- *Corresponding Author:
- I. A. Ghazi
Department of Plant Sciences, School of Life Sciences, University of Hyderabad, Prof. C. R. Rao Road, Gachibowli, Hyderabad-500 046, India
E-mail: irfan@uohyd.ernet.in
| Date of Submission | 06 September 2016 |
| Date of Revision | 07 March 2017 |
| Date of Acceptance | 03 September 2017 |
| Indian J Pharm Sci 2017;79(6): 872-884 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Medicinal plants serve as unlimited source for phytoconstituents that possess potent antioxidant and antiproliferative properties. Artemisia nilagirica L. is a potent medicinal plant widely found in India and has been used to treat human diseases for centuries. The present investigation was conducted to investigate the antioxidant and antiproliferative abilities of different crude extracts of A. nilagirica. A detailed study was performed on the antioxidant activity and antiproliferative activity of the methanol extract of A. nilagirica by in vitro chemical analysis. The chemical composition of extracts, studied in terms of phenolics, total flavonoids, triterpenoids, tannins and alkaloids were also determined. Results suggested that the amounts of different phytoconstituents in extracts vary based on polarity of solvents. The extracts possessed different antioxidant and radical-scavenging activities in different assays. Among the extracts, ethyl acetate and alcohol extracts showed the most potent radical-scavenging activities. Ethyl acetate and alcohol extracts showed cytotoxicity towards eight different human cancer cell lines in vitro with IC50 values ranging between 30.43±0.86 and 982.46±14.40 μg/ml and they were less toxic to normal cell lines. The findings of this study provide evidence that A. nilagirica extracts can be used potentially as ready accessible and valuable bioactive source for isolation of antioxidant and anticancer agents.
Keywords
Medicinal plants, Artemisia nilagirica, ROS, extracts, antioxidant, antiproliferative
Free radicals like various reactive oxygen species (ROS) are usually the products of normal cellular metabolism in the living systems. However, excessive production of ROS due to disruption of the delicate balance between the antioxidant system and ROS generation is responsible for oxidative stress which results in impairment of cellular macromolecules, like lipids, DNA and proteins [1]. This imbalance is eventually associated with the aetiology of various disorders such as cancers, inflammation, Parkinson's disease, Alzheimer's disease, hypertension, diabetes, atherosclerosis, cardiovascular diseases, immunological disorders and the aging process [2]. ROS are considered as tumorigenic due to their capacity to enhance cell growth, survival and cellular migration. Moreover, they are also responsible for DNA damage, which manifests in the form of genetic abnormalities that induce tumorigenicity and sustain subsequent tumour progression [3]. Malignancy is one of the most deadly diseases and foremost public health concern in both developing and developed world. According to the American Cancer Society, mortalities resulting due to this fatal disease comprise 2-3% of the annual deaths globally [4]. Additionally, present-day approaches of cancer treatment such as radiotherapy, chemotherapy, immunosuppression and surgery are associated with high mortality rate. In spite of a considerable progress in cancer treatment during the recent past, drug resistance and the hostile side effects of the established cytostatic compounds is a primary cause of concern. Moreover, the outcome of currently available methods of treatment for various cancers is gloomy, especially when the cancer has progressed till the metastatic stage. This has necessitated an indispensable requirement for the development of novel treatment methodologies, which are safe as well as effective in preventing, arresting or reversing the molecular and cellular processes of carcinogenesis.
Antioxidants provide a cellular defence mechanism against oxidative stress within the human body. They have the potential to quench ROS by donating hydrogen atom or electrons, chelating transitional metal ions, activation of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) and also inhibiting the oxidising entities. However, these defensive mechanisms are sometimes insufficient to prevent the damage entirely due to the various pathological processes, therefore, antioxidant supplements are vital to combat oxidative damage [5]. Investigations on medicinal plants have reclaimed significance in the modern times due to their increasing comprehensive biological properties and emerging recognition of their origin, function and structural diversity [6]. Medicinal plants are receiving great attention because of their significant antioxidant potential, minimal adverse effects and economic affordability [7]. They contain a wide variety of chemical constituents, which may act individually or synergistically to cure diseases associated with oxidative stress and improve health [8]. Medicinal plant materials (and their derived phytochemicals) have been extensively used to treat various types of cancers [9]. In contrast to cytotoxic agents that induce damage to cancer cells, antioxidants prevent the initiation of cancer during the process of carcinogenesis and therefore, are considered beneficial to the cells. Approximately 60% of all the approved drugs used in the treatment and/or prevention of cancers find their origin from natural products or natural product derivatives, of which plants contribute around 25% [10]. This has invoked the quest for efficient antioxidant and antiproliferative entities from different natural sources especially medicinal and aromatic plants [11].
Artemisia nilagirica (syn. A. vulgaris, Asteraceae) is an aromatic perennial shrub, which grows in the hilly areas of India up to 1500 m elevation [12]. Traditionally, in Ayurveda and Siddha, this plant is often used for the treatment of variety of ailments such as diabetes, epilepsy, asthma, psychoneurosis, depression, anxiety and stress [13]. The plant is also useful for leucorrhoea, threatened abortion, haemoptysis, skin diseases, vomiting, colic, rheumatism and fever [12]. Few experimental studies regarding the therapeutic activity of A. nilagirica reported a plethora of pharmacological properties of this plant including; antibacterial [14], antifungal [15], cytotoxic [16], antioxidant [17]. This prompted us to hypothesize that this plant could demonstrate broader antioxidant and antiproliferative activities. Therefore, the present study was designed to investigate the various extracts obtained through successive extraction for antioxidant activities using a series of in vitro and antiproliferative effects on multiple cancer cell lines. The extracts were also subjected to quantify the presence of phytochemical constituents such as total phenolics, total flavonoids, tannins, and titerpenoids.
Materials and Methods
The analytical grade chemicals were procured from HiMedia and Merck, India. Standard drugs, RPMI- 1640, Dulbecco’s modified Eagle’s medium (DMEM) and foetal bovine serum (FBS) from Gibco (USA) and Sigma Aldrich Chemicals Co. (USA). The cell lines were obtained from National Centre for Cell Sciences (NCCS), Pune, India whereas, the peritoneal macrophages were isolated from female BALB/C mice after official permission from University of Hyderabad, School of Life Science’s Animal Ethics Committee, vide approval No. LS/IAEC/IAG/11/10, dated April 15, 2011.
Plant material
The leafy aerial parts of A. nilagirica plants were collected in the month of July-September, 2012 from Survey of Medicinal Plant Unit, Central Research Institute of Unani Medicine, Hyderabad. A voucher specimen (UoH/VS/AN-3) has been preserved for future references.
