- *Corresponding Author:
- Yang Cheng
Institute of Liver Diseases, Shuguang Hospital Ailiated to Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
E-mail: drchengyang@163.com
| Date of Received | 25 January 2023 |
| Date of Revision | 17 October 2023 |
| Date of Accepted | 03 May 2024 |
| Indian J Pharm Sci 2024;86(3):1058-1068 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Huangqi decoction, one of the traditional Chinese medicine prescriptions, has been found useful for the treatment of liver fibrosis. This study focused on investigating Huangqi decoction serum effect on growth, migration and angiogenesis of rat liver sinusoidal endothelial cells. We administered Huangqi decoction (20 mg/100 g) into Sprague-Dawley rats orally once daily for 3 d consecutively. Then, Huangqi decoction serum of rats was obtained. Initial Huangqi decoction serum was considered a high concentration. The initial Huangqi decoction serum was diluted one time and three times respectively to create a medium and low concentration. Liver sinusoidal endothelial cells were isolated from Sprague-Dawley rats, and the Huangqi decoction serum was obtained. Liver sinusoidal endothelial cells without any treatment were used as control. Liver sinusoidal endothelial cells treated by vascular endothelial growth factor A (40 ng/ml) served as vascular endothelial growth factor A group. Huangqi decoction serum hindered protein kinase B and mammalian target of rapamycin protein phosphorylation and microtubule-associated protein light chain 3 II/microtubule-associated protein light chain 3 I and Beclin-1 protein expression in rat liver sinusoidal endothelial cells (p<0.01). SC79 and 3MA treatment reversed high-concentration of Huangqi decoction serum inhibition on liver sinusoidal endothelial cells proliferation, migration, angiogenesis, and promotion on autophagy in rat liver sinusoidal endothelial cells (p<0.01). Huangqi decoction serum hindered proliferation and migration of rat liver sinusoidal endothelial cells via angiogenesis inhibition by promoting protein kinase B/mammalian target of rapamycin-dependent autophagy. Huangqi decoction might be applied to be an effective drug to treat patients' liver fibrosis in the future.
Keywords
Huangqi decoction, protein kinase B/mammalian target of rapamycin pathway, nitric oxide, endothelin-1, light chain 3
Liver Sinusoidal Endothelial Cells (LSECs), the endothelial cells with high specialization degree, can generate liver sinusoids wall[1,2]. LSECs account for around 15 %-20 % of liver cells, however, just about 3 % of total liver volume[3]. LSECs have a special morphology and organization, which typically manifest as a porous-like fenestration cell membrane structure and the endothelium without basement membrane[4,5]. The special structure of LSECs has important functions, such as the transfer of nutrients (including lipids and lipoproteins) [6]. However, under pathological conditions, the continuous thickening, defenestration, and basement membrane formation of LSECs lead to hepatic sinusoidal capillaries[7]. Hepatic sinusoidal capillaries may induce hepatocyte damage, such as enhancement of damage by oxygen free radicals, reduction of oxygen and nutrient uptake, obstacles to the free exchange of blood components between liver cells and liver sinusoids, and disorders of lipid metabolism and endocrine function. Eventually, this damage results in the occurrence of fatty liver and stimulate the process of liver fibrosis caused by fatty liver[8-10]. Moreover, as shown in some studies, sinusoidal capillarization occurs before liver fibrosis caused by different hepatopathies, which include nonalcoholic fatty liver disease[11,12]. Therefore, preventing the proliferation and angiogenesis of LSECs is greatly significant for treating liver fibrosis.
Huangqi Decoction (HQD), the traditional Chinese medicine, consists of Astragalus (Astragalus membranaceus Bunge (Fabaceae)) and licorice (Glycyrrhiza glabra Linn (Fabaceae)). Studies have indicated that HQD exerts a prominent role in treating liver fibrosis[13-15]. Liu et al.[16] discovered that HQD makes a protective impact against liver fibrosis. Moreover, Astragalus polysaccharides promote the fenestration structure of LSECs[17]. Nevertheless, there is still a lack of deep understanding of its internal mechanisms. Recently, Yang et al.[18] explored a novel method involving the use of HQD serum to investigate its effect on nephrotic syndrome. A similar method has also been used in diabetic retinopathy research[19]. Thus, this study mainly concentrated on the mechanism by which HQD serum regulates angiogenesis in LSECs. Previous study had revealed that, pathological angiogenesis was a main event during liver fibrosis occurrence and autophagy inhibition in LSECs could suppress the pathological angiogenesis[20]. Therefore, this research investigated whether HQD serum mediates angiogenesis via regulating autophagy in LSECs. The goal of this study was to discover a novel strategy and a theoretical basis for liver fibrosis treatment with HQD serum.
Materials and Methods
Preparation of HQD:
HQD, a mixture of Astragalus root and licorice root, was prepared at a weight ratio of 5:1 and boiled for a 40 min period within water. Then, this resultant aqueous extract was subjected to dry spraying to collect the powder. The powder was preserved at -20°. Before use, purified water was added to dissolve the powder at 2 g in 100 ml
Preparation of HQD serum:
A total of 20 w, 8 w old Sprague-Dawley (SD) rats (weighing 180-220 g) were provided by Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai, China). Animal studies gained approval from Ethics Committee of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine. Rats were maintained in a room at 22° (12 h/12 h light/ dark cycle) and allowed to eat food and drink water freely.
The rats were selected randomly and administered HQD at a concentration of 20 mg/100 g. The remaining 10 rats were administered purified water at the same volume. The administration was performed once daily for 3 d consecutively. On d 3, blood samples from each rat were gathered from the abdominal aorta 2 h following the last administration. Blood samples were centrifuged to collect serum. The serum was subjected to filter sterilization and then preserved at -80° until use.
Isolation of rat LSECs:
We bought male SD rats (n=3, 8 w old, average weight of 195 g) in Shanghai Experimental Animal Center, Chinese Academy of Sciences (Shanghai, China). The involved animal studies gained approval from Ethics Committee of Shuguang Hospital affiliated to Shanghai University of Traditional Chinese Medicine.
We first separated primary LSECs in the 3 male SD rats according to the previous description[12]. LSECs were cultivated within Dulbecco’s Modified Eagle Medium (DMEM) which included 10 % Fetal Bovine Serum (FBS) and 1 % double antibiotics (penicillin and streptomycin) at 37° and 5 % Carbon dioxide (CO2). DMEM, FBS, penicillin, and streptomycin were provided by Solarbio (Beijing, China).
LSECs treatment:
HQD serum instead of HQD extract was used to treat LSECs. The purpose was to simulate the human body’s absorption of drugs. After orally taken, the active ingredients reached LSECs through blood circulation.
