMARCA5, suggesting that the anti-tumor function of houttuyn berberine may be related to the regulation of miR-670-5p and circ-SMARCA5. Circ-SMARCA5 is a negative regulator of cancer progression. It is reported that high expression of circ-SMARCA5 results in better overall survival of melanoma patients. However, overexpression of circ-SMARCA5 inhibits melanoma cell proliferation and promotes apoptosis[11]. The expression of circ-SMARCA5 decreased in liver cancer tissues. Restoring the expression level of circ-SMARCA5 promotes liver cancer cell apoptosis, inhibits liver cancer cell migration, invasion, as well as liver cancer progression[12,13]. Dysregulation of miRNA expression is associated with cell proliferation, metastasis, apoptosis and other tumor progression processes. Targeted binding of circRNA to miRNAs has been identified as the main mechanism by which circRNAs participate in regulating cancer progression[14,15]. It has been documented that circ-SMARCA5 inhibits miR-620 expression, induces cell cycle arrest, and reduces cervical cancer cell migration, invasion and proliferation[16]; circ-SMARCA5 has proapoptotic and anti-proliferative effects on nonsmall cell lung cancer cells by binding miR-670- 5p[17]. miR-346 plays a role as a tumor promoting factor in a variety of cancers. Studies have reported that miR-346 is up-regulated in NPC and breast cancer, and its down-regulation can hinder tumor cell invasion and migration[18]. LncRNA DGCR5 inhibits hepatocellular carcinoma progression by targeting miR-346[19]. This study confirmed that miR-346 is a target miRNA of circ-SMARCA5, and miR-346 expression is negatively regulated by circ-SMARCA5. Based on functional analysis, overexpression of circ-SMARCA5 could reduce the viability and clone formation, invasive and migratory abilities of C666-1 cells, and improve the apoptosis ability and the expression of cleaved caspase-3 protein, which was consistent with the anti-tumor effect of houttuyn berberine on C666- 1 cells. The recovery experiment showed that the effect of houttuyn berberine on the apoptosis, proliferation, invasion and migration of C666- 1 cells was reversed by inhibiting the expression of circ-SMARCA5, suggesting that the anti-NPC effect of houttuyn berberine is related to the increase of circ-SMARCA5/miR-346. In conclusion, houttuyn berberine plays an anticancer role in NPC progression by promoting the expression of circ-SMARCA5 and reducing the expression of miR-346, blocking NPC cells’ migration, invasion and proliferation, inducing apoptosis, which indicates that houttuyn berberine is a potential therapeutic drug for NPC and provides a potential target for molecular therapy of NPC. Author’s contributions: Dongbao Yang and Xiaodong Wen have contributed equally to this work. Conflict of interests: The authors declared no conflict of interests. [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed] [Crossref] [Google Scholar] [PubMed]
Group
circ-SMARCA5
miR-346
OD value
Clone formation numbers
Apoptosis rate %
Cleaved caspase-3
Wound healing rate %
Invasive numbers
Control
1.00±0.00
1.00±0.00
1.15±0.09
129.67±3.30
8.09±0.55
0.15±0.01
61.82±2.12
167.33±5.56
Houttuyn berberine
3.64±0.04a
0.13±0.01a
0.49±0.02a
63.33±1.25a
21.66±0.57a
0.69±0.05a
25.47±0.87a
73.00±0.82a
Houttuyn berberine+si-circ-SMARCA5
1.17±0.03b
0.78±0.04b
1.01±0.05b
112.00±2.94b
11.22±0.49b
0.24±0.02b
53.25±1.73b
146.33±3.68b
F
7859.640
1083.350
98.946
503.552
523.808
251.100
394.186
489.152
p
0.000
0.000
0.000
0.000
0.000
0.000
0.000
0.000
Group
miR-346
OD value
Clone formation numbers
Apoptosis rate %
Cleaved caspase-3
Wound healing rate %
Invasive numbers
Control
1.00±0.00
1.16±0.08
131.42±5.41
8.12±0.61
0.13±0.01
62.36±3.02
162.86±7.15
Houttuyn berberine
0.15±0.01a
0.47±0.03a
60.68±2.33a
22.14±0.96a
0.68±0.06a
23.49±1.11a
74.61±3.11a
Houttuyn berberine+miR-346
0.84±0.06b
1.03±0.06b
119.58±4.06b
12.34±0.57b
0.22±0.02b
50.91±2.37b
135.29±4.28b
F
496.297
111.000
252.446
287.664
191.049
224.852
231.888
p
0.000
0.000
0.000
0.000
0.000
0.000
0.000
References