Preparation of extracts
The plant material (leafy aerial portion of A. nilagirica) was dried in the shade, coarsely powdered in a mechanical blender and then subjected to successive extraction in a Soxhlet apparatus using the solvents of n-hexane, ethyl acetate, ethanol and water at room temperature. The material was allowed to dry in hot air oven at 40° each time before the next extraction with another solvent. After the successive extraction, the extracts were filtered and dried with the aid of rotary evaporator (Buchii, USA) under reduced pressure at temperatures below 45° and water was removed by lyophilisation. Different extracts were designated as hexane extract (ANH), ethyl acetate extract (ANE), ethanol extract (ANA) and water extract (ANW) and the resulting crude extracts were stored at –20° until assayed. The stock solution of 20 mg/ml of each extract was prepared in dimethyl sulfoxide (DMSO) and further diluted with phosphate-buffered saline (PBS, pH 7.4) to yield specific working concentrations.
Determination of phytoconstituents
The quantification of phytoconstituents in different solvent extracts of A. nilagirica were carried out using standard quantitative methods as described previously [5,18-20]. The components analysed for phytochemicals were total phenolics, flavonoids, triterpenoids, tannins and alkaloids.
Antioxidant ability assays
Antioxidant potential of different extracts from A. nilagirica was determined by employing series of methods like phosphomolybdenum method, the ferric reducing power (FRAP) assay, Fe2+ and Cu2+ chelating ability.
Phosphomolybdenum assay
The total antioxidant potential of different extracts was evaluated by the green phosphomolybdenum complex formation according to the previously described method [21]. Briefly, an aliquot of 10 μl of sample solution was mixed with 1 ml of reagent solution (0.6 M sulphuric acid, 28 mM ammonium molybdate) in micro centrifuge tubes. The tubes were incubated in a dry thermal bath at 95° for 90 min. Subsequently, the absorbance of the mixture solution was measured at λ 695 nm against black. Ascorbic acid was used as positive control. The antioxidant capacity was estimated using following Eqn., antioxidant effect=(control absorbance–sample absorbance)/ (control absorbance)×100. The reducing capacities of extracts were expressed as mg of ascorbic acid equivalents (mg AAE)/g of dry weight (dw).
Ferric-reducing/antioxidant power (FRAP) assay
The Fe3+ reducing power of the extracts was determined by the described method with slight modifications [22]. Briefly, extracts and standard solution of ascorbic acid in 1 ml of appropriate solvents were mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide (1%). The mixtures were incubated separately at 50-60° for 30 min. Later, 2.5 ml of trichloroacetic acid (10%) was added to each mixture, which was then centrifuged at 5000 rpm for 10 min. Finally, 2.5 ml of the upper layer solution of each mixture were mixed with 2.5 ml of distilled water and 0.1 ml of FeCl3 (0.1% w/v). The absorbance was measured at λ 700 nm. The antioxidant power was calculated using the following Eqn., antioxidant effect= [(control absorbance–sample absorbance)/ (control absorbance)]×100.
Chelating activity on Cu2+
The Cu2+-chelating activities of extracts were assessed according to previous methods [23,24]. Concisely, 60 μl of 20 mM CuSO4 aqueous solution was added to hexamine HCl buffer (30 mM; pH 5.3) containing 30 mM KCl and 0.20 mM murexide. After incubation at room temperature for 1 min, an addition of 10 μl sample solutions was added to the mixture. The final volume was adjusted to 1.5 ml with methanol. Then, the mixture was vortexed vigorously and further incubated at room temperature for 10 min. Finally the absorbance of the solutions was then measured by UV/Vis spectrophotometer at λ 485 nm and λ 520 nm. The absorbance ratio (A485/A520) reflected the free Cu2+ content. Hence, the percentage of cupric chelating effect was calculated by the following Eqn., relative chelating effect (%)=(A485/A520)max–(A485/A520)sample/ (A485/A520)max–(A485/A520)min×100, where, (A485/A520)sample is the absorbance ratio of the mixtures with sample, (A485/A520)max is the maximum absorbance ratio without sample and (A485/A520)min is the minimum absorbance ratio of mixture without CuSo4 aqueous solution and sample in the test.
Chelating activity on Fe2+
The extracts were further evaluated for their capability to compete with ferrozine for iron (II) ions in a free solution as per the protocol reported [25]. Extracts (50-500 μg/ml) were dissolved in a solution of 0.1 ml of 2 mM FeCl2.4H2O. The reaction was triggered by adding 0.2 ml of 5 mM ferrozine and the reaction mixture was shaken briskly and left standing at room temperature for 10 min to allow the solution to reach the equilibrium. Absorbance of the solution was then taken at λ 562 nm against the blank prepared in similar manner with FeCl2 and water. EDTA at the concentration range of 1-25 μg/ml used as the positive control and sample mixture devoid of extract or EDTA served the negative control. The percentage of inhibition of ferrozine-Fe2+ complex formation was calculated using the following Eqn., chelating activity (%)=(Acontrol-Asample/Acontrol)×100.
Radical scavenging ability assays
Radical scavenging capability of the extracts was determined and compared to that of 2,2´-azinobis [3-ethylbenzthiazoline]-6-sulfonic acid (ABTS), 2-diphenyl-2-picryl hydrazyl hydrate (DPPH), •OH, NO•, SO2•- and lipid peroxidation methods. Antioxidant potential of the extracts of A. nilagirica was measured by ABTS assay [26]. Briefly, 7 mM ABTS was made by dissolving it in double distilled water and subsequently mixed with a 2.45 mM potassium persulphate solution to yield a radical cation (ABTS*+). The reaction mixture was left to settle in dark at room temperature for 12-16 h. Prior to the experiment, freshly prepared ABTS+• solution was diluted with ethanol to achieve an absorbance of 0.700±0.02 at λ 734 nm wavelength. About 10 μl of each extract or standard was added to each well containing 190 μl of ABTS*+ solution. The reaction mixture was gently shaken and incubated for 15 min under dark conditions at room temperature. Subsequently, the absorbance was measured spectrophotometrically at λ 734 nm using a microtiter plate reader (TECAN, Switzerland). The ABTS+• scavenging capacity of the extracts was compared with those of ascorbic acid and the percentage inhibition calculated as: ABTS radical scavenging activity (%)=(Abscontrol– Abssample)/Abscontrol×100. Radical scavenging activity of different extracts against stable DPPH was measured as per our previous report [27].
The scavenging activity of different extracts of A. nilagirica (40-400 μg/ml) on hydroxyl radical activity was measured according to the previously described method [5]. The colour intensity formed was measured spectrophotometrically at λ 532 nm against the blank sample. The hydroxyl radical scavenging activity of the extracts was determined as percentage of antioxidant activity.
The nitric oxide radical quenching potential was further confirmed by sodium nitroprusside method [28]. The reaction solution of 50 μl containing 10 mM sodium nitroprusside in PBS (pH 7.0) was mixed with different concentration (40-400 μg/ml) of extracts and incubated at 37° for 20 min under light conditions. Thereafter, the samples were further mixed with 300 μl of Griess reagent (1% sulphanilamide, 2% H3PO4). All the samples were again incubated for 30 min at ambient temperature followed by the addition of 0.1% N- (1-naphthyl) ethylenediamine dihydrochloride. The absorbance was recorded at λ 546 nm and the results were expressed as percent of scavenged nitric oxide with respect to the negative control without addition of any antioxidant.
