- *Corresponding Authors:
- N. K. Lee
Department of Oriental Medicine Resources, Chonbuk National University, Iksan 54596, Korea - K-H. Park
Department of Oriental Pharmaceutical Development, Nambu University, Gwangju, 62271, Korea
E-mail: khpark@nambu.ac.kr
| Date of Submission | 23 March 2017 |
| Date of Revision | 26 October 2017 |
| Date of Acceptance | 29 May 2018 |
| Indian J Pharm Sci 2018;80(4):749-755 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of the present study was to investigate the immunomodulating property of Polygonum multiflorum extracts using various in vitro and in vivo tests. In this study, Polygonum multiflorum extracts were shown to exhibit immunomodulating activities such as cytokine production in normal rat splenocyte and cyclophosphamide-induced immunosuppression model. The immunostimulant effects of Polygonum multiflorum extracts were further tested for various immunological markers. Polygonum multiflorum extracts significantly enhanced cytokines production activity in rat splenocyte. Consistently oral administration of Polygonum multiflorum extracts enhanced blood cytokine production and ameliorated spleen damage in cyclophosphamide immunosuppressed rat. In conclusion, the present investigation revealed that Polygonum multiflorum extracts possessed immunomodulating and immunostimulating properties.
Keywords
Polygonum multiflorum extracts, immunomodulation, immunostimulation, splenocytes, cyclophosphamide, emodin
Polygonum multiflorum is a traditionally highly efficient plant species that is cultivated in many countries and widely used as a source of Chinese medicine to treat various diseases like liver injury, cancer, diabetes, alopecia, atherosclerosis, neurodegenerative [1], asthma, cerebral ischemia, steatosis, and Alzheimer’s disease [2-5]. Many studies showed that the major bioactive components of P. multiflorum are flavonoids and polyphenols including emodin, resveratrol, and 2,3,5,4'-tetrahydroxylstilbene-2-O-β-D-glucoside [6,7].
However, lesser is known about the enhancing immune response property of P. multiflorum extract (PME). Cancer and other infectious diseases treated by chemotherapies with immunosuppressive drugs such as cyclophosphamide lead to immunosuppressive response in patients [8]. Cyclophosphamide is generally used to treat lymphocytic leukaemia, lymphoma, and specific solid tumours like bladder and ovarian cancer [9]. However, treatment with cyclophosphamide often leads to serious side effects such as decrease in lineage of blood cells and its functional products including cytokines. Therefore, cyclophosphamide can be used as an immunosuppressive agent both in vitro and in vivo models [10,11].
Immunomodulation-related cytokines such as interleukin-2 (IL-2), IL-12, tumor necrosis factor-α (TNF-α), Interferon gamma (IFN-γ), are well-identified targets used for amelioration of various immune diseases. Based on the evaluation of immunity, cyclophosphamide-induced immunomodulated mice exhibited significant immune-related differences compared to untreated mice, such as reduced activity of splenocytes, and repressed productions of TNF-α, IL-2 and IL-12 in serum and in splenocytes, suggesting that cyclophosphamide is a