LSECs from the 3 male SD rats were cultured to the logarithmic growth phase, followed by being collected. Then LSECs were divided into the control, Vascular Endothelial Growth Factor A (VEGFA), VEGFA+serum, VEGFA+HQD-L, VEGFA+HQD-M, VEGFA+HQD-H, VEGFA+HQD-H+SC79, and VEGFA+HQD H+3MA groups. LSECs in the control group were cultivated with DMEM which included 10 % FBS and 1 % double-antibiotics. Those in VEGFA group were cultivated with DMEM including 10 % FBS, 1 % double-antibiotics, and 40 ng/ ml VEGFA. LSECs in the VEGFA+serum group were cultivated with DMEM including 10 % FBS, 1 % double-antibiotics, 40 ng/ml VEGFA, and 10 % rat serum (without HQD treatment), whereas the LSECs in the VEGFA+HQD-L group were cultivated with DMEM including 10 % FBS, 1 % double-antibiotics, 40 ng/ml VEGFA, and a 10 % low concentration of HQD serum. LSECs in the VEGFA+HQD-M group were cultured with DMEM including 10 % FBS, 1 % double antibiotics, 40 ng/ml VEGFA, and a 10 % medium concentration of HQD serum and those in the VEGFA+HQD-H group were cultured with DMEM including 10 % FBS, 1 % double antibiotics, 40 ng/ml VEGFA, and a 10 % high concentration of HQD serum. LSECs in the VEGFA+HQD-H+SC79 group were cultivated with DMEM containing 10 % FBS, 1 % double antibiotics, 40 ng/ml VEGFA, a 10 % high concentration of HQD serum, and 5 μg/ml SC79 (an Protein Kinase B (AKT) agonist)[21]. Finally, LSECs of the VEGFA+HQD-H+3MA group were cultivated with DMEM containing 10 % FBS, 1% double antibiotics, 40 ng/ml VEGFA, a 10 % high concentration of HQD serum, and 5 mM 3MA (an autophagy inhibitor)[22].
The initial HQD serum was considered a high concentration and was diluted three times using blank serum to create a low concentration. The initial HQD serum was diluted one time using blank serum to create a medium concentration.
3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl Tetrazolium Bromide (MTT) assay:
LSECs from each group were cultivated within 96-well plates containing 100 μl corresponding medium (1×104 LSECs per well) under 37° and 5 % CO2 conditions for 48 h. MTT solution (dissolved into phosphate-buffered saline, 5 mg/ml, 20 μl per well) was then supplemented in every well for 4 h cell treatment under 37°. Residual liquid in each well was substituted by Dimethyl Sulfoxide ((DMSO); 150 μl/well). Then, 96-well plates were gently shaken for 10 min. By using the microplate reader (Molecular Devices, Sunnyvale, California, United States of America (USA)), the absorbance values of diverse wells were determined at 490 nm. Five duplicate wells were set for the LSECs of each group. Additionally, the toxicity of HQD serum to rat LSECs was tested using MTT assay as described above.
Transwell experiment:
LSECs migration ability was detected by Transwell experiment. LSECs from each group were gathered and suspended within serum-free medium at 1×106 LSECs/ml. The 6-well inserts were seeded with 500 μl of the LSECs suspension in the upper chambers (8 μm pore size). DMEM (600 μl, with 10 % FBS and 1 % double-antibiotics) was supplemented to bottom chambers. LSECs were cultivated under 37° and 5 % CO2 conditions for 24 h. Migration cells were fixed using 4 % paraformaldehyde prior to 15 min staining using 0.1 % crystal violet. Later, we applied the microscope (Olympus, Tokyo, Japan) in counting migrating LSECs number.
Tube formation assay:
Angiogenesis of LSECs was determined by using tube formation assay. To be specific, Corning Matrigel Basement Membrane Matrix (150 μl, 10 μg/ml) was pre-coated into 24-well plates. LSECs (1×105) were seeded into 24-well plates before culture within corresponding medium <37° and 5 % CO2. After 12 h, LSECs were observed under a microscope. The tube length was identified with ImageJ software (National Institutes of Health, Bethesda, Maryland, USA)[23].
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR):
LSECs in every group were collected at 48 h post-culture prior to treatment with TRIzol reagent (Solarbio, Beijing, China) for extracting total Ribonucleic Acid (RNA) following specific instructions. A total RNA purification kit (LC Sciences, Houston, Texas, USA) was used following specific protocols. Total RNA was reversetranscribed with high-capacity complementary Deoxyribonucleic Acid (cDNA) transcription Kit (Applied Biosystems, Foster City, California, USA). Thereafter, qRT-PCR was carried out with Power SYBR Green Master Mix (Applied Biosystems, Foster City, California, USA) under conditions below, 5 min under 94°; 30 s under 95°, 30 s under 55°, and 5 min under 72° for 40 cycles. The primers used were; Platelet endothelial Cell Adhesion Molecule-1 (CD31), forward 5 -ATTGCAGTGGTTATCATCGGAGTG-3; reverse 5 - C T C G T T G T T G G A G T T C A G A A G T G G - 3 ′ ; endothelial Nitric Oxide Synthase (eNOS); forward 5′-CTCGTCCCTGTGGAAAGACAA-3′, reverse 5′ -TGACTTTGGCTAGCTAGCTGGTAACTGT-3′; Endothelin-1 (ET-1), forward 5′-TCCTCTGCTGGTTCCTGACT-3′; reverse 5′-CAGAAACTCCACCCCTGTGT-3′. Platelet- Derived Growth Factor-BB (PDGF-BB), forward 5′-CCTCGGCCTGTGACTAGAAG-3′, reverse 5′-CCTTGTCATGGGTGTGCTTA-3′. Angiopoietin 2, forward 5′-ACTGTTCTTCCCACTGCAA-3′, reverse 5′-CTGTGTTCTCTCCAGGCAT-3′. VEGFR 2, forward 5′-ATTGGCAGTTGGAGGAAG-3′, reverse 5′-CGCTTGGATAACAAGGGT-3′. G l y c e r a l d e h y d e - 3 - P h o s p h a t e Dehydrogenase (GAPDH), forward 5′-CAATGACCCCTTCATTGACC-3′ and reverse 5′-CTCGTCCCTGTGGAAAGACAA-3′. Using 2−ΔΔCt approach with GAPDH being the control, relative messenger RNA (mRNA) expression of CD31, eNOS, and ET-1 was calculated.
