Kaushita Banerjee1, N.Thiagarajan1 and Padma Thiagarajan*
School of Biosciences and Technology, VIT University, Vellore- 632 014
1Material Science Division, National Aerospace Laboratories, Bangalore-560 017, India
- *Corresponding Author:
- Padma Thiagarajan
School of Biosciences and Technology, VIT University, Vellore- 632 014
E-mail: padmadk4@gmail.com
Received date: 16 Sep 2015; Revised date: 21 May 2016; Accepted date: 26 May 2016
Abstract
Azadirachta indica A. Juss (Neem) is attested to be an important medicinal tree whose parts and extracts are known to cure several ailments since the Vedic era. But knowledge regarding their concoctions and dosages has remained largely esoteric. Dilute neem oil emulsions are used to deliver active ingredients to body parts by the topical route of administration. This possibly attenuates its dose dumping and concentration related noxious effects to a large extent. However, almost all such products incorporate synthetic organic and bio hazardous chemicals for purposes of formulation and stability, posing ultimate risks to the user. Hence in the present study, an emollient cream using 10% neem oil and an arachidyl glucoside emulsifier of completely biological origin has been formulated. Octa and hexadecanoic acid derivatives were the major fatty acid components identified in the oil. The creamy white product showed a mean particle size of 137 nm and a Z average of 19 nm, with a polydispersity index of 0.245. Zeta potential and electrophoretic mobility were measured as -47.2 and -0.000328 cm2/Vs, respectively thus conferring good stability. FTIR analysis revealed the incidence of extensive hydrogen bonding in its structure and SEM image captured its undulating surface topography. The emollient cream was not susceptible to cracking, creaming or phase separation even after a period of 180 days, when stored at 37° or under low speed centrifugation. Similar results were observed when it was stored at 40°, 4° and -18° for three days and brought to 37° for three cycles. It is concluded that this novel potentially non-toxic neem oil emollient cream can either be used per se or as a base matrix for loading active ingredients and hence function as an efficient delivery system for the same.
Keywords
Azadirachta indica, neem oil, arachidyl glucoside, emollient cream, delivery system
Azadirachta indica A. Juss, commonly known as neem or Indian lilac, belongs to Meliaceae family. Most parts of this plant, including its seed oil, have been traditionally valuable for their potent antimicrobial, anti-inflammatory, antipyretic, analgesic, immunostimulatory, antiulcerous and antioxidant effects[1,2]. Its vital constituents are the triterpenoid azadirachtin complex, protomeliacins, limonoids, gedunin and its derivatives, vilasinin and c-secomeliacins like nimbin, salanin and dihydrochalcone, flavonoids, coumarin and tannins[3,4]. Along with a dozen minor azadirachtin analogs, they contribute to the overall efficacy of the oil when incorporated in cosmetic, cosmeceutical and pharmaceutical formulations[5]. The oil has been considered non-toxic and safe to mammalian species till a dosage of 5000 mg/kg of body weight. But the probability of risk due to its usage in concentrated form is of considerable concern as reported from several studies[6-9]. Hence, its crude and concentrated forms may pose limitations with respect to handling, usage and dose dumping, raising toxicity issues. This could possibly be the reason for its underutilization in thrust areas of potential applications. Its emulsions have been used as a feasible alternative approach, to a limited extent maybe to overcome the possible risk due to inadvertent over dosages[10]. These also facilitate considerable dilution of the bioactive ingredients to an optimal concentration for the required applications. But such formulations incorporate organic and polymeric emulsifiers like tweens, spans and sulfonates for emulsification[11]. Solvents like petroleum ether and toluene are also added to improve their physicochemical properties and lower the energy required for the emulsification process[12]. Synthetic antioxidants like butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) form part of these emulsions, as they are expected to confer enhanced stability against long term oxidative damage[13]. Although dilute emulsions may lower the toxic effects of the oil (at times making it ineffective too), several of these synthetic chemicals pose additional risks to humans, animals and the environment.