The scavenging activity in terms of superoxide anions was evaluated by the nitro-blue tetrazolium (NBT) reduction method [29] with little modifications. Briefly, the superoxide anions were generated in 2 ml of phosphate buffer (100 mM, pH 7.4) containing 500 μl of 156 μM NBT solution, 500 μl of 468 μM nicotinamide adenine dinucleotide (NADH) solution. The mixture was treated with different extracts at a concentration range of 40-400 μg/ml. The reaction was initiated by adding 100 μl of 60 μM phenazine methosulfate (PMS) to the mixture and after 5 min of incubation at room temperature, the absorbance was measured at λ 560 nm against blank. DMSO and ascorbic acid were used as solvent and positive controls, respectively.
Inhibition of lipid peroxidation assay
A modified thiobarbituric acid-reactive species (TBARS) assay [30] was employed to evaluate inhibition of lipid peroxidation using rat liver homogenate as lipid rich medium. Healthy Wister albino rats (250 g) were sacrificed (procedure was reviewed and approved by the University of Hyderabad, School of Life Sciences’ Animal Ethics Committee) and liver was perfused with 0.15 M KCl, homogenate was centrifuged at 800 g for 15 min at 4° and the supernatant was used for thiobarbituric acid assay [5].
Antiproliferative activity
A panel of eight cancer cell lines namely; (a) human acute monocytic leukaemia cell line-THP-1, (b) human T-cell lymphoblastic lymphoma- SupT1, (c) human B-cell lymphoma leukaemia-JM1, (d) human hepatocellular carcinoma cells-HepG2, (e) human cervix adeno carcinoma-HeLa, (f) human gastrocarcinoma cells- AGS, (g) rat tumour glial cells-C6, (h) human chronic myelogenous leukaemia-K562 were used to study the antiproliferative activity. The murine peritoneal macrophages were used as a model for normal cells.
The cell lines HepG2, HeLa, C6, were cultured in DMEM, supplemented with 4.5 g/l D-glucose, L-glutamine; THP-1, K562, AGS, SupT1 were grown in Roswell Park Memorial Institute medium (RPMI) 1640 supplemented with L-glutamine. JM1 cells were cultured in Iscove's Modified Dulbecco's Medium (IMDM) supplemented with L-glutamine and 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 2-mercaptoethanol. In addition, all the three media were supplemented with 10% (v/v) heat inactivated FBS, 100 U/ml penicillin and 100 μg/ml streptomycin, antibiotics.
Thioglycollate-elicited mouse peritoneal exudate cells were obtained from mice following intraperitoneal injection of 3 ml thioglycollate medium (3 g/100 ml) and lavage of the peritoneal cavity with 5 ml of ice cold PBS (10 mM, pH 7.2) 3-4 d later [31]. Cells were washed, resuspended in HEPES-buffered RPMI-1640 medium (supplemented with 10% heat inactivated FBS and antibiotics: 100 U/ml penicillin G and 100 mg/ml streptomycin and 50 mM 2-mercaptoethanol. These cells were seeded (5×104 cells/ml) in sterile disposal plates (60 mm). Cells were maintained in a humidified incubator with 5% CO2 at 37° during growth and treatments.
In vitro cell viability assay
Cells were seeded in 96-well plates at a density of 1×104 cells/ml in 200 μl of complete media and incubated for 24 h at 37° in 5% CO2 atmosphere. Cytotoxicity was evaluated by means of a growth inhibition using the 3- [4,5-dimethylthiazol-2yl]-2,5- diphenyltetrazolium bromide (MTT) assay [32]. Briefly the sample extracts (25-200 μg/ml) initially dissolved in DMSO (not exceeding the concentration of 1%) and doxorubicin (standard drug) were further diluted in cell culture medium and added into a 96-well plate. After 48 h of incubation, 20 μl of MTT reagent (5 mg/ml) were added and mixtures were re-incubated for 4 h. Following incubation, the media were removed and the purple formazan precipitate in each well was dissolved in 200 μl DMSO. Absorbance was measured using microplate reader at λ 570 nm and results were expressed as percentage viability which is directly proportional to metabolically active cell number. Control group containing without treatment was run in each assay. Percent viability was calculated as: percent viability=OD in sample well/OD in control well×100.
Statistical analysis
The results were expressed as mean±standard deviation (SD) values average from 3 to 4 independent experiments performed in triplicate. IC50 value (the concentration of the extracts required to scavenge/ inhibit 50% of radicals/cell growth) was calculated for different extracts. The statistical calculations were performed using GraphPad Prism version 6. Statistical differences between the samples were evaluated using appropriate statistical tests (one-way ANOVA, repeated measures ANOVA, Student’s t-test). A P-value of ≤0.05 was considered significant where probability values were found to be equal to or less than 0.05. The graphical representation of the results was performed using GraphPad Prism version 6 (San Diego, USA) and Sigma plot version 11.0 (USA) software’s.
Results and Discussion
Total phenolic content (TPC) of four different extracts of A. nilagirica were measured using the Folin-Ciocalteu method, and the results are shown in Table 1. The TPC ranged from 0.44±0.04 to 12.13±0.20 mg GAE/g. ANA showed the highest phenolic content (12.13±0.20 mg GAE/g), followed by ANW (4.00±0.03 mg GAE/g), ANE (3.52±0.09 mg GAE/g), whereas ANH showed the lowest phenolic content (0.44±0.04 mg GAE/g) of these extracts.
| Sample | Phenols (mg GAE/g dw) |
Flavonoids (mg QE/g dw) |
Tannins (mg TAE/g dw) |
Triterpenoids (mg UAE/g dw) |
Alkaloids (mg/g dw) |
Total antioxidant activityf (mg AAE/g dw) |
FRAP (mg AE/g dw) |
|---|---|---|---|---|---|---|---|
| ANH | 0.44±0.04ab | 3.02±0.05b | -- | 20.56±1.09a | -- | 0.49±0.97b | 1.27±0.02b |
| ANE | 3.52 ±0.09b | 16.63±1.18a | 1.09±0.11b | 13.07±1.58ab | 2.14±0.27b | 15.92±0.44a | 3.69±0.13a |
| ANA | 12.13±0.20a | 5.0±0.46b | 2.70±0.19ab | 7.56±0.67b | 1.31±0.31b | 8.16±1.45a | 4.43±0.11a |
| ANW | 4.00±0.03b | 4.30±0.10b | 10.11±0.34a | 2.87±0.28b | 1.67±0.05b | 5.58±1.23b | 1.94±0.03b |
Each value is represented as mean±SD (n=3). aHighest value and significantly different (P≤0.05) from other values (ab and b) of each row; absignificantly different (P≤0.05) from b only
Table 1: Phytoconstituents and antioxidant power of A. nilagirica extracts
The quantitative analysis of total flavonoid content of various crude extracts of A. nilagirica revealed that the ANE contained highest amount of TPC (16.63±1.18 mg QE/g). The moderate amounts were recorded in ANA (5.0±0.46 mg QE/g) and ANW (4.30±0.10 mg QE/g) and least amount of TFC was found in ANH (3.02±0.05 mg GAE/g). The results are presented in Table 1.