Western blotting:
LSECs in every group were gathered at 48 h post-culture before incubation with Radioimmunoprecipitation Assay (RIPA) lysis buffer that contained the protease inhibitor cocktail (Solarbio, Beijing, China) for extracting total proteins. In addition, we used the Bicinchoninic Acid (BCA) protein assay kit (Beyotime, Shanghai, China) for determining total protein content in line with specific protocols. Protein separation was completed through Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis (SDS-PAGE), followed by transfer onto a Polyvinylidene Fluoride (PVDF) membrane and blocked using 5 % nonfat milk for 1 h under ambient temperature. Next, we added rabbit anti-primary antibodies for probing proteins overnight at <4°. Primary antibodies included rabbit anti-CD31 (1:1000, ab28364, Abcam, Cambridge, United Kingdom); anti-ET-1 (1:1000, NY-174490M, Anyan Biotechnology Co., Ltd., Nanjing, China); anti-eNOS (1:1000, ab5589), anti-p-AKT (1:500, ab8805), anti-AKT (1:1000, ab126811), anti-p-AKT (1:500, ab8805), anti-mammalian Target of Rapamycin (mTOR) (1:1000, ab2732), anti-p-mTOR (1:500, ab63552), anti-microtubule-associated protein Light Chain 3-I (LC3I) (1:500, ab51520), anti-microtubule associated protein LC 3II (LC3II) (1:500, ab48394), anti-Beclin-1 (1:1000, ab62557), and anti-GAPDH (1:1000, ab9485, Abcam). Subsequently, we added Horseradish Peroxidase (HRP)-labeled goat antirabbit secondary antibody (1:2000, ab97051, Abcam) in cells and incubated under ambient temperature for a 2 h period. Protein blots were identified with the Enhanced Chemiluminescence (ECL) kit (Millipore, Billerica, Massachusetts, USA) in line with specific protocols. Protein bands were quantified with the use of ImageJ software (National Institutes of Health (NIH), Bethesda, Maryland, USA). GAPDH served as a control.
Statistical analysis:
Each assay was performed in triplicate. Results were shown to be mean±standard deviation and processed with Statistical Package for the Social Sciences (SPSS) 21.0 statistical software (SPSS, Chicago, Illinois, USA). Between-group comparison was completed with a paired Student's t-test, while three or more groups were compared through one-way Analysis of Variance (ANOVA) plus Tukey’s post hoc test. p<0.05 stood for significant difference.
Results and Discussion
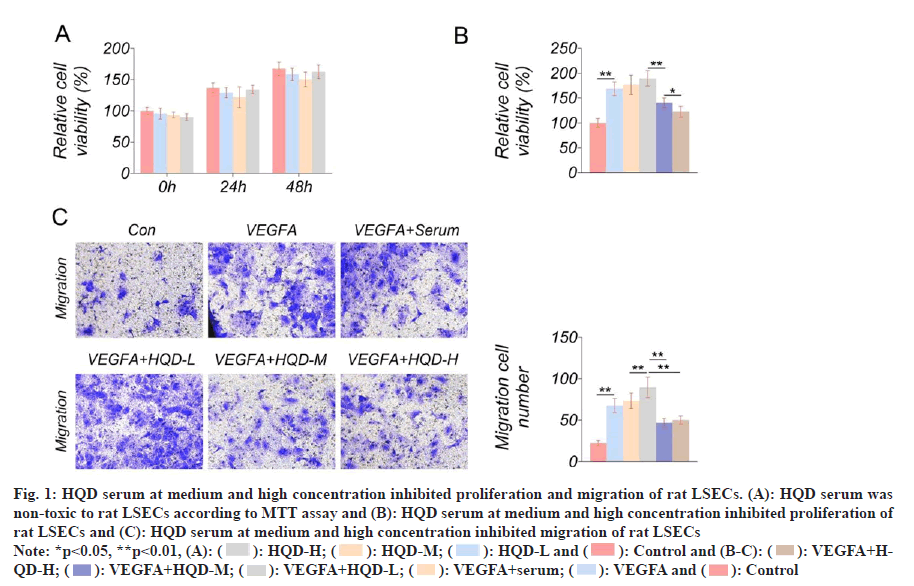
The toxicity of HQD serum to rat LSECs was tested using MTT assay. Matched with control group, the cell viability of LSECs of HQD-L group, HQD-M group and HQD-H group was not obviously changed at 0, 24 and 48 h (fig. 1A). This result indicating that HQD serum with the three different concentrations was non-toxic to rat LSECs. After treatment with HQD serum, rat LSECs proliferation was investigated using the MTT assay. Fig. 1B displayed the results. Relative to the control group, much higher proliferation occurred for the LSECs in VEGFA group (p<0.01). The LSECs in VEGFA+HQD-L group exhibited notably higher proliferation than that of the VEGFA+serum group (p<0.05). Relative to VEGFA+HQD-L group, proliferation of LSECs in VEGFA+HQD-M group was obviously lowered (p<0.01). Significantly lower proliferation was observed in LSECs of the VEGFA+HQD-H group compared with VEGFA+HQD-M group (p<0.01). As revealed by Transwell assay, the significantly increased migrated number could be detected in LSECs of VEGFA group relative to control group (p<0.01). Additionally, migrating cell number of VEGFA+HQD-L group markedly increased relative to VEGFA+serum group (p<0.01). On the contrary, the significantly lower number of cells migrated of the LSECs of the VEGFA+HQD-M group and the VEGFA+HQD-H group in comparison with VEGFA+HQD-L group (p<0.01) (fig. 1C). Therefore, it was concluded that low-dose HQD serum enhanced proliferation and migration of rat LSECs. However, HQD serum at medium and high concentration had an inhibitory effect on rat LSECs proliferation and migration.
