- *Corresponding Author:
- Y. M. Yousafzai
Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow, United Kingdom
E-mail: yasar.yousafzai@kmu.edu.pk
| This article was originally published in a special issue, “Advanced Targeted Therapies in Biomedical and Pharmaceutical Sciences” |
|
Indian J Pharm Sci 2023:85(1) Spl Issue “51-56” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Coronavirus disease 2019 mass vaccination has led to drastic reduction in hospitalizations and mortality. A number of case reports have emerged reporting coronavirus disease 2019 infection within days following vaccination. There is a need to understand development of immune antibodies in the early post-vaccination period. A prospective analysis of immunoglobulin M and immunoglobulin G kinetics was conducted during the first 28 d following vaccination with either CanSino or Sinovac vaccines in a cohort of 40 healthy volunteers. Serial blood samples were collected from the volunteers right before the first dose of vaccine (d 0) and then on d 4, d 7, d 14, d 21, d 24 and d 28 post-vaccination. Using enzyme-linked immunosorbent assay, circulating anti-severe acute respiratory syndrome coronavirus 2 receptor binding domain immunoglobulin M and immunoglobulin G antibodies were analyzed. Most vaccine recipients (31/40) did not develop any circulating immunoglobulin M. The remaining 9 recipients showed a typical immunoglobulin M curve with antibodies appearing on d 4, peaking on d 7 and declining on d 21 and beyond. Immunoglobulin G response was more typical within 38/40 recipients showing the appearance of immunoglobulin G on d 4, which continued till the end of the study period. This study demonstrates that vaccine-induced immunoglobulin M-based immunity cannot be relied during the first few days following vaccination and more time is needed to have a better picture of the real situation.
Keywords
Immunoglobulin M and immunoglobulin G kinetics, CanSino, Sinovac, enzyme-linked immunosorbent assay, coronavirus disease 2019
Coronavirus Disease 2019 (COVID-19) has emerged at the end of 2019 from China and less than half a year was declared as a pandemic[1]. A rapid increase in the number of cases and deaths urged the scientific community to respond quickly and deal with the virus in a dual strategy: Treat the sick and find ways to halt the spread of the disease[2]. Vaccines got the spotlight and with an exceptional pace, different vaccine candidates were launched[3]. The incidence of COVID-19 related hospitalizations and deaths was reduced drastically upon the use of vaccines[4].
Vaccines were thought at first to prevent from Severe Acute Respiratory Syndrome Coronavirus 2 (SARS- CoV-2) infection[5]. This seemed quite desirable and achievable at the start. But as more and more people got vaccinated, certain breaches occurred, though less common but significant importance was noted. Breakthrough infections were one such concern that shifted the focus much on symptomatology of the disease after many studies reported cases of infections within 1-7 d following vaccination[6,7]. It was established that “the vaccine provides protection against developing serious symptoms once one gets infected” would be a more realistic statement. Breakthrough infection is defined as the detection of SARS-CoV-2 Ribonucleic Acid (RNA) or antigen in a respiratory specimen collected from a person 14 d after receiving a dose of an authorized COVID-19 vaccine[8]. This added a new aspect to the ongoing research on immunological response initiated by the vaccine.
The breakthrough infections are labelled on d 15 or later and after receiving the vaccine. The short-term immunological response to COVID-19 vaccination, which is poorly understood, needs further elucidation. As far as the chronology of immunological response to SARS-CoV-2 infection is concerned, it is generally understood that Immunoglobulin M (IgM) antibodies provide an early response to infection before the appearance of high affinity levels of Immunoglobulin G (IgG) antibodies[9]. However, the kinetics of early IgM and IgG in response to vaccination is still under intensive investigation which could, probably, provide an answer to the emergence of breakthrough infections[10,11].