The total triterpenoid content of the four crude extracts was evaluated by calorimetry with ursolic acid as the standard reference. The total triterpenoid content of the four extracts varied widely from 2.87±0.28 to 20.56±1.09 mg ursolic acid/g dw of extract. The lowest total triterpenoid content was found in ANW extract, whereas the ANH extract provided the highest triterpenoid content of the extracts. This result suggested that ANH had highest concentration of triterpenoid compounds out of the four extracts.
The results for tannin content in different extracts are presented in Table 1. In total tannin contents were maximum in ANW extract (10.11±0.34 mg TAE/g dw extract), followed by ANA (2.70±0.19 mg TAE/g dw). The least amount of tannins was obtained from ANE extract (1.09±0.11 mg TAE/g dw). ANH did not show any presence of tannins in the experimental conditions. Therefore, our results suggested that the tannins content were higher in ANW as compared to ANA and ANE.
The alkaloid content of extracts of A. nilagirica ranged from 1.31±0.31 to 2.14±0.27 mg/g dw). The ANE extract had more alkaloid content i.e. 2.14±0.27 mg/g than other extracts. The results are shown in Table 1.
Total antioxidant potential of the different extracts of A. nilagirica was evaluated by phosphomolybdenum method and was presented as AAE per gram of plant extract. The investigated extracts were significantly active in reduction of Mo(VI) to Mo(V). Among the tested extracts, the highest total antioxidant capacity was observed for ANE with a value of 15.92±0.44 mg AAE/g dw. of extract. The ANH showed least antioxidant capacity (0.49±0.97), whereas other two extracts showed moderate phosphomolybednum reduction (Table 1).
FRAP assay was used to evaluate antioxidant capacities of extracts. In general, the extracts under study had significant antioxidant abilities. As indicated in Table 1, the FRAP values varied from 1.27±0.02 to 4.43±0.11 mg AAE/g dw. ANA had the highest FRAP value (4.43±0.11 mg AAE/g dw.) and ANH showed the lowest FRAP value (1.27±0.02 mg AAE/g dw.) among the tested extracts.
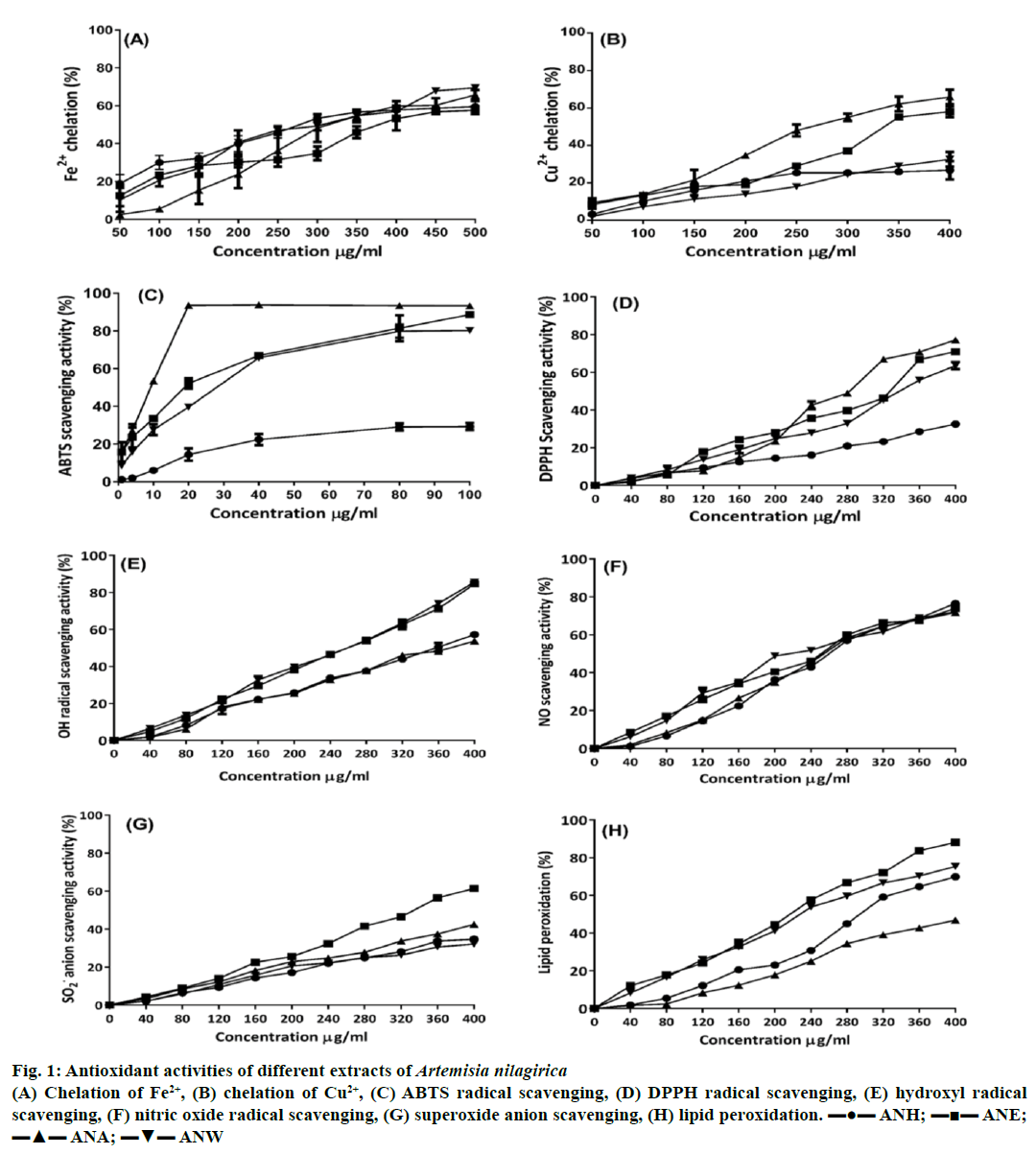
The results of chelating activity on Fe2+ established that the formation of ferrozine-Fe2+ complex is inhibited in presence of extracts and standard compound (Table 2 and Figure 1A). In this experiment, the extracts and the EDTA interfered with the formation of ferrozine and ferrous complex suggesting that they possess efficient chelating activity and are able to capture ferrous ion before ferrozine. The extracts decreased the absorbance of the complex solution in a concentration dependent manner. The iron chelating property of the plant extracts at the highest concentration of 500 μg/ml was observed in accordance with the order of their IC50 values (Table 2).