Fig. 1: HQD serum at medium and high concentration inhibited proliferation and migration of rat LSECs. (A): HQD serum was non-toxic to rat LSECs according to MTT assay and (B): HQD serum at medium and high concentration inhibited proliferation of rat LSECs and (C): HQD serum at medium and high concentration inhibited migration of rat LSECs
Note: *p<0.05, **p<0.01, (A): ( ): HQD-H; (
): HQD-H; ( ): HQD-M; (
): HQD-M; ( ): HQD-L and (
): HQD-L and ( ): Control and (B-C): (
): Control and (B-C): ( ): VEGFA+HQD- H; (
): VEGFA+HQD- H; ( ): VEGFA+HQD-M; (
): VEGFA+HQD-M; ( ): VEGFA+HQD-L; (
): VEGFA+HQD-L; ( ): VEGFA+serum; (
): VEGFA+serum; ( ): VEGFA and (
): VEGFA and ( ): Control
): Control
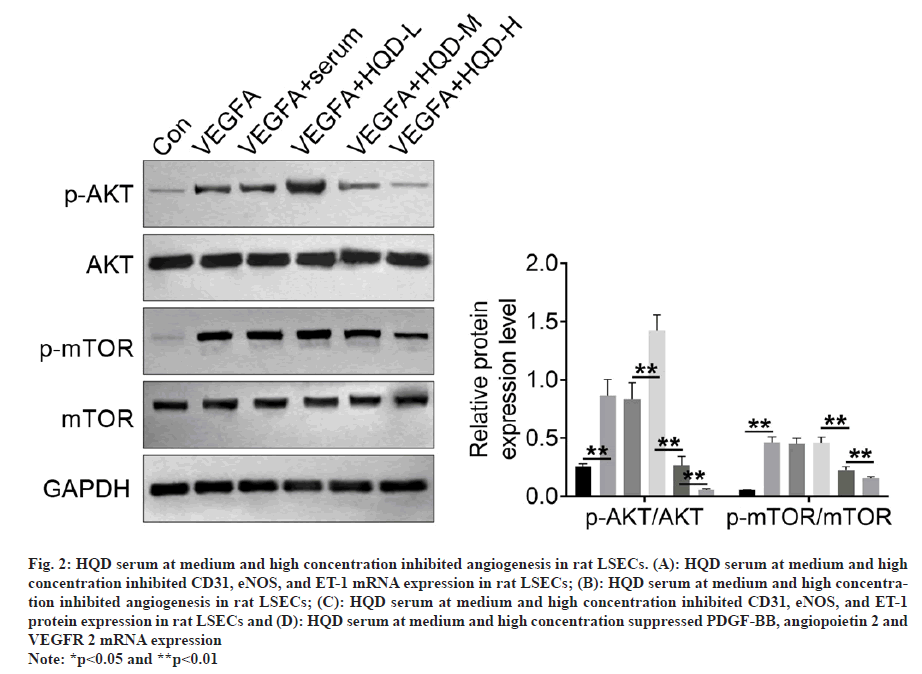
Angiogenesis-related gene expression was explored by qRT-PCR, including CD31, eNOS, and ET-1. LSECs of the VEGFA group had much higher CD31, eNOS, and ET-1 mRNA expression than that of the control group (p<0.01). Relative to VEGFA+serum group, VEGFA+HQD-L group exhibited much lower CD31 mRNA expression and higher eNOS and ET-1 mRNA expression (p<0.01). However, LSECs of the VEGFA+HQD-M group displayed lower CD31, eNOS, and ET-1 mRNA expression than the VEGFA+HQD-L group (p<0.01). Furthermore, a dramatic decrease in CD31, eNOS, and ET-1 mRNA expression occurred in the LSECs of the VEGFA+HQD-H group compared with VEGFA+HQD-M group (p<0.01) (fig. 2A). A tube formation assay was used for identifying angiogenesis in rat LSECs. As presented in fig. 2B, the tube length of LSECs in VEGFA group significantly increased relative to control group (p<0.01). A significantly increased tube length was observed in the LSECs of the VEGFA+HQD-L group relative to VEGFA+serum group (p<0.01). Interestingly, LSECs of the VEGFA+HQD-M and VEGFA+HQD-H groups exhibited remarkably lower tube lengths than the VEGFA+HQD-L group (p<0.01). Western blotting was conducted to explore CD31, eNOS, and ET-1 protein levels in LSECs. Results are shown in fig. 2C. Much higher CD31, eNOS, and ET-1 protein levels occurred within LSECs of the VEGFA group relative to control group (p<0.01). Compared with LSECs of VEGFA+serum group, CD31 and eNOS protein expression were similar in the VEGFA+HQD-L group. However, much higher ET-1 protein level could be observed within LSECs of VEGFA+HQD-L group than in the VEGFA+serum group (p<0.01). Relative to VEGFA+HQD-L group, CD31, eNOS, and ET-1 protein expression was significantly declined within LSECs in VEGFA+HQD-M group (p<0.01).
Fig. 2: HQD serum at medium and high concentration inhibited angiogenesis in rat LSECs. (A): HQD serum at medium and high concentration inhibited CD31, eNOS, and ET-1 mRNA expression in rat LSECs; (B): HQD serum at medium and high concentration inhibited angiogenesis in rat LSECs; (C): HQD serum at medium and high concentration inhibited CD31, eNOS, and ET-1 protein expression in rat LSECs and (D): HQD serum at medium and high concentration suppressed PDGF-BB, angiopoietin 2 and VEGFR 2 mRNA expression
Note: *p<0.05 and **p<0.01
Furthermore, CD31, eNOS, and ET-1 protein level significantly decreased in VEGFA+HQD-H group relative to VEGFA+HQD-M group (p<0.01). Additionally, this work explored how HQD serum affected pro-angiogenic factors expression using qRT-PCR, including PDGF-BB, angiopoietin 2 and VEGFR 2. As exhibited in fig. 2D, much higher mRNA expression of PDGF-BB, angiopoietin 2 and VEGFR 2 was monitored in LSECs of VEGFA group in comparison with control group (p<0.01). Matched with VEGFA+serum group, LSECs of VEGFA+HQD-L group exhibited obviously higher PDGF-BB mRNA expression and lower VEGFR 2 mRNA expression (p<0.01). Markedly lower PDGF-BB mRNA expression was monitored in LSECs of VEGFA+HQD-M group in comparison with VEGFA+HQD-L group (p<0.01). Simultaneously, distinctly lower mRNA expression of PDGF-BB, angiopoietin 2 and VEGFR 2 was observed in LSECs of VEGFA+HQD-H group when relative to LSECs of VEGFA+HQD-M group (p<0.05 or p<0.01). Hence, medium and highconcentration HQD serum inhibited angiogenesis in rat LSECs.
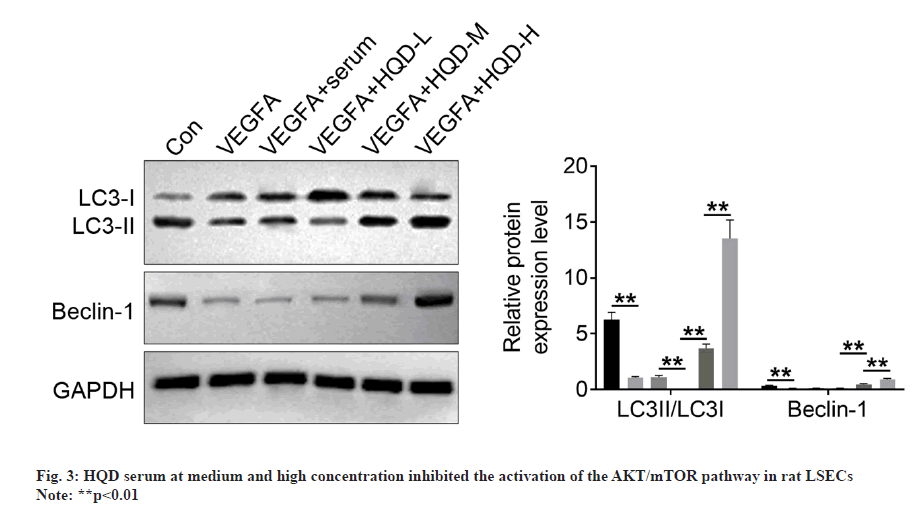
We conducted Western-blotting for analyzing how HQD serum affected AKT/mTOR pathway activity. From fig. 3, significantly higher p-AKT/ AKT and p-mTOR/mTOR protein expression could be detected within the LSECs in the VEGFA group than control group (p<0.01). p-AKT/AKT and p-mTOR/mTOR protein expression was not significantly different in VEGFA+serum vs. VEGFA+HQD-L groups. However, relative to the VEGFA+HQD-L group, obviously reduced p-AKT/ AKT and p-mTOR/mTOR protein levels was observed within LSECs of the VEGFA+HQD-M group (p<0.01). Additionally, p-AKT/AKT and p-mTOR/mTOR protein levels were lower within LSECs of the VEGFA+HQD-H group in relative to those of the VEGFA+HQD-M group (p<0.01). Therefore, medium and high-concentration HQD serum hindered AKT/mTOR pathway activation in rat LSECs.