The World Health Organization (WHO) established a Target Product Profile (TPP) for COVID-19 vaccines, with the preferred vaccine demonstrating at least 70 % efficacy[12]. The most common vaccines provided in Pakistan include the CoronaVac (Sinovac Biotech Ltd) and CanSino (CanSino Biologics Inc.). CanSino vaccine, developed by CanSino Biologics in collaboration with Beijing Institute of Biotechnology, developed the vaccine using replication deficient human Adenovirus type 5 vector-novel Coronavirus (Ad5-nCoV) expressing the SARS-CoV-2 spike (S)-protein[13]. In February 2021, global data from phase III trials and 101 COVID cases showed that the vaccine had a 65.7 % efficacy in preventing moderate symptoms and 91 % efficacy in preventing severe disease. CoronaVac is an inactivated virus of COVID-19 vaccine developed by Sinovac (China) [14]. It has been evaluated in phase III clinical trial in Turkey. Phase III results from Turkey showed an efficacy of 84 %, based on the data from 10 218 participants in the trials[15].
This vaccine induced a Receptor Binding Domain (RBD) with antibodies developing in 94 %-100 % of the participants. The single dose efficacy against all symptomatic and severe COVID-19 cases was 68.83 % and 95.47 %, respectively, on d 14 post vaccination. Some common adverse reactions were noted with no serious concerns. A phase III clinical trial showed that the efficacy of CoronaVac was 51 % against symptomatic and 100 % against severe SARS-CoV-2 infection after the 2nd dose. Another study showed that the CoronaVac induced a significant humoral response with the anti-RBD IgG antibodies to be of significant importance[3]. Most common adverse reactions were those of no significant or serious consequences. However, a study on a large population in Hong Kong reported that the vaccine was associated with Bell’s palsy in some of the participants[16]. However, serological studies particularly investigating early humoral responses among the recipients to these vaccines are lacking.
This study was designed with the objective of investigating the short-term immunological response to CanSino and CoronaVac vaccines on a group of adult volunteers. The adult participants enrolled and included in the study were checked for their anti-spike IgM and IgG response following the administration of either CanSino or Sinovac vaccine. Humoral response was evaluated at seven different time points during a 28 d period post vaccination.
Materials and Methods
This prospective follow up study was conducted at the Institute of Pathology and Diagnostic Medicine (IPDM), Khyber Medical University (KMU), Peshawar, Pakistan in collaboration with scientists from King Abdulaziz University in Jeddah, SaudiArabia. The study population comprised adults living in the Peshawar district. This study was approved by the ethics committee of Khyber Medical University-Advance Studies and Research board with the ethical approval number of DIR/KMU- ASRB/A1/001436. After ethical approval, participants with their signed informed consent were enrolled in this study. Participants with the history of immunodeficient disease and known COVID-19 patients were excluded from this study.
Levels of circulating anti-SARS-CoV-2-RBD IgM and IgG antibodies in the serum were assessed at 7 different time points, firstly, just immediately before the administration of the vaccine (d 0) and then on d 4, d 7, d 14, d 21, d 24 and d 28 post-vaccinations. Blood samples were collected in Ethylenediaminetetraacetic Acid (EDTA) containing tubes (category number: 367856, BD Biosciences, New Jersey, United States of America (USA)) for complete blood counts at each time point. Samples for IgM and IgG assessment were collected in Z-serum clot activator tubes (category number: 367983, BD Biosciences, New Jersey, USA), centrifuged at 1500 g for 20 min and the supernatant serum transferred to an Eppendorf tube.
SARS-CoV-2 IgM kit (category number: 41A246R) and IgG kit (category number: 41A235) were used (ImmunoDiagnostics (IMD) kits, Sha Tin, Hong Kong). These kits work based on the principal of indirect, Enzyme-Linked Immunosorbent Assay (ELISA) to detect antibodies against S1 region of the SARS-CoV-2 virus including the RBD.
IMD SARS-CoV-2-IgM ELISA kit is a two steps incubation immunoassay kit used for the detection of anti-spike protein IgM antibodies. The micro-wells of the plate are coated with SARS-CoV-2 S1 protein, so that they form a complex (Antigen-antibody complex). At 1 h incubation, the antibodies present in serum were captured by immobilized SARS-CoV-2 S1 protein while the unbound component was removed by washing. After washing away the unbound materials, the enzyme- linked secondary antibody (anti-human polyclonal antibodies) was added, which then conjugate with the Horseradish Peroxidase (HRP) and attach to the primary antibody. Once the 1 h incubation time is completed, the unbound antibody was removed and a HRP-substrate solution was added which is chromogenic containing 3,3’,5,5’-Tetramethylbenzidine (TMB). This resulted in a chemical reaction which forms blue color in the wells. The excessive blue color is stopped by adding 2 M Sulphuric acid (H2SO4) to the wells which converts the blue color to yellow in the wells. The yellow color was then quantified by measuring its absorbance in the micro plate reader at 450 nm
Statistical analysis:
Data was entered and analyzed by using Statistical Package for the Social Sciences (SPSS) version 25.0. Frequency and percentages were calculated for categorical data and mean with Standard Deviation (SD) were calculated for continuous data. Independent t-test was used to compare the mean age between IgM responders and non-responders, while chi-square test was used for categorical variables. A p-value less than 0.05 were considered statistically significant. Multiple linear regression was used to identify the determinants of IgG level at 28 d. The results were presented as adjusted and unadjusted beta (β) and 95 % Confidence Interval (95 % CI).