In the present study, a neem oil emollient cream (EC) has been formulated exclusively with a natural, nonionic arachidyl glucoside (APG) emulsifier, in an aqua base after its fatty acid methyl esters (FAME) analysis by GC-MS. APG emulsifiers have been recently gaining substantial importance as they present very encouraging toxicological, ecological and dermatological profiles[14]. The smooth and creamy EC after ultrasonication has been characterized for its mean particle size diameter, Z average, polydispersity index, zeta potential and electrophoretic mobility. Fourier transform infrared (FTIR) spectrum and scanning electron microscopy (SEM) images have been recorded. Its shelf-life stability at 37° for 180 days and under low speed centrifugation, in terms of cracking, creaming or phase separation has been assessed. Stability under heating-cooling and freezing-thawing conditions has also been studied. The results favor its use as a very promising product per se or as a base matrix for loading active ingredients for cosmeceutical and pharmaceutical applications by the topical route. To the best of our knowledge, such a stable water based neem oil and APG emollient cream has not been formulated, characterized and reported so far for any applications.
Materials And Methods
Neem oil was purchased from a reputed dealer in the local market. APG emulsifier, marketed under the trade name of Montonov 202, was procured from SEPPIC, Bangalore.
FAME analysis of neem oil:
Neem oil was esterified using methanol and sodium hydroxide[15] and subjected to GC-MS analysis (Joel GCMATE II) with a HP 5Ms ultra inert column of very low bleeding characteristics. Pure helium was used as a carrier gas (1 ml/min). The GC inlet temperature was set to 220° and the oven temperature was ramped from 50° to 250° at a rate of 10°/minute. The GC interface and ion chamber temperature were both set to 250°. Electron impact ionization (70 eV) mode was employed with a scan rate of 50 to 600 amu. Quadruple double focusing mass analyzer and photo multiplier tube were used for the analysis. NIST library was used to identify the compounds.
Formulation of EC:
About 10 ml of neem oil was preheated to 45° and 2 g of the powdered APG emulsifier was slowly dissolved in it. Then 30 ml of distilled water was gradually added with constant stirring at 1200 rpm using a magnetic stirrer (MLH series, REMI) to obtain the EC. It was ultrasonicated (Vibra Cell Model, Sonics and Materials Inc., USA) for 15 min. thereafter, 10 ml water was then added every 5 min under continuous sonication, till the total volume reached 100 ml. Sonication was continued for another 20 min for achieving particle size reduction. The EC was then stored undisturbed in a brown bottle for 48 h at 37° before further characterization.
Characterization studies:
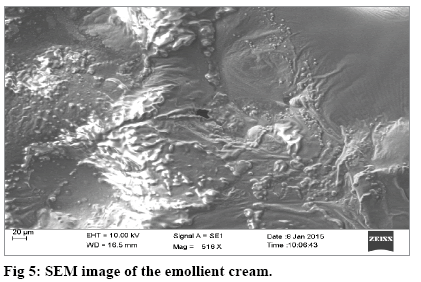
FTIR spectra of both the oil and EC were recorded between 4000 and 500 cm-1 using attenuated total reflection technique and with diamond crystal (Bruker, Optics, Germany). Mean particle diameter, zeta potential, polydispersity index and electrophoretic mobility were recorded, after 1/100 dilution of the EC at 25° with a scattering angle of 90° (Horiba scientific SZ 100) and by using a Windows [Z type] version 2.00 Software. For obtaining the SEM image, the EC was coated onto a 1×1 cm glass slide, presmeared with a thin layer of egg albumin and air dried. It was sputter coated with Au/Pd (SC7620 Sputter coater, Quorum Technologies), under a vacuum pressure of -6.5 mbar for 30 min with a process current of 10 mV. It was then mounted on a SEM sample stub and imaged at 516× with LaB6 filament (Carl Zeiss EVO 18 Research model).
Stability tests[16]:
EC (10 g) was taken in various beakers and maintained at 40° and -18° for 48 h. After this time interval, the beakers were brought to 37° and the EC were observed for any signs visual destabilization like creaming, cracking, oil droplet formation and phase separation. The test procedures were repeated for three cycles. The shelf life stability was assessed by maintaining the EC at 37° for a period of 120 days and observing for any signs visual destabilization. The EC was also centrifuged (REMI Microprocessor Research Centrifuge PR-24) for 30 min at 5000 rpm, and observed visually for any signs of destabilization.
Results And Discussion
The versatile medicinal properties of A. indica A. Juss and its oil, that includes its anti-fertility, immunostimulatory, antimicrobial, antipyretic and acaricidal activities are well known[1,11]. But concurrently, deleterious effects focusing on the oil’s (over) dosage have been reported[6-9]. These effects seem to vary across test subjects, organs, tissues, cells and conditions studied. A better understanding of the optimal dosage levels that are required to derive its benefits without toxicity concerns is the need of the hour and is presently a subject of intense research. Hence the essentiality of a suitable and safe delivery system for neem oil, devoid of harmful effects, has been necessitated to make use of its full potential. Neem oil based formulations in the form of emulsions have been reported[10,11]. But the additional synthetic chemicals incorporated into such systems, for synthesis and stability, make their own noxious contributions.