Autophagy-related proteins (LC3-I, LC3-II, and Beclin-1) expression was determined using Western blotting. It should be noted that the LSECs of the VEGFA group had much lower LC3-II/LC3-I and Beclin-1 protein levels than control group (p<0.01). In comparison with VEGFA+serum group, LC3-II/LC3-I protein levels significantly decreased within LSECs of VEGFA+HQD-L group (p<0.01). However, Beclin-1 protein expression was of no significant difference in VEGFA+serum compared with VEGFA+HQD-L groups. Additionally, Beclin-1 and LC3-II/LC3-I protein levels within LSECs in VEGFA+HQD-M group significantly increased compared with VEGFA+HQD-L group (p<0.01), while their protein levels were significantly higher within the LSECs in VEGFA+HQD-H group in relative to those of the VEGFA+HQD-M group (p<0.01) (fig. 4). Thus, HQD serum at medium and high concentration promoted autophagy in rat LSECs.
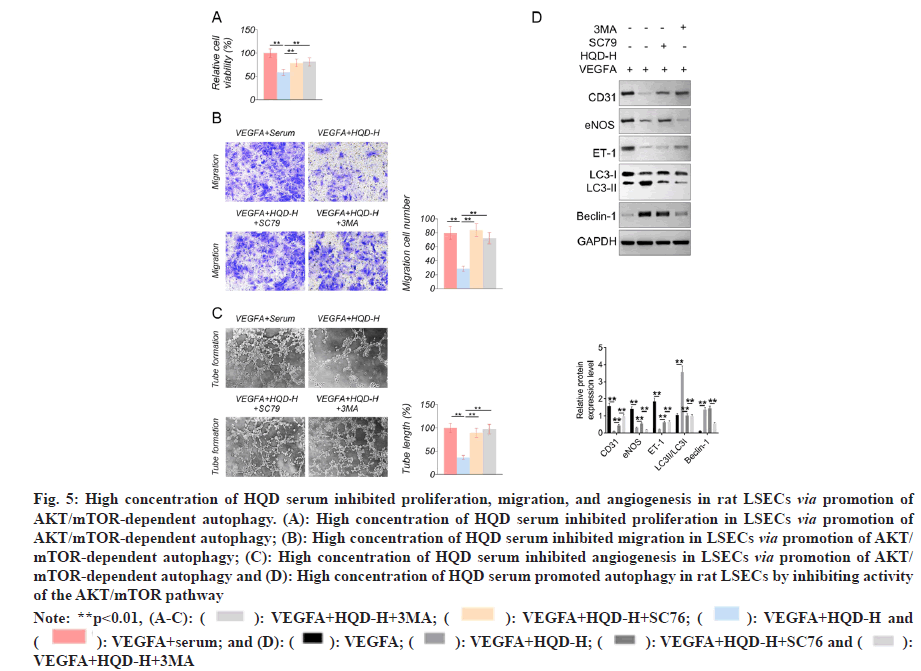
The MTT assay showed that, compared with the VEGFA+serum group, much lower proliferation occurred in the LSECs of the VEGFA+HQD-H group (p<0.01). Nevertheless, compared with VEGFA+HQD-H group, proliferation of LSECs in the VEGFA+HQD-H+SC79 group and VEGFA+HQD-H+3MA group markedly increased (p<0.01) (fig. 5A). Based on Transwell experiment, remarkably lower migration cell numbers were observed in the LSECs of the VEGFA+HQD-H group in relative to VEGFA+serum group (p<0.01). On the contrary, compared with VEGFA+HQD-H group, a markedly increased migration cell number was found in the LSECs of the VEGFA+HQDHQDH+ SC79 and VEGFA+HQD-H+3MA groups (p<0.01) (fig. 5B). The tube formation assay showed significantly lower tube length in the LSECs of the VEGFA+HQD-H group relative to VEGFA+serum group (p<0.01). By contrast, a markedly longer tube length was observed in the LSECs of the VEGFA+HQD-H+SC79 group and VEGFA+HQD-H+3MA group compared with VEGFA+HQD-H group (p<0.01) (fig. 5C). Western blotting showed much lower CD31, eNOS, and ET-1 protein expression and higher Beclin-1 and LC3-II/LC3-I protein levels within the LSECs in VEGFA+HQD-H group than in the VEGFA+serum group (p<0.01). Compared with VEGFA+HQD-H group, LSECs of VEGFA+HQD-H+SC79 group exhibited significantly higher CD31, eNOS, and ET-1 protein expression and lower LC3-II/LC3-I protein levels (p<0.01). Additionally, relative to VEGFA+HQD-H+SC79 group, markedly higher CD31 and ET-1 protein expression and lower eNOS, Beclin-1 and LC3-II/LC3-I protein levels were observed in VEGFA+HQD-H+3MA group (p<0.01) (fig. 5D). The obtained findings indicated that high concentration of HQD serum might inhibit proliferation, migration, and angiogenesis in rat LSECs by promoting AKT/mTOR-dependent autophagy.
Fig. 5: High concentration of HQD serum inhibited proliferation, migration, and angiogenesis in rat LSECs via promotion of AKT/mTOR-dependent autophagy. (A): High concentration of HQD serum inhibited proliferation in LSECs via promotion of AKT/mTOR-dependent autophagy; (B): High concentration of HQD serum inhibited migration in LSECs via promotion of AKT/ mTOR-dependent autophagy; (C): High concentration of HQD serum inhibited angiogenesis in LSECs via promotion of AKT/ mTOR-dependent autophagy and (D): High concentration of HQD serum promoted autophagy in rat LSECs by inhibiting activity of the AKT/mTOR pathway
Note: **p<0.01, (A-C): ( ): VEGFA+HQD-H+3MA; (
): VEGFA+HQD-H+3MA; ( ): VEGFA+HQD-H+SC76; (
): VEGFA+HQD-H+SC76; ( ): VEGFA+HQD-H and (
): VEGFA+HQD-H and ( ): VEGFA+serum; and (D): (
): VEGFA+serum; and (D): ( ): VEGFA; (
): VEGFA; ( ): VEGFA+HQD-H; (
): VEGFA+HQD-H; ( ): VEGFA+HQD-H+SC76 and (
): VEGFA+HQD-H+SC76 and ( ): VEGFA+HQD-H+3MA
): VEGFA+HQD-H+3MA
Pathological angiogenesis belongs to one of the main factors for chronic liver disease development, including liver fibrosis, cirrhosis, or even liver cancer[24]. Both clinical and experimental conditions have been significantly associated with liver fibrosis development[25]. LSECs form fenestrated wall of the liver sinusoid, which are non-parenchymal liver cells involved in pathological liver angiogenesis[26]. Endothelial cell growth and migration represent the early signs of angiogenesis, followed by capillary angiogenesis[27]. LSECs exert an important effect on the pathobiology of angiogenesis and accelerate liver fibrosis progression[28,29]. Therefore, the inhibition of angiogenesis in LSECs may be a novel method to treat liver fibrosis.