Results and Discussion
A total of 40 Pakistani participants were enrolled in this study. Out of whom 28 were males and 12 were females. The mean age of the participants was 47.8±7.8 y. 3 participants had a known comorbidity. 14 participants were Punjabi by ethnicity and 26 were Pushtoons (Table 1).
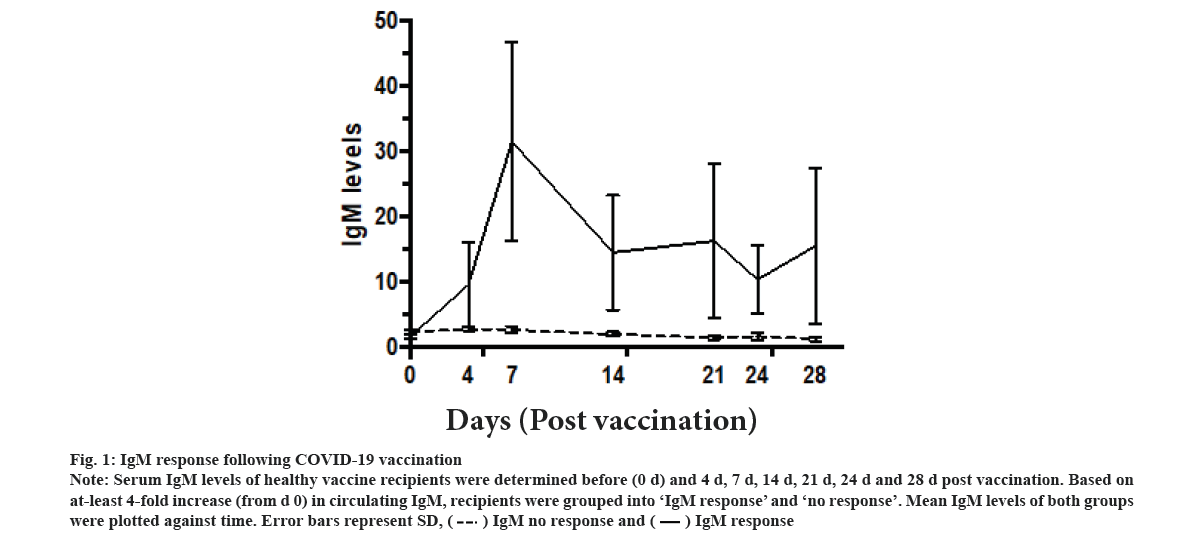
IgM response following vaccination was explained in this study. The IgM levels of the vaccinated recipients were assessed on d 0 (just before taking the vaccine) and on d 4, d 7, d 14, d 21 and d 28 thereafter. Two different groups were identified. Only 9 out of 40 participants showed at least 4-fold increase in IgM levels above the baseline (fig. 1). They were grouped as IgM responders. The mean IgM levels in these 9 participants showed IgM response curve. IgM levels increased progressively on d 4 and d 7 post-vaccination, and then started to decline on d 14, and then they reached a plateau level till d 28. However, these differences were statistically non-significant. The remaining 31 were labelled as ‘non-responders’ did not show any increase in the cut-off criteria at any point after vaccination. There was no statistically significant difference between the IgM responders and non-responders in terms of age, gender, ethnicity, presence of comorbidities or the type of vaccine used (Table 1).