| Scavenging/Chelating assay | Type of Extract | ||||
|---|---|---|---|---|---|
| ANH | ANE | ANA | ANW | Standard compound | |
| DPPH• | 713.50±6.23 | 305.25±1.32 | 266.13±1.21 | 337.95±4.21 | 6.86±0.92# |
| ABTS | 171.48±8.00 | 19.45±0.81 | 9.37±0.18 | 25.28±0.60 | 2.13±0.13# |
| •OH | 345.06±9.11 | 247.52±8.71 | 335.51±3.91 | 232.02±7.41 | 62.40±3.72# |
| H2O2 | 193.23±2.34 | 115.12±3.67 | 88.23±5.11 | 187.67±2.15 | 245.30±4.60# |
| NO• | 209.20±7.33 | 257.30±6.78 | 264.60±1.71 | 258.09±5.43 | 19.90±2.30# |
| •O2 | 609.99±8.12 | 330.32±7.12 | 509.94±1.71 | 536.12±4.33 | 32.86±3.78# |
| LPO | 297.35±5.78 | 229.64±7.89 | 451.06±4.95 | 236.21±9.00 | 48.72±3.20# |
| Fe2+ | 883.27±17.85 | 303.44±16.67 | 357.70±9.78 | 342.12±8.34 | 6.44±0.44≠ |
| Cu2+ | 748.65±26.43 | 550.45±19.71 | 260.52±5.80 | 620.31±21.13 | 275.69±6.52† |
Each value is represented as mean±SD (n=3) and expressed in μg/ml of extract. #Ascorbic acid; ≠EDTA; †sodium citrate
Table 2: IC50 of A. nilagirica extracts on tested radicals
Figure 1: Antioxidant activities of different extracts of Artemisia nilagirica
(A) Chelation of Fe2+, (B) chelation of Cu2+, (C) ABTS radical scavenging, (D) DPPH radical scavenging, (E) hydroxyl radical
scavenging, (F) nitric oxide radical scavenging, (G) superoxide anion scavenging, (H) lipid peroxidation. ▬●▬ ANH; ▬■▬ ANE;
▬▲▬ ANA; ▬▼▬ ANW
The ability to chelate cupric ions varied widely for the different extracts tested. ANH at the concentration chelated only 26.71±4.87 of Cu2+ ions while ANA was the most potent Cu2+ ion chelator exhibiting an IC50 of 260.52±5.80 μg/ml. The results about the Cu2+ chelating activities of the plant extracts and the control are listed in Table 2; Figure 1B. The Cu2+ binding ability of the extracts is an indication of the fact that the extracts possess significant amounts antioxidant compounds.
Variations for the ABTS•+ radical cation scavenging activities of each extract and positive control, ascorbic acid was observed in our study (Table 2; Figure 1C). Among various extracts of A. nilagirica, ANA possessed the highest ABTS radical scavenging activity (93.30±0.20%) with IC50 value of 9.37±0.18 μg/ml while ANH displayed lowest ABTS radical scavenging activity (29.26±1.94%) with IC50 value of 171.48±8.00 μg/ml) at the highest concentration of 100 μg/ml. The percentage inhibition of other two extracts, ANE and ANW was found to be 88.60±1.32 and 80.20±1.36%.
The percentage inhibition of samples was assayed by DPPH radical scavenging method and the corresponding percentage inhibitions and IC50 values were calculated. As displayed in Table 2, IC50 values for the different extracts showed wide variability, ranging between 266.13±1.21 and 713.50±6.23 μg/ml. The order of DPPH scavenging against the tested extracts was found to be ANA>ANE>ANW>ANH. ANA with an IC50 value of 266.13±1.21 μg/ml, showed particularly high free-radical scavenging activity, followed by ANE (305.25±1.32) and ANW (337.95±4.21). ANH (713.50±6.23) exhibited the lowest activity among the examined extracts, since it required much higher concentrations to reduce 50% of free-radical concentrations. Ascorbic acid-positive control, exhibited highest scavenging effects at minimal concentrations (Table 2; Figure 1D).
The extracts of A. nilagirica scavenged •OH radical in a concentration dependent manner. The IC50 values ANW, ANE, ANA and ANH were 232.02±7.41; 247.52±8.71; 335.51±3.91 and 345.06±9.11 μg/ml, respectively. ANW was found to be more potent with the percentage inhibition of 85.55±1.14% at the highest concentration of 400 μg/ml compared to other extracts. ANE also quenched the •OH in a significant manner with percentage inhibition reaching close to ANW (84.60±0.98. However, ANA and ANH were weak scavengers compared to ANW and ANE as evident from their IC50 values (Table 2; Figure 1E).
The scavenging of NO• by A. nilagirica extracts was concentration-dependent, exhibiting significant decrease in the NO•. ANH with an IC50 value of 209.20±7.33 μg/ml inhibited a maximum of 76.47±0.41% of NO• at the highest concentration of 400 μg/ml. Furthermore, considerable inhibition of NO• was exhibited by ANE, ANW and ANA extracts with maximum inhibition of 73.93±1.19, 72.11±0.57 and 71.75±1.23%, respectively at the same concentration of 400 μg/ml. The effective concentrations at which 50% of the NO• radicals were inhibited by these extracts (257.30±6.78, 258.09±5.43, 264.60±1.71 μg/ml, respectively) were not statistically different (Table 2; Figure 1F).
We also investigated the capacity of A. nilagirica extracts to scavenge the superoxide and thus diminish the reduction of NBT. The reduction in absorbance at 560 nm upon the addition of extracts as well as of the reference compound indicated the consumption of superoxide anion in the reaction mixture. The scavenging activity of superoxide anion radicals was enhanced by raising the concentration of extracts from 40 to 400 μg/ml, as illustrated in Table 2 and Figure 1G. Among the extracts, the ANA was found to be potent superoxide radical scavenger with the percentage inhibition of 88.23±5.11 while ANH was the least efficient in terms of quenching activity with percentage inhibition of 34.68±0.50. Other extracts, ANE and ANW also presented significant scavenging activity towards the superoxide anions. As evident, ANA extract was a better scavenger of superoxide anion as its counterparts.
The effect of extracts in retaining MDA production by percent antilipid peroxidation was conducted in 10 different concentrations on liver homogenate which was induced by Fe2+ ion (Table 2; Figure 1H). All the tested extracts demonstrated that the antioxidant activities were in concentration dependent manner against Fe2+ induced lipid peroxidation. Of all the extracts, ANE revealed maximum antioxidant activity with percentage inhibition of 88.15±0.99% with the IC50 value of 229.64±7.89 μg/ml. The potential antioxidant was indicated in the following decreasing order ANE>ANW>ANH>ANA. The result showed that extracts had the capacity to protect the liver membrane phospholipids from undergoing oxidative deterioration.