The present work investigated the efficacy of HQD serum in rat LSECs angiogenesis. VEGFA is a well-known angiogenesis-promoting factor[30]. This study used exogenous VEGFA to treat LSECs, which was used as a positive control. It was shown that HQD serum at medium and high concentration could suppress rat LSEC growth, migration and angiogenesis in vitro. Additionally, medium and high-concentration HQD serum suppressed the expression of CD31, eNOS, and ET-1 proteins. As we know, CD31, eNOS and ET-1 are important genes that promote[31-33]. Thus, medium and high concentration of HQD serum could weaken angiogenesis in LSECs by reducing CD31, eNOS and ET-1 proteins expression. However, the effect of low concentration of HQD serum on LSECs proliferation, migration and angiogenesis was opposite to that of medium and high concentration. Low concentration of HQD serum promoted LSECs proliferation and migration, and enhanced angiogenesis by up-regulating CD31, eNOS and ET-1 proteins. Additionally, this study monitored that HQD serum at high concentration effectively reduced PDGF-BB, angiopoietin 2 and VEGFR 2 mRNA expression in LSECs. PDGF-BB, angiopoietin 2 and VEGFR 2 are three important pro-angiogenic factors[34-36]. Hence, HQD serum at high concentration might weaken angiogenesis in LSECs via reducing PDGF-BB, angiopoietin 2 and VEGFR 2 expression. However, HQD serum at low concentration could facilitate angiogenesis in LSECs by increasing the expression of PDGFBB. HQD is a traditional Chinese medicine. Its active ingredients are absorbed by the body, followed by being reached to the blood circulatory system to reach organs or tissues. This research revealed that HQD serum with low, medium and high concentrations was non-toxic to rat LSECs. Importantly, HQD serum at medium and high concentrations could inhibit LSECs growth, migration and angiogenesis in vitro. Thus, HQD serum might be effective and safety for the clinical use of HQD to treat liver fibrosis.
Beclin-1 and LC3-II represent important autophagypromoting genes, which up-regulation has relieved liver fibrosis[37,38]. Additionally, inhibiting autophagy can promote the defenestration of LSECs[39]. As reported by Ruart et al.[40], impaired autophagy in LSECs facilitated liver fibrosis through aggravating oxidative stress response. Previously, HQD is reported with protection against liver fibrosis[41]. However, the detailed molecular mechanism of HQD in inhibiting liver fibrosis has not yet been completely explained. In the present study, HQD serum at medium- and highconcentration could enhance Beclin-1 and LC3-II/ LC3-I protein levels in rat LSECs. However, using 3MA (an autophagy inhibitor) partially reversed inhibition of high concentration HQD serum on LSECs proliferation, migration and angiogenesis. Thus, it might be that HQD serum at medium and high concentration inhibited LSECs proliferation, migration and angiogenesis by promoting the autophagy.
In this study, HQD serum at medium and high concentration suppressed AKT and mTOR phosphorylation. Interestingly, using an AKT agonist (SC79) reversed the inhibitory effect of high concentration HQD serum on LSECs proliferation, migration and angiogenesis. The AKT/mTOR pathway activation could promote angiogenesis within hepatocellular carcinoma[42]. Furthermore, AKT pathway activation could accelerate endothelial cell growth and migration, and finally form stable blood vessels[43,44]. Autophagy can promote endothelial cell death, leading to attenuated angiogenic behavior in endothelial cells[45]. According to Shen et al.[46], inducing autophagy could alleviate liver fibrosis. Inhibiting AKT/mTOR pathway is essential to activate autophagy. This study demonstrated that high concentration of HQD serum could weaken LSECs angiogenesis. Simultaneously, high concentration of HQD serum induced autophagy in LSECs via suppressing the AKT/mTOR pathway activity. Thus, it was revealed that high concentration of HQD serum might attenuate angiogenesis in rat LSECs by promoting autophagy via reducing AKT/ mTOR pathway activity. A previous study found that HQD could relieve renal tubulointerstitial fibrosis of mice through suppressing Wnt/β- catenin pathway[47]. Nevertheless, few studies have concentrated on the impacts of HQD on the AKT/mTOR pathway in liver fibrosis. This was the first time that HQD serum was found to inhibit angiogenesis in rat LSECs by inducing autophagy via suppressing AKT/mTOR pathway activity. Moreover, the obtained findings offer important theoretical basis for the use of HQD to treat liver fibrosis clinically.
This study had several limitations. The HQD serum at medium and high concentration had an inhibiting effect on rat LSECs proliferation, migration, angiogenesis, and autophagy. However, a low concentration of HQD serum showed the opposite results. The reason for this result will be investigated in further research.
To conclude, medium and high-concentration HQD serum inhibited rat LSECs proliferation, migration, angiogenesis and autophagy. HQD serum could promote autophagy in rat LSECs by suppressing the AKT/mTOR pathway activity. Mechanically, high concentration of HQD serum inhibited proliferation and migration of rat LSECs via angiogenesis inhibition by promoting AKT/ mTOR-dependent autophagy. Thus, the utilization of HQD might be a novel strategy to treat liver fibrosis clinically. This study provided novel strategy and theoretical foundation for using HQD to treat liver fibrosis. Certainly, more research is required in the future.
Acknowledgement:
This study is supported by National Natural Science Foundation of China (No: 81774098).
Conflict of interests:
The authors declared no conflict of interests.
References
- Hammoutene A, Rautou PE. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J Hepatol 2019;70(6):1278-91.
[Crossref] [Google Scholar] [PubMed]
- Poisson J, Lemoinne S, Boulanger C, Durand F, Moreau R, Valla D, et al. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol 2017;66(1):212-27.
[Crossref] [Google Scholar] [PubMed]
- Maslak E, Gregorius A, Chlopicki S. Liver sinusoidal endothelial cells (LSECs) function and NAFLD; NO-based therapy targeted to the liver. Pharmacol Rep 2015;67:689-94.