| Variables | Number (%) (n=40) | IgM responders (n=9) | IgM non-responders (n=31) | p-value |
|---|---|---|---|---|
| Age (mean±SD) | 47.8±7.8 | 46.2±6.4 | 48.2±8.2 | 0.512 |
| Gender (n=40) | ||||
| Male | 28/40 (70 %) | 6 (21.4) | 22 (78.6) | 1 |
| Female | 12 (30 %) | 3 (25) | 9 (75) | |
| Comorbidities (n=3) | 3 (7.5 %) | 2 (66.7) | 1 (33.3) | 0.545 |
| Ethnic group (40) | ||||
| Punjabis | 14 (35 %) | 4 (28.6) | 10 (71.4) | 0.694 |
| Pushtoons | 26 (65 %) | 5 (19.2) | 22 (84.8) | |
| Vaccine type (40) | ||||
| CanSino | 20 (50 %) | 4 (20) | 16 (80) | 0.5 |
| Sinovac | 20 (50 %) | 5 (25) | 15 (75) | |
Table 1: Baseline Characteristics of the Study Population
Figure 1: IgM response following COVID-19 vaccination Note: Serum IgM levels of healthy vaccine recipients were determined before (0 d) and 4 d, 7 d, 14 d, 21 d, 24 d and 28 d post vaccination. Based on at-least 4-fold increase (from d 0) in circulating IgM, recipients were grouped into ‘IgM response’ and ‘no response’. Mean IgM levels of both groups were plotted against time. Error bars represent SD, 
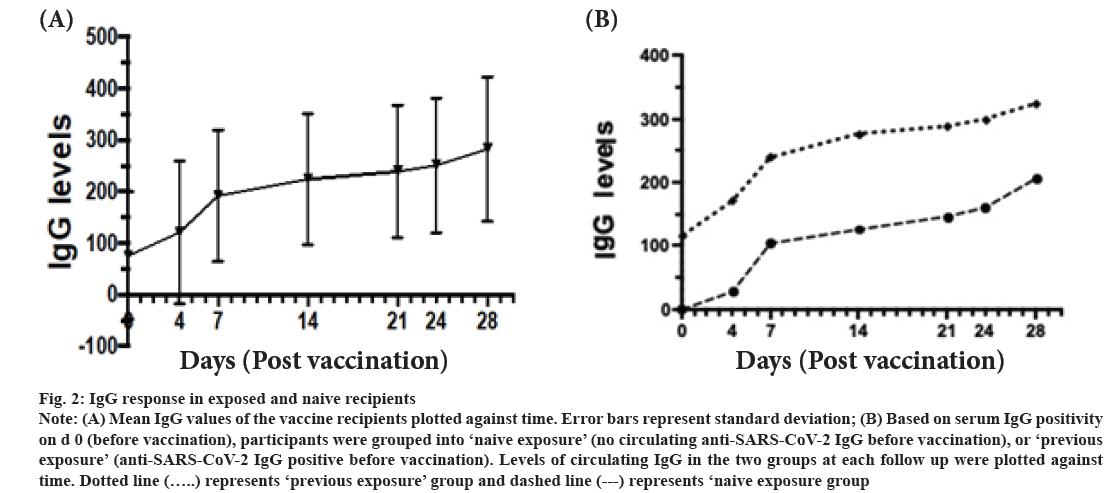
IgG response following vaccination was shown here. The IgG levels of recipients increased progressively during the follow up period was shown in fig. 2A. Serum IgG levels of healthy vaccine recipients were determined before (0 d) and 4 d, 7 d, 14 d, 21 d, 24 d and 28 d post vaccination. Based on presence of COVID-19 IgG antibodies on d 0 (before vaccination), participants were divided into ‘previous exposure’ and ‘naive exposure’. In both groups, the IgG levels increased progressively till d 28 (fig. 2B). Only 2 participants showed no detectable levels of IgG over the 28 d period. Using logistic regression analysis, ‘previous exposure’ and vaccination with Sinovac had a significantly higher levels of COVID-19 IgG levels (p<0.001 and p<0.002 respectively) (Table 2).