In the present study, the screening of cytotoxic effect of plant extracts from A. nilagirica on eight different cancer cell lines was analysed by conducting MTT cell viability assay. Cultures of selected cell lines were treated with different concentrations of extracts (50, 100, 200 μg/ml) and the effect on the survival of cells is shown in Table 3. The present report shows that ANE and ANA exert inhibitory effects on selected cell lines in a concentration dependent manner with significant difference in selectivity (P<0.05). On the other hand, ANH and ANW extracts failed to inhibit the proliferation of selected cancer cells considerably, therefore suggesting their non-cytotoxic properties (Table 3).
| Cell line | Sample | Percentage inhibition (200 µg/ml) |
IC50 Value (µg/ml) |
|---|---|---|---|
| THP-1 | ANE | 89.25±1.22* | 38.21±7.37** |
| ANA | 61.21±12.00 | 132.41±7.19 | |
| Doxorubicin | 91.80±0.58 | 1.63±0.58 | |
| SUPT-1 | ANE | 76.28±4.01 | 59.02±5.90** |
| ANA | 78.18±6.00 | 112.24±3.66 | |
| Doxorubicin | 92.93±0.52 | 1.89±0.52 | |
| JM-1 | ANE | 77.33±1.63* | 35.87±1.68* |
| ANA | 68.80±4.45 | 40.91±9.78* | |
| Doxorubicin | 92.93±0.52 | 1.79±1.66 | |
| K562 | ANE | 93.35±3.18** | 30.43±0.86** |
| ANA | 66.41±2.81 | 126.45±3.56* | |
| Curcumin | 83.96±2.79 | 10.81±0.68 | |
| HepG2 | ANE | 73.96±3.15** | 101.01±2.46* |
| ANA | 39.72±3.09 | 164.36±5.36 | |
| Doxorubicin | 85.84±3.04 | 0.47±0.08 | |
| C6 | ANE | 95.27±0.15** | 31.57±3.08** |
| ANA | 10.18±0.15 | 982.46±14.40 | |
| Doxorubicin | 87.67±1.81 | 0.79±0.01 | |
| HeLa | ANE | 67.09±13.52* | 51.67±9.84* |
| ANA | 56.88±4.72* | 92.25±5.55 | |
| Doxorubicin | 74.73±1.52 | 6.69±1.52 | |
| AGS | ANE | 82.86±0.59 | 36.35±1.32 |
| ANA | 88.40±2.12 | 47.98±0.41* | |
| Doxorubicin | 92.29±1.60 | 2.09±0.09 | |
| Peritoneal macrophages | ANE | 2.87±0.89 | -- |
| ANA | 4.34±1.23 | -- |
Values expressed in μg/ml of extract. Significant P value (*P<0.05 was obtained by Student’s t-test analysis. Composite treatments were compared using one-way analysis of variances (ANOVA) and probability values were found to be equal to or less than 0.05 for all the eight cell lines
Table 3: A. nilagirica extracts exhibiting in vitro cytotoxicity in eight selected cancer cell lines
ANE was more potent than ANA for all the cells exhibiting an IC50 value of 38.21±7.37, 59.02±5.90, 35.87±1.68, 101.01±2.46, 51.67±9.84, 36.35±1.32, 31.57±3.08, 30.43±0.86 for THP-1, SupT1, JM1, HepG2, HeLa, AGS, C6 and K562 cells, respectively. It is also worth to mention that the percentage of inhibition by ANE ranged from 39.72±3.09% in HepG2 cells to 95.27±0.15 in C6 cells. Similarly the percentage of inhibition by ANE at the highest concentration of 200 μg/ml ranged between 10.18±0.15% in C6 to 88.40±2.12% in AGS cells. However, the corresponding IC50 values of ANA were higher than ANE in all the tested cell lines (Table 3). Doxorubicin, a known anticancer drug used as a positive control, exhibited IC50 values ranging from 0.47±0.08 μg/ml in HepG2 cells to 2.09±0.09 μg/ ml in AGS cells. Curcumin was also used as positive control for K562 cells that showed a percentage inhibition of 83.96±2.79% at highest concentration of 50 μg/ml with an IC50 value 10.81±0.68 μg/ml.
An important criterion in the search of anticancer compound(s) with therapeutic potential is to determine whether they show toxic effects on normal cells. It is well known that adverse side effects associated with extracts might hinder their usage. Therefore, in order to define whether the strong inhibitory effects of active crude extracts A. nilagirica (ANE and ANA) were specific to cancer cells, the inhibitory effects of these extracts towards the proliferation of normal cells were subsequently monitored. For this purpose, a test of cytotoxicity to peritoneal macrophages was performed in order to determine the selectivity to biological activity. The results of this study clearly indicated that assayed extracts were virtually non-toxic and had negligible inhibitory effects on cell proliferation against peritoneal macrophages and there was minimal reduction in cell survivability (Table 2).
During the recent past, the emphasis on medicinal plant research has augmented all over the world and significant evidences have been collected to demonstrate the immense potential of medicinal plants used in different traditional systems of medicine. Numerous plants have been investigated using modern scientific approaches wherein the results shown the potential of medicinal plants in the field of pharmacology. Besides, it was also concluded that medicinal plants can be involved in preventing or hindering the progression of many diseases. The herbal drugs/formulations apart from being considered as free from serious adverse effects are obtained from natural world and are easily accessible [33-35]. Despite the plethora of drugs developed by pharmaceutical industry, herbal remedies have proved to be effective alternative or complementary treatments for different ailments [36].
Plant phytochemicals such as phenols, flavonoids, alkaloids, terpenoids, saponins, etc. have been involved in varied functional roles including curative properties, are reflected as main contributors of antioxidant properties [37-39]. The phytochemicals act as antioxidants, by acting as reducing agents, hydrogen donators, singlet oxygen quenchers as well as metal chelators owing to their redox abilities [27]. The present study is in continuation of our search for small medicinal active molecules and evaluation of their biological activity. The aim of this work was to evaluate the antioxidant and antiproliferative activity of crude extracts against eight different human tumor cells. We also aimed to assess the phytochemical profile of these extracts to support the therapeutic use of A. nilagirica plant as an antioxidant and antiproliferative agent in the Indian system of medicine. In this study, ANA and ANE showed highest quantities of polyphenols and flavonoids. However, the higher concentration of tannins and triterpenoids were detected in aqueous and ANH respectively. The extracts of A. nilagirica also possessed minute quantities of alkaloid content except the ANH. Significant (P<0.05) differences in terms of extract yield obtained from different solvents might be due to differences in solvent polarity as well as the presence of extractable components of the plant [40,41].