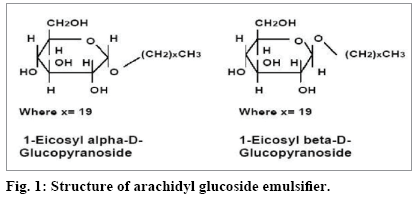
In this context, the neem emollient cream formulated in this study did not employ any synthetic emulsifiers and stabilizers. The natural and biocompatible APG emulsifier (fig. 1) used here was non-ionic, biodegradable and of 100% vegetable origin[17]. It was derived from corn /manioc and contained no solvents or preservatives. It is known to aid emulsification of oils at low concentrations of 1 to 2% and across a wide range of pH. It stabilizes formulations by obstructing creaming and phase separations. By varying its concentration, emulsions and gels of required viscosities can be obtained. It promotes growth of liquid crystals and retains the skin moisturization for hours after the initial application. It can be used on oily as well as sensitive skin. It also provides an evanescent effect.
FAME analysis of the oil revealed the presence of seven fatty acids. Their lipid numbers have been further identified and their relative percentages recorded by mass spectrometry as shown in Table 1. These are mainly substituted and methylated octanoic and decanoic acids as also reported by other workers[18]. Undecanoic acid is an active ingredient of medications used in the treatment of Candida albicans skin infection[19] and has antiseborrheic property. It actively inserts and positions itself into the phospholipid layer of the fungal membrane and deranges it thus elevating the membrane fluidity. This finally results in conformational disintegration of the membrane proteins and cytoplasmic disordering, thus releasing the intracellular components and eventually inducing cell collapse. Hexadecanoic and octanoic acids are the most important antifungal fatty acids that target cell membranes as well as protein synthesis. They cause an elevation in the fluidity and rapid exo osmosis that result in exudation of the intracellular components along with cell senescence[20]. Myristic acid analogues also hinder protein synthesis and topoisomerase activity in fungi[21]. Hexadecanoic acid exhibits antibacterial activity against Mycobacterium tuberculosis[22]. 10-octadecenoic acid has been used in treating dermatomycosis caused by Trichophyton rubrum, Epidermophyton inguinale and Microsporum audouini. It is also employed to cure tineapedis caused by Trichophyton mentagrophytes and Trichophyton rubrum. The fatty acid suppresses hyphal formation in C. albicans and prevents its overgrowth in the human host by hindering acyl-CoA during lipid synthesis. Methylated and hydroxyl fatty acids, derived from plant extracts, pose low environmental risks and exhibit a high degree of specificity that may be due to the inhibition of β-oxidation, resulting in increased halflives of the fatty acids. This would allow them to be incorporated more efficiently into the membrane and cause methoxylation[23,24]. It has been contemplated that the presence of these fatty acids in neem oil will confer the EC with anti-arthritic, antipyretic hypoglycemic, antigastric, spermicidal, diuretic, antimalarial, antibacterial, anti-inflammatory, immunomodulatory and antifungal properties. The antifungal efficiencies of fatty acids are directly proportional to their chain length. The hydrophobic group of tridecanoic acid plays an important role in bioactivity. However, the increase of hydrophobicity with longer chain length may reduce their solubility in aqueous systems.
| Lipid number of fatty acids | Relative percentage of fatty acids |
|---|---|
| C8 | 0.17 |
| C11 | 0.68 |
| C13 | 0.96 |
| C16 | 15.80 |
| C16:1(9) | 13.48 |
| C18:1(10) | 39.58 |
| C18:1(16) | 28.65 |
TABLE 1: FAME ANALYSIS OF NEEM OIL
In the present study, the emollient cream was formulated using 10% neem oil in an aqua base by a simple procedure. The APG emulsifier serves to coat the oil droplets and disperse them in the water medium. It also ensures stability of the EC by preventing droplet aggregation by steric based mechanisms. After ultrasonication, the EC presented a creamy appearance with a smooth silky feel. It was stored undisturbed in a brown bottle for 48 h at 37° to attain stability.