[Crossref] [Google Scholar] [PubMed]
- Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circul Res 2008;102(4):488-96.
[Crossref] [Google Scholar] [PubMed]
- Wisse E, Braet F, Luo D, de Zanger R, Jans D, Crabbe E, et al. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol 1996;24(1):100-11.
[Crossref] [Google Scholar] [PubMed]
- Miyao M, Kotani H, Ishida T, Kawai C, Manabe S, Abiru H, et al. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Lab Invest 2015;95(10):1130-44.
[Crossref] [Google Scholar] [PubMed]
- Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: A review. Comp Hepatol 2002;1(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Deleve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest 2013;123(5):1861-6.
[Crossref] [Google Scholar] [PubMed]
- Xu GF, Wang XY, Ge GL, Li PT, Jia X, Tian DL, et al. Dynamic changes of capillarization and peri-sinusoid fibrosis in alcoholic liver diseases. World J Gastroenterol 2004;10(2):238.
[Crossref] [Google Scholar] [PubMed]
- Zhao X, Zhao Q, Luo Z, Yu Y, Xiao N, Sun X, et al. Spontaneous immortalization of mouse liver sinusoidal endothelial cells. Int J Mol Med 2015;35(3):617-24.
[Crossref] [Google Scholar] [PubMed]
- Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: A translational success story. Gut 2015;64(5):830-41.
[Crossref] [Google Scholar] [PubMed]
- Xie G, Choi SS, Syn WK, Michelotti GA, Swiderska M, Karaca G, et al. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut 2012;62(2):299-309.
[Crossref] [Google Scholar] [PubMed]
- Yang X, Wang Z, Kai J, Wang F, Jia Y, Wang S, et al. Curcumol attenuates liver sinusoidal endothelial cell angiogenesis via regulating Glis-PROX1-HIF-1α in liver fibrosis. Cell Prolif 2020;53(3):e12762.
[Crossref] [Google Scholar] [PubMed]
- Song YN, Zhang GB, Lu YY, Chen QL, Yang L, Wang ZT, et al. Huangqi decoction alleviates dimethylnitrosamine-induced liver fibrosis: An analysis of bile acids metabolic mechanism. J Ethnopharmacol 2016;189:148-56.
[Crossref] [Google Scholar] [PubMed]
- Xu W, Xu YN, Zhang X, Xu Y, Jian X, Chen JM, et al. Hepatic stem cell number gene is a potential target of Huang Qi decoction against cholestatic liver fibrosis. Sci Rep 2020;10(1):17486.
- Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu X, et al. Huangqi decoction inhibits apoptosis and fibrosis, but promotes Kupffer cell activation in dimethylnitrosamine-induced rat liver fibrosis. BMC Complement Alternat Med 2012;12:51-8.
[Crossref] [Google Scholar] [PubMed]
- Lu WL, Li JM, Yang J, Xu CG, Zhang SS, Yan J, et al. Effects of Astragalus polysaccharide on mechanical characterization of liver sinusoidal endothelial cells by atomic force microscopy at nanoscale. Chin J Integrat Med 2018;24:455-9.
[Crossref] [Google Scholar] [PubMed]
- Yang L, Li A, Chen M, Yan Y, Liu Y, Li K, et al. Comprehensive investigation of mechanism and effective ingredients of Fangji Huangqi Tang by serum pharmacochemistry and network pharmacology. Biomed Chromatogr 2020;34(4):e4785.
[Crossref] [Google Scholar] [PubMed]
- Luo XX, Yang M, Tai PP, Zhang DH. Effects of Yougui pills serum on expressions of VEGF, VEGFR-2, PI3K and Akt in rat retinal microvascular endothelial cells under high glucose and hypoxia. China J Tradit Chin Med 2019;34:1675-8.
- Gao L, Yang X, Liang B, Jia Y, Tan S, Chen A, et al. Autophagy-induced p62 accumulation is required for curcumol to regulate KLF5-mediated angiogenesis in liver sinusoidal endothelial cells. Toxicology 2021;452:152707-18.
- Zhang Y, Li F, Liu L, Jiang H, Jiang X, Ge X, et al. Salinomycin-induced autophagy blocks apoptosis via the ATG3/AKT/mTOR signaling axis in PC-3 cells. Life Sci 2018;207:451-60.
[Crossref] [Google Scholar] [PubMed]
- Liang L, Hui K, Hu C, Wen Y, Yang S, Zhu P, et al. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J Exp Clin Cancer Res 2019;38:38-83.
[Crossref] [Google Scholar] [PubMed]
- Deng F, Zhou R, Lin C, Yang S, Wang H, Li W, et al. Tumor-secreted dickkopf2 accelerates aerobic glycolysis and promotes angiogenesis in colorectal cancer. Theranostics 2019;9(4):1001.
[Crossref] [Google Scholar] [PubMed]
- Li N, Liu C, Ma G, Tseng Y, Pan D, Chen J, et al. Asparaginyl endopeptidase may promote liver sinusoidal endothelial cell angiogenesis via PI3K/Akt pathway. Rev Esp Enferm Dig 2019;111:214-22.
[Crossref] [Google Scholar] [PubMed]
- Coulon S, Heindryckx F, Geerts A, van Steenkiste C, Colle I, van Vlierberghe H. Angiogenesis in chronic liver disease and its complications. Liver Int 2011;31(2):146-62.
[Crossref] [Google Scholar] [PubMed]
- Zhang GB, Song YN, Chen QL, Dong S, Lu YY, Su MY, et al. Actions of Huangqi decoction against rat liver fibrosis: A gene expression profiling analysis. Chin Med 2015;10(1):39-49.
[Crossref] [Google Scholar] [PubMed]
- Folkman J. Angiogenesis. J Biol Chem 1992;267:10931-4.
- Sakata K, Eda S, Lee ES, Hara M, Imoto M, Kojima S. Neovessel formation promotes liver fibrosis via providing latent transforming growth factor-β. Biochem Biophys Res Commun 2014;443(3):950-6.
[Crossref] [Google Scholar] [PubMed]
- Zeng XQ, Li N, Pan DY, Miao Q, Ma GF, Liu YM, et al. Kruppel-like factor 2 inhibit the angiogenesis of cultured human liver sinusoidal endothelial cells through the ERK1/2 signaling pathway. Biochem Biophys Res Commun 2015;464(4):1241-7.
[Crossref] [Google Scholar] [PubMed]
- Kashyap AS, Schmittnaegel M, Rigamonti N, Pais-Ferreira D, Mueller P, Buchi M, et al. Optimized antiangiogenic reprogramming of the tumor microenvironment potentiates CD40 immunotherapy. Proc Natl Acad Sci 2020 ;117(1):541-51.