The results indicated that apart from phenolics as antioxidant constituents, the extracts of the plant under study possess other phytochemical entities which may possess some other medicinal applications. The profile of phytochemicals in different extracts and their mix ratios determine the joint action of the compounds, with either synergistic, antagonistic and/or additive effects. The efficacy of a combination of different phenolic compound structures might be greater than that of other combinations on a kind of activity. Consequently, different kinds of activities of a phenolic mixture may also depend on the mix ratios etc. The variation in the phytochemical profile compared to earlier reports [42,43] in the present work may be the result of the involvement of these compounds in different functional roles in the plants collected from different places of the world. Earlier reports have also confirmed that the antioxidant potential of certain bioactive compounds is associated with their reducing abilities, therefore, the reducing power assays may act as an important indicator of probable antioxidant activity in a plant extracts [44,45]. Furthermore, antioxidant capacity of the plant extracts mainly depends on both the composition of the extracts as well as the test system. It can be influenced by number of factors and cannot be comprehensively evaluated by a single method. It is imperative to perform more than one type of antioxidant capacity measurement to take into account the various mechanisms of antioxidant action [46], hence, we employed series of in vitro assays to get a broader prospective of antioxidant potential of this plant. A. nilagirica extracts possessed a significant antioxidant potential as indicated by phosphomolybednum assay and reduction of ferric to ferrous ions in FRAP assay. Transition metals such as Fe and Cu upon reacting with perioxides form harmful alkoxy radicals by donating electrons. Chelation of these metals gives a good assessment of the protective antioxidant potential of the plant extracts/compounds [47]. The build-up of toxic iron causes tissue damage and leads to various complications in human beings. Chelation therapy decreases iron-related complications and hence progresses the quality of life and overall survival [48,49]. All the extracts except ANH chelated Fe and Cu ions considerably in a concentration dependent manner in vitro again justifying their antioxidant capability.
O2-•, •OH, NO•, H2O2 and lipid peroxidation plays a substantial role in promoting pathological conditions and diseases governed by oxidative stress in human beings [50]. O2-• and other ROS species resulting from it (•OH and H2O2) are responsible for homeostatic disruption and subsequently damages the myocardial tissues in ischemia/reperfusion injury. Moreover, they also plays a significant role in the pathogenesis of many disorders such as hypertension, chronic obstructive pulmonary disease, diabetic complications [50,51]. The DNA damage induced by the •OH radicals is responsible for the initiation, progression and development of carcinogenesis [52]. Studies have indicated that enhanced formation of nitric oxide by iNOS have been responsible for inflammatory disorders of the joints, atherosclerosis, cancer and diabetes [53]. Increased formation of lipid peroxides and aldehydes is critical in the progression of atherosclerosis, arthritis, cancer and several neurodegenerative diseases and other immunological disorders [54]. Moreover, the presence of phenolic compounds in this study support the hypothesis that phenolic compounds contribute significantly to the total antioxidant capacity of the examined plant species, in accordance to the earlier findings where a linear correlation was observed between the content of phenolic compounds and antioxidant capacity of several plant species [55,56]. In view of these facts, the evaluation of antioxidant potential in the extracts of A. nilagirica may extend the use of this potent medicinal plant in the direction of prevention and treatment of disorders associated with oxidative stress.
Cancer remains one of the leading causes of mortality worldwide. There were about 14.1 million new cases of cancer and 8.2 million cancer deaths and 32.6 million people living with cancer (within 5 y of diagnosis) estimated in the year 2012 globally. By 2025, it has been estimated that mortality can reach as high as 12 million with a substantive increase to 19.3 million new cancer cases per year [57]. Consequently, lot of progress has been made in the field of cancer research to develop various approaches to reduce the mortality caused by cancer. The introduction of active agents derived from medicinal plants into the cancer therapy that have proven to be more potent with less toxicity has changed the natural history of many types of human cancer [58,59]. Earlier studies have suggested that plant derived polyphenols and flavonoids obstruct the growth and angiogenesis of tumour cells in vitro [60]. The mechanistic aspects of bioactive components may prevent cancer through various mode of action including antioxidant activity, inhibition of cell proliferation, induction of apoptosis, inhibition of cell invasion and subcellular signalling pathways [61,62]. Several laboratory evidences from chemical, cell culture and animal studies indicate that antioxidants may slow or possibly prevent the development of cancer [63]. In this study, we have also shown that two extracts of A. nilagirica possessed significant cytotoxic effects on the panel of eight different cancer cell lines in a concentration dependent manner. The active crude extract with an IC50 value of <30 μg/ml is considered to be cytotoxic according to the standard National Cancer Institute criteria [64]. The active extracts exhibited different activity on different cell lines. This variation in activity could be due to the different sensitivity of cell line towards the bioactive compounds in the extract or to tissue specific response [65]. The antiproliferative activity of active extracts may not be attribute to their toxicity as there was no apparent reduction in viability of controlled cultures of peritoneal macrophages at the same concentration. Hence, our results suggested that these extracts may have potential to be further developed into therapeutic treatments.
In the present study, the phytochemical and detailed antioxidant activities of A. nilagirica were demonstrated using series of in vitro antioxidant models. Our results provide evidence that extracts of A. nilagirica are efficient quenchers of free radicals, thereby inhibiting free chain reaction, act as proficient reducing agents and metal ion chelators to terminate the initiation and cease free radical formation.
In addition, our results further conclude that the ethyl acetate and alcoholic extracts strongly inhibited the growth of 8 different human cancer cell lines and were nontoxic to normal peritoneal macrophages. Substantial antioxidant and antiproliferative capacities of A. nilagirica extracts may be mainly attributed to the presence of the high level of phytoconstituents. Consequently, A. nilagirica may have a great relevance in the prevention and therapy of diseases in which free radicals and oxidants are implicated. However, further work is still needed to identify and characterise the inherent phytocompounds from active extracts and to investigate the antioxidant and antiproliferative efficacy in vivo.
Acknowledgement
This research was supported by University Grant Commission sponsored, University of Hyderabad UPE Phase-II research grant to IAG (UH/UGC/UPE Phase II/Interface Studies/Research Projects/R-58). The authors acknowledge the financial support from CSIR and UGC, India respectively in the form of SRF fellowships. The authors would also like to thank Central Research Institute of Unani Medicine, Hyderabad, Telangana for providing the plant material. Authors would also like to extend thanks to the Mr. Chanderasekaran, Department of Animal Biology, School of Life Sciences, UoH for his assistance in cell culture studies. Authors are also thankful to DBT-CREBB, DBT-FIST level I & II, DST-PURSE phase I & II and UGC-SAP - CAS, UGCXI plan seed money for supporting infra structural facilities to Department of Plant Sciences and School of Life Sciences, University of Hyderabad, Hyderabad, India.
Conflict of interest
The authors declare that there is no conflict of interests.
Financial support and sponsorship
Nil.
References
- Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 1984;219:1-14.
- Morris D, Khurasany M, Nguyen T, Kim J, Guilford F, Mehta R, et al. Glutathione and infection. Biochim Biophys Acta 2013;1830:3329-49.
- Storz P. Reactive oxygen species in tumor progression. Front Biosci 2005;10:1881-96.
- Prakash O, Kumar A, Kumar P, Ajeet A. Anticancer potential of plants and natural products: A Review. Am J Pharm Sci 2013;1:104-15.
- Gul MZ, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA. Antioxidant and antiproliferative activities of Abrus precatorius leaf extracts-an in vitro study. BMC Complement Altern Med 2013;13:53.
- Sen T, Samanta SK. Medicinal plants, human health and biodiversity: a broad review. Adv Biochem Eng Biotechnol 2014;147:59-110.
- Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, et al. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol 2003;84:131-8.