| Contents | Unadjusted model β (95 % CI) | p-value | Adjusted model β (95 % CI) | p-value |
|---|---|---|---|---|
| Age | -2.5 (-12.6-7.4) | 0.60 | -2.5 (12.5-7.4) | 0.14 |
| Gender | -83 (-109.3-89.2) | 0.84 | -83 (-195-29) | 0.79 |
| Previous infection | 118.3 (31.2-205.4) | 0.009* | 151 (67-235.1) | 0.001* |
| Vaccine | 81.5 (-5.5-168.4) | 0.06 | 138.100 (64.6-211.6) | 0.001* |
Note: *p<0.05
Table 2: Multinomial Logistic Regression Analysis for Post Sars-Cov-2 Vaccine Recipients
Figure 2: Note: (A) Mean IgG values of the vaccine recipients plotted against time. Error bars represent standard deviation; (B) Based on serum IgG positivity on d 0 (before vaccination), participants were grouped into ‘naive exposure’ (no circulating anti-SARS-CoV-2 IgG before vaccination), or ‘previous exposure’ (anti-SARS-CoV-2 IgG positive before vaccination). Levels of circulating IgG in the two groups at each follow up were plotted against time. Dotted line
We report that in the majority of Sinovac and CanSino COVID-19 vaccine recipients, IgM response was not detected in the participant’s blood. In those with detectable response, peak circulating IgM levels were detected on d 7 post vaccination. IgG response, on the other hand, showed a robust increase that began as early as d 4 and kept increasing till d 28.
It is well known that IgM will be detected in blood of vaccinated individuals as early as d 4 post infection, peaking rapidly during the following week and then declining sharply thereafter[17]. Regarding SARS-CoV-2 infection, some studies have reported that typical IgM kinetics with detectable levels of antibodies appearing as early as d 4 post infection and declining to below baseline levels in around, d 28[18]. However studies suggested that infection with SARS- CoV-2 is peculiar in this sense that Immunoglobulin A (IgA) and IgG develop before IgM[19]. Along the same line, studies on the vaccine report demonstrated that as many as half of recipients do not develop IgM response. They also indicated that a group of recipients developed the IgM response after the IgG response[20]. These differences might be because of the type of vaccine administered or the antibody detection method used. Nevertheless, based on the data obtained in this study, vaccine recipients should not be considered as immune until the emergence of anti-spike IgG.
IgG is a biomarker for sustainable longer-term immunity[21]. The IgG response in our cohort was more robust. Recipients with previous, relatively recent, exposure to SARS-CoV-2 showed higher levels of IgG at each time point. This is intuitive. An argument for considering COVID-19 as an immunizing event and considering vaccination as a booster vaccine has previously been recommended[22]. Our findings support this hypothesis. Sinovac induced a different IgG response compared to CanSino. Whether the observed differences in IgG levels during the first 28 d are narrowed down during the corresponding weeks it could not be seen.
This pilot study is limited by its small sample size. Additionally, we could not test neutralizing activity of the antibodies. Nevertheless, anti-RBD antibody levels have shown close correlation with neutralizing activity. Availability of multiple measurements along the follow up period provided important perspectives on the kinetics of immune response following COVID-19 vaccination.
Author’s contributions:
Rajaa Mohammad Al-Raddadi and Atif Rehman contributed equally to this work.
Acknowledgements:
This research work was funded by Institutional Fund Projects under grant no (IFPRC-052-248-2020). Therefore, authors gratefully acknowledge technical and financial support from Ministry of Education and King Abdulaziz University, Jeddah, Saudi Arabia.
Conflict of interests:
The authors declared no conflict of interest.
References
- Umakanthan S, Sahu P, Ranade AV, Bukelo MM, Rao JS, Abrahao-Machado LF, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J 2020;96(1142):753-8.
[Crossref] [Google Scholar] [PubMed]
- Kaur SP, Gupta V. COVID-19 vaccine: A comprehensive status report. Virus Res 2020;288:198114.
[Crossref] [Google Scholar] [PubMed]
- Rahman M, Masum M, Ullah H, Wajed S, Talukder A. A comprehensive review on COVID-19 vaccines: Development, effectiveness, adverse effects, distribution and challenges. Virusdisease 2022;33(1):1-22.
[Crossref] [Google Scholar] [PubMed]
- Moghadas SM, Vilches TN, Zhang K, Wells CR, Shoukat A, Singer BH, et al. The impact of vaccination on Coronavirus Disease 2019 (COVID-19) outbreaks in the United States. Clin Infect Dis 2021;73(12):2257-64.