[Crossref] [Google Scholar] [PubMed]
- Nadeau V, Potus F, Boucherat O, Paradis R, Tremblay E, Iglarz M, et al. Dual ETA/ETB blockade with macitentan improves both vascular remodeling and angiogenesis in pulmonary arterial hypertension. Pulm Circul 2017;8(1):2045893217741429.
[Crossref] [Google Scholar] [PubMed]
- Nasirzadeh M, Rasmi Y, Rahbarghazi R, Kheradmand F, Karimipour M, Aramwit P, et al. Crocetin promotes angiogenesis in human endothelial cells through PI3K-Akt-eNOS signaling pathway. EXCLI J 2019;18:936-49.
[Google Scholar] [PubMed]
- Yang M, Li CJ, Sun X, Guo Q, Xiao Y, Su T, et al. miR-497∼195 cluster regulates angiogenesis during coupling with osteogenesis by maintaining endothelial Notch and HIF-1α activity. Nat Commun 2017;8(1):16003.
[Crossref] [Google Scholar] [PubMed]
- Lin B, Song X, Yang D, Bai D, Yao Y, Lu NA. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene 2018;654:77-86.
[Crossref] [Google Scholar] [PubMed]
- Komici K, Gnemmi I, Sangiorgi C, Ricciardolo FL, Rinaldi M, di Stefano A, et al. Indexes of angiogenic activation in myocardial samples of patients with advanced chronic heart failure. Medicina 2019;55(12):766.
[Crossref] [Google Scholar] [PubMed]
- Yehya AH, Asif M, Petersen SH, Subramaniam AV, Kono K, Majid AM, et al. Angiogenesis: Managing the culprits behind tumorigenesis and metastasis. Medicina 2018;54(1):8-27.
[Crossref] [Google Scholar] [PubMed]
- Ma JQ, Sun YZ, Ming QL, Tian ZK, Yang HX, Liu CM. Ampelopsin attenuates carbon tetrachloride-induced mouse liver fibrosis and hepatic stellate cell activation associated with the SIRT1/TGF-β1/Smad3 and autophagy pathway. Int Immunopharmacol 2019;77:105984-92.
[Crossref] [Google Scholar] [PubMed]
- Sun H, Yu J, Wen Z, Wang M, Chen W. Decreased expression of Beclin-1 in patients with hepatocellular carcinoma. J BUON 2019;24(2):634-41.
[Google Scholar] [PubMed]
- Luo X, Wang D, Zhu X, Wang G, You Y, Ning Z, et al. Autophagic degradation of caveolin-1 promotes liver sinusoidal endothelial cells defenestration. Cell Death Dis 2018;9(5):576.
[Crossref] [Google Scholar] [PubMed]
- Ruart M, Chavarria L, Campreciós G, Suárez-Herrera N, Montironi C, Guixe-Muntet S, et al. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J Hepatol 2019;70(3):458-69.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Zhang F, Lu Y, Zheng S. Update on implications and mechanisms of angiogenesis in liver fibrosis. Hepatol Res 2015;45(2):162-78.
[Crossref] [Google Scholar] [PubMed]
- Li W, Tan D, Zhang Z, Liang JJ, Brown RE. Activation of Akt-mTOR-p70S6K pathway in angiogenesis in hepatocellular carcinoma. Oncol Rep 2008;20(4):713-9.
[Google Scholar] [PubMed]
- Nakao T, Shiota M, Tatemoto Y, Izumi Y, Iwao H. Pravastatin induces rat aortic endothelial cell proliferation and migration via activation of PI3K/Akt/mTOR/p70 S6 kinase signaling. J Pharmacol Sci 2007;105(4):334-41.
[Crossref] [Google Scholar] [PubMed]
- Yu P, Yu DM, Qi JC, Wang J, Zhang QM, Zhang JY, et al. High D-glucose alters PI3K and Akt signaling and leads to endothelial cell migration, proliferation and angiogenesis dysfunction. Zhonghua Yi Xue Za Zhi 2006;86(48):3425-30.
[Google Scholar] [PubMed]
- Liang P, Jiang B, Li Y, Liu Z, Zhang P, Zhang M, et al. Autophagy promotes angiogenesis via AMPK/Akt/mTOR signaling during the recovery of heat-denatured endothelial cells. Cell Death Dis 2018;9(12):1152.
[Crossref] [Google Scholar] [PubMed]
- Shen M, Guo M, Wang Z, Li Y, Kong D, Shao J, et al. ROS-dependent inhibition of the PI3K/Akt/mTOR signaling is required for Oroxylin A to exert anti-inflammatory activity in liver fibrosis. Int Immunopharmacol 2020;85:106637.
[Crossref] [Google Scholar] [PubMed]
- Jiang MQ, Wang L, Cao AL, Zhao J, Chen X, Wang YM, et al. Huangqi decoction improves renal tubulointerstitial fibrosis in mice by inhibiting the up-regulation of Wnt/β-catenin signaling pathway. Cell Physiol Biochem 2015;36(2):655-69.
[Crossref] [Google Scholar] [PubMed]
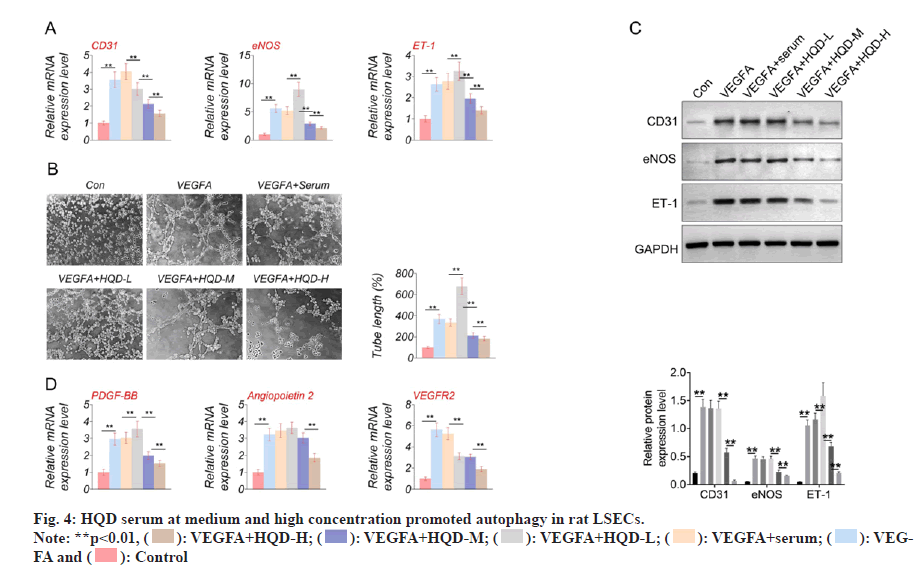
 ): VEGFA+HQD-H; (
): VEGFA+HQD-H; ( ): VEGFA+HQD-M; (
): VEGFA+HQD-M; (