- Bhatt I, Rawat S, Rawal R Antioxidants in Medicinal Plants. In: Chandra S, Lata H, Varma A, editors, Biotechnology for Medicinal Plants. Heidelberg: Springer Berlin; 2013, p. 295-326.
- Hartwell JL. Plants used against cancer. A survey. Lloydia 1971;34:386-425.
- Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci 2005;78:431-41.
- Katiyar C, Gupta A, Kanjilal S, Katiyar S. Drug discovery from plant sources: An integrated approach. Ayu 2012;33:10-9.
- Gaur RD. Flora of the District Garhwal, North West Himalaya (with ethnobotanical notes). Honolulu: TransMedia; 1999.
- Trivedi PC. Medicinal Plants: Traditional Knowledge. New Delhi: I.K. International Publishing House; 2006.
- Ahameethunisa AR, Hopper W. Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC Complement Altern Med 2010;10:6.
- Sati SC, Sati N, Ahluwalia V, Walia S, Sati OP. Chemical composition and antifungal activity of Artemisia nilagirica essential oil growing in northern hilly areas of India. Nat Prod Res 2012;27:45-8.
- Naik SK, Mohanty S, Padhi A, Pati R, Sonawane A. Evaluation of antibacterial and cytotoxic activity of Artemisia nilagirica and Murraya koenigii leaf extracts against mycobacteria and macrophages. BMC Complement Altern Med 2014;14:87.
- Suseela V, Gopalakrishnan VK, Varghese S. In vitro Antioxidant Studies of Fruits of Artemisia nilagirica (Clarke) Pamp. Indian J Pharm Sci 2010;72:644-9.
- Fan JP, He CH. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J Pharm Biomed Anal 2006;41:950-6.
- Hagerman AE, Butler LG. Protein precipitation method for the quantitative determination of tannins. J Agric Food Chem 1978;26:809-12.
- Sreevidya N, Mehrotra S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff's reagent in plant materials. J AOAC Int 2003.86:1124-7.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem1999;269:337-41.
- Sun J, Liu SF, Zhang CS, Yu LN, Bi J, Zhu F, Yang QL. Chemical composition and antioxidant activities of Broussonetia papyrifera fruits. PLoS One 2012;7:e32021.
- Li X, Lin J, Gao Y, Han W, Chen D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem Cent J 2012;6:140.
- Koksal E, Gulcin I, Beyza S, Sarikaya O, Bursal E. In vitro antioxidant activity of silymarin. J Enzyme Inhib Med Chem 2009;24:395-405.
- Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 1994;315:161-9.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231-7.
- Gul MZ, Bhakshu LM, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA. Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC Complement Altern Med 2011;11:64.
- Sreejayan, Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol 1997;49:105-7.
- Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 1972;46:849-54.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8.
- Bhattacharjee S, Gupta G, Bhattacharya P, Mukherjee A, Mujumdar SB, Pal A, et al. Quassin alters the immunological patterns of murine macrophages through generation of nitric oxide to exert antileishmanial activity. J Antimicrob Chemother 2009;63:317-24.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods1983;65:55-63.
- Dahech I, Farah W, Trigui M, Hssouna AB, Belghith H, Belghith KS, et al. Antioxidant and antimicrobial activities of Lycium shawii fruits extract. Int J Biol Macromol 2013;60:328-33.
- Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis 2007;39:293-304.
- Vlase L, Parvu M, Parvu EA, Toiu A. Chemical constituents of three Allium species from Romania. Molecules 2012;18:114-27.
- Phillipson JD. Phytochemistry and medicinal plants. Phytochemistry 2001;56:237-43.
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry 2000;55:481-504.
- Ferrazzano GF, Amato I, Ingenito A, Zarrelli A, Pinto G, Pollio A. Plant polyphenols and their anti-cariogenic properties: a review. Molecules 2011;16:1486-507.
- Quideau S, Deffieux D, Douat-Casassus C, Pouysegu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl 2011;50:586-621.
- Shabir G, Anwar F, Sultana B, Khalid ZM, Afzal M, Khan QM, Ashrafuzzaman M.Antioxidant and antimicrobial attributes and phenolics of different solvent extracts from leaves, flowers and bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf]. Molecules 2011;16:7302-19.
- Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009;14:2167-80.
- Temraz A, El-Tantawy WH. Characterization of antioxidant activity of extract from Artemisia vulgaris. Pak J Pharm Sci 2008;21:321-6.
- Erel SB, Reznicek G, Şenol SG, Yavaşoğulu NUK, Konyalioğlu S, Zeybek AU. Antimicrobial and antioxidant properties of Artemisia L. species from western Anatolia. Turkish J Biol 2012;36:75-84.
- Jayaprakasha GK, Singh RP, Sakariah KK. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem 2001;73:285-90.
- Panda SK, Padhi LP, Mohanty G. Antibacterial activities and phytochemical analysis of Cassia fistula (Linn.) leaf. J Adv Pharm Technol Res 2011;2:62-7.
- Frankel EN, Meyer AS. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J Sci Food Agr 2000;80:1925-41.
- Cai H, Xie Z, Liu G, Sun X, Peng G, Lin B, et al. Isolation, identification and activities of natural antioxidants from Callicarpa kwangtungensis Chun. PLoS One 2014;9:e93000.
- Shinar E, Rachmilewitz EA. Oxidative denaturation of red blood cells in thalassemia. Semin Hematol 1990;27:70-82.
- Hebbel RP, Leung A, Mohandas N. Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood1990;76:1015-20.
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 2010;4:118-26.
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997;82:291-5.
- Lipinski B. Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev 2011;2011:809696.
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315-424.
- Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014;2014:360438.
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 2004; 74:2157-84.
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem 2006;97:654-60.
- Seffrin JR. Cancer control as a human right. Lancet Oncol 2008;9(5):409-11.
- da Rocha AB, Lopes RM, Schwartsmann G. Natural products in anticancer therapy. Curr Opin Pharmacol 2001;1:364-9.
- Pan L, Chai H, Kinghorn AD. The continuing search for antitumor agents from higher plants. Phytochem Lett 2010;3:1-8.
- Yaglıoglu A, Akdulum B, Erenler R, Demirtas I, Telci I, Tekin S. Antiproliferative activity of pentadeca-(8E, 13Z) dien-11-yn-2-one and (E)-1,8-pentadecadiene from Echinacea pallida (Nutt.) Nutt. roots. Med Chem Res 2013;22:2946-53.
- Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr 2004;134:3479s-85s.
- Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr 2006;46:161-83.
- Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst 1993; 85:1483-92.
- Momtazi-Borojeni AA, Behbahani M, Sadeghi-Aliabadi H. Antiproliferative activity and apoptosis induction of crude extract and fractions of Avicennia marina. Iran J Basic Med Sci 2013;16:1203-8.
- Kirana C, Record IR, McIntosh GH, Jones GP. Screening for antitumor activity of 11 Species of Indonesian Zingiberaceae using human MCF-7 and HT-29 cancer cells. Pharm Biol 2003;41:271-6.