[Crossref] [Google Scholar] [PubMed]
- Mehrotra DV, Janes HE, Fleming TR, Annunziato PW, Neuzil KM, Carpp LN, et al. Clinical endpoints for evaluating efficacy in COVID-19 vaccine trials. Ann Intern Med 2021;174(2):221-8.
[Crossref] [Google Scholar] [PubMed]
- Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: A prospective, community-based, nested, case-control study. Lancet Infect Dis 2022;22(1):43-55.
[Crossref] [Google Scholar] [PubMed]
- Keehner J, Horton LE, Pfeffer MA, Longhurst CA, Schooley RT, Currier JS, et al. SARS-CoV-2 infection after vaccination in health care workers in california. N Engl J Med 2021;384(18):1774-5.
[Crossref] [Google Scholar] [PubMed]
- Hirsh J, Htay T, Bhalla S, Nguyen V, Cervantes J. Breakthrough SARS-CoV-2 infections after COVID-19 immunization. J Investig Med 2022;70(6):1429-32.
[Crossref] [Google Scholar] [PubMed]
- Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020;75(7):1564-81.
[Crossref] [Google Scholar] [PubMed]
- Carr EJ, Kronbichler A, Graham-Brown M, Abra G, Argyropoulos C, Harper L, et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep 2021;6(9):2292-304.
[Crossref] [Google Scholar] [PubMed]
- Speer C, Goth D, Benning L, Buylaert M, Schaier M, Grenz J, et al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin J Am Soc Nephrol 2021;16(7):1073-82.
[Crossref] [Google Scholar] [PubMed]
- Koff WC, Schenkelberg T, Williams T, Baric RS, McDermott A, Cameron CM, et al. Development and deployment of COVID-19 vaccines for those most vulnerable. Sci Transl Med 2021;13(579):eabd1525.
[Crossref] [Google Scholar] [PubMed]
- Asghar N, Mumtaz H, Syed AA, Eqbal F, Maharjan R, Bamboria A, et al. Safety, efficacy, and immunogenicity of COVID-19 vaccines: A systematic review. Immunol Med 2022;45(4):225-37.
[Crossref] [Google Scholar] [PubMed]
- Jin L, Li Z, Zhang X, Li J, Zhu F. CoronaVac: A review of efficacy, safety, and immunogenicity of the inactivated vaccine against SARS-CoV-2. Hum Vaccin Immunother 2022;18(6):2096970.
[Crossref] [Google Scholar] [PubMed]
- Akova M, Unal S. A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): A structured summary of a study protocol for a randomised controlled trial. Trials 2021;22(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Wong ICK, Wan EYF, Chui CSL, Li X, Chan EWY. Adverse event reporting and Bell's palsy risk after COVID-19 vaccination-Author’s reply. Lancet Infect Dis 2021;21(11):1492-3.
[Crossref] [Google Scholar] [PubMed]
- Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect 2004;10(12):1062-6.
[Crossref] [Google Scholar] [PubMed]
- Zhou C, Bu G, Sun Y, Ren C, Qu M, Gao Y, et al. Evaluation of serum IgM and IgG antibodies in COVID-19 patients by enzyme linked immunosorbent assay. J Med Virol 2021;93(5):2857-66.
[Crossref] [Google Scholar] [PubMed]
- Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect 2004;10(12):1062-6.
[Crossref] [Google Scholar] [PubMed]
- Ruggiero A, Piubelli C, Calciano L, Accordini S, Valenti MT, Carbonare LD, et al. SARS-CoV-2 vaccination elicits unconventional IgM specific responses in naive and previously COVID-19-infected individuals. eBioMedicine 2022;77:103888.
[Crossref] [Google Scholar] [PubMed]
- Alzaabi AH, Ahmed LA, Rabooy AE, Zaabi AA, Alkaabi M, AlMahmoud F, et al. Longitudinal changes in IgG levels among COVID-19 recovered patients: A prospective cohort study. PLoS One 2021;16(6):e0251159.
[Crossref] [Google Scholar] [PubMed]
- Kent SJ, Juno JA. Vaccination after prior COVID-19 infection: Implications for dose sparing and booster shots. eBioMedicine 2021;72:103586.
[Crossref] [Google Scholar] [PubMed]