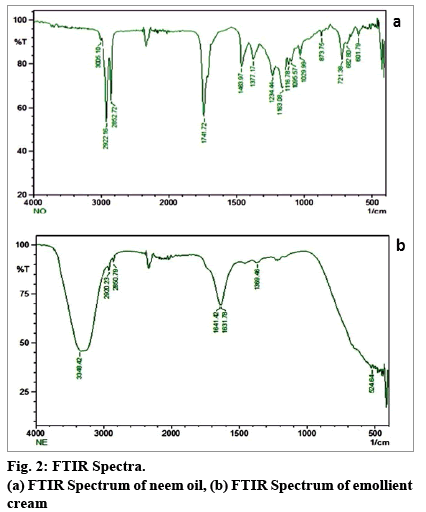
FTIR spectroscopy is a valuable technique for the detection of functional groups in components of medicinal and edible oils[25]. FTIR spectra of the oil and the EC are depicted in fig. 2a and 2b, respectively. Alkenes (=C-H stretch at 3005.10 cm-1 along with =C-H bend at 873.75 cm-1 and 721.38 cm-1), alkanes (C-H stretch at 2922.26 cm-1 and 2852.72 cm-1 along with C-H rock at 1377.17 cm-1 and C-C stretch at 1463.97 cm-1), aromatics (C-H: C-H bend; C-H "oop" at 682.80 and C-C stretch in ring at 1463.97 cm-1) esters (C-O stretches at 1741.72 cm-1, 1234.44 cm-1, 1163.08 cm-1, 1116.78 cm-1, 1095.57 cm-1 and 1029.99 cm-1) and alcohols (3005.10 cm-1 and 2922.26 cm-1) have been identified in the oil. These functional groups correspond to the compounds in the oil revealed by mass spectral analysis. Similar results have been reported by Tanvar et al[26]. In case of the EC, an extensive hydrogen bonding has been observed at 3348.42 cm-1. The other functional groups correspond to the skeletons of constituent fatty acids found in the neem oil.
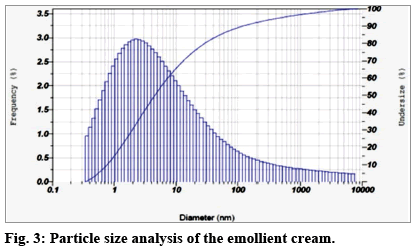
Important properties of particulate materials like quality, rheology and performance are determined by its mean particle diameter. This parameter also decides the extent of their textural feel and visual appeal. It is well established that nanometer sized particles ensure their effective end applications in any chosen field[27]. Hence in this study, ultrasonication was adopted as the method of choice to achieve particle size reduction of oil droplets. The same technique has been reported for the synthesis neem seed oil nanoemulsion using Tween 20 to achieve particle sizes between 50 and 169 nm[28]. The mean diameter of the particles in the EC after adopting this technique in our study was found to be 137 nm (fig. 3). This serves as a valuable indicator to ensure its superior quality and performance. Further, the EC is a polydisperse system that incorporates both the oil droplets and the constituent APG molecules. In this context, its Z average, viz., harmonic intensity averaged particle diameter and the mode value, viz., the particle size (or size range) that is most commonly found in the distribution analysis, assume great significance. These were found to be 1.9 nm and 2.2 nm, respectively. The fact that maximum number of particles are in the size range of 2.2 nm is attested by the polydispersity index (PI) that was found to be 0.245. The PI ensures long term stability of the EC, protecting it against destabilization mechanisms such as Ostwald’s ripening[29]. Hence although a fraction of the particles do show high individual diameters that lead to significant standard deviation from the mean value, these are relatively few in number. They are mainly due to free APG molecules that are not involved in oil droplet encapsulation. These molecules however cannot be dispensed with during the EC formulation as they play a very important role in the steric based stabilization of the overall system. They fill the gaps in the medium between any two oil droplets and prevent their aggregation/agglomeration by restricting their mobility. This aspect was confirmed here by measuring the mean electrophoretic mobility of the droplets that depicts their movement under the influence of an electric field. For this EC it was found to be -3.28 cm2/Vs. This favorable value is probably due to APG induced charge stabilization of the oil droplets as well as by their tight steric packing in the aqueous phase thus restricting movement of particles. Hence, destabilization mechanisms that are dependent on migrations of constituent particles in standing systems, do not function effectively here. Long term stability of the EC is thus ensured. Kabri et al. have reported similar results for emulsions formulated with Tween 80 and soy lecithin[30]. It is concluded that the lipophilic and hydrophilic characteristics of these emulsifiers are responsible for maintaining the equilibrium between positive and negative charges. A similar inference is made for the APG emulsifier used here in the formulation of our EC.
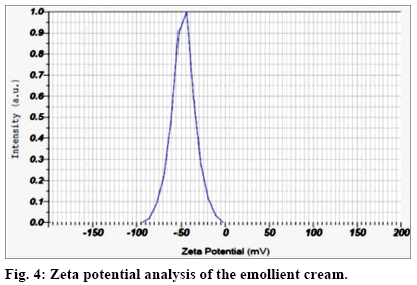
Mean zeta potential, that is a measure of the extent of charge distribution in the diffuse layer around the constituent droplets, was found to be -47.2 mV (fig. 4). A mean value of above or below ±30 is considered a reasonable cut off for systems to retain their stability by maintaining their constituent droplets in the dispersed state[31]. Zeta potential acts as an important determinant for delivery systems[32] and can be applied in favorable context in this study.
The SEM image of the emollient cream surface, captured at a magnification of 516× showed a undulating and wave like morphology (fig. 5). Sample preparation was done using egg albumin and this technique is particularly useful for opaque compounds that cannot be contemplated in detail using an optical microscope[33].
No signs of any visual destabilization in terms of creaming, cracking, oil droplet formation or phase separation were seen in the EC after low speed centrifugation or when stored at 37° for a period of 120 days. The color and texture also remained unchanged. Similar results were observed after the heating-cooling and freezing-thawing tests. Such results have been reported as indicators of stability in case of palm oil in water emulsions, formulated by employing span 83, tween 20, whey protein isolates and casein as emulsifiers[16]. It can be inferred that the EC is stable under the conditions tested in the study.
The water based neem oil and APG emollient cream synthesized here does not incorporate any chemical constituents in the formulation. The procedure adopted was very simple and the end product was stable with favorable structural indices as reported for other such formulations that incorporate numerous chemical constituents. Fatty acids and other reported components present in neem oil have been well established for their medicinal properties. They are probably also responsible for imparting stability to this product against time dependent oxidative damage. The potential of this emollient cream to serve as a therapeutically important product on its own or as a delivery system for other active pharmaceutical and cosmeceutical ingredients is tremendous. These applications are being currently explored in our laboratory with promising preliminary results.
Acknowledgments:
The authors thank the Management of VIT University, Vellore and Director, NAL, CSIR, Bangalore, for providing the infrastructure for successful completion of this study. We also acknowledge DST-FIST, Government of India for the SEM facility.
Financial support and sponsorship:
Nil.
Conflicts of interest:
There are no conflicts of interest.
References
- Tiwari R, Verma AK, Chakraborty S, Dhama K, Singh SV. Neem (Azadirachtaindica) and its potential for safeguarding health of animals and humans: A Review. Journal of Biological Sciences 2014;14:110-23.
- Dua VK, Pandey AC, Raghavendra K, Gupta A, Sharma T, Dash AP. Larvicidal activity of neem oil (Azadirachtaindica) formulation against mosquitoes. Malaria Journal 2009;8:1-6.
- Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem(Azadirachtaindica). Current Sci 2002;82:1336-45.
- Govindachari TR. Chemical and biological investigations on Azadirachtaindica. Current Sci 1992;63:117-22.
- Koul O, Isman MB, Ketkar CM. Properties and uses of neemAzadirachtaindica. Canadian J Bot 1990;68:1-11.
- Raizada BR, Srivastava MK, Kaushal RA, Singh RP. Azaridachtin, a neembiopesticide: subchronic toxicity assessment in rats.FoodChemToxicol2001;39:477-83.
- Sampathraj R, Narayanan PBS, VanithaKumari C. Effect of neem oil: structural and functional changes in the epididymis of rats. In: World Neem Conference, India, 1993, 1173-81.
- Deng YX, Cao M, Shi DX, Yin ZQ, Jia R Xu J. Toxicological evaluation of neem (Azadirachtaindica) oil: Acute and subacute toxicity. Environ ToxicolPharmacol 2013;35:240-46.
- Winkaler UE, Santos TRM, Machado-Neto JG, Martinez CBR. Acute lethal and sublethal effects of neem leaf extract on the neotropical freshwater fish Prochiloduslineatu.Comp BiochemPhysiol C PharmacolToxicolEndocrinol2007;145:236-44.
- Batra CP, Mittal PK, Adak T, Sharma VP. Efficacy of neem-water emulsion against mosquito immatures. Indian J Malariol 1998;35:15-21.
- Singh A, Hamme JDV, Ward OP. Surfactants in Microbiology and Biotechnology: Part 2 Application aspects. BiotechnolAdv 2007;25:99-121.
- Siddiqui BS, Rasheeda M, Ilyas F, Gulzar T, Tariq RM, Naqvi SM. Analysis of insecticidal AzadirachtaindicaA. Juss. Fractions. Z. Naturforsch 2004;59:104-12.
- Frankel EN, Huang SW, Kanner J, German JB. Interfacial phenomena in the evaluation of antioxidants: bulk oils vs emulsions. J Agric Food Chem1994;42:1054-9.
- Holmberg K. Natural surfactants. CurrOpin Colloid Interface Sci 2001;6:148-59.
- Durga KK., Vijaya R. Statistical affirmative testing tool for various adulterants. Res J ApplSci, EngTechnol 2012;4:5357-60.
- Thanasukarn P, Pongsawatmanit R, Mc Clements DJ. Influence of emulsifier type on freeze-thaw stability of hydrogenated palm oil-in-water emulsions. Food Hydrocolloids 2004;18:1033-43.
- National Industrial Chemicals Notification and Assessment Scheme. (NICNAS), Full Public Report. ArachidylGlucoside 2007, 131.
- SandanasamyJ,Nour AZ, Tajuddin SNB, Nour AH. Fatty acid composition and antibacterial activity of neem (Azadirachtaindica) seed oil. Open ConfProc J 2013;4:43-8.
- McLain N, Ascanio R, Baker C, Strohaver RA, Dolan JW. Undecylenic acid inhibits morphogenesis of Candida albicans. AntimicrobAgentsChemother2001;6:2873-5.
- Liu S, Weibin R, Jing L, Hua X, Jingan W, Wang J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008;166:93-102.
- Parang K, Knauss EE, Wiebe LI, Sardari S, Daneshtalab M, Csizmadia F. Synthesis and antifungal activities of myristic acid analogs. Archiv der Pharmazie 1996;329:475-82.
- Verschoor JA, Baird, MS, Grooten J. Towards understanding the functional diversity of cell wall mycolic acids of Mycobacterium tuberculosis. Prog Lipid Res 2012;51:325-39.
- Walters D, Raynor L, Mitchell A, Walker R, Walker K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004;157:87-90.
- Bergsson G, Arnfinnsson J, Steingrimson O, Thormar H. In vitro killing of Candida albicansby fatty acids and Monoglycerides. AntimicrobAgentsChemother 2001;45:3209-12.
- Siddiqui N, Ahmad A. Infrared spectroscopic studies on edible and medicinal oils. Int J Sci Environ Technol 2013;2:1297-306.
- Tanwar D, Ajayta, Sharma A, Mathur YP. Production and characterization of neem oil methyl ester. Int J Eng Res Technol 2013;2:1896-903.
- Vijayan V, Aafreen S, Sakthivel S, Reddy KR. Formulation and characterization of solid lipid nanoparticles loaded neem oil for topical treatment of acne. J Acute Disease 2013;2:282-6.
- Ghotbi RS, Khatibzadeh M, Kordbacheh S. Preparation of neem seed oil nanoemulsion. Proceedings of the 5th International Conference on Nanotechnology: Fundamentals and Applications, Prague, Czech Republic, Paper No. 150, 2014, 11-13.
- Yilmaz E, Borchert HH. Design of a phytosphingosine-containing, positively-charged nanoemulsion as a colloidal carrier system for dermal application of ceramides. Eur J Pharm Biopharm 2005;60:91-8.
- Kabri T, Tehrany EA, Belhaj N, Linder M. Physicochemical characterization of nanoemulsions in cosmetic matrix enriched on omega-3. J Nanobiotechnol2011;9:1-8.
- Wiacek A, Chibowski E. Zeta potential, effective diameter and multimodal size distribution in oil/water emulsion. Colloids Surf A PhysicochemEng Asp 1999;159:253-61.
- McClements DJ, Decker EA, Weiss J. Emulsion based delivery systems for lipophilic bioactive components. J Food Sci 2007;72:109-24.
- Rajendran R, Radhai R , Balakumar C, Ahamed HAM, Vigneswaran C, Vaideki, K. Synthesis and characterization of neem chitosan nanocomposites for development of antimicrobial cotton textiles. J Eng Fibers Fabrics 2012;7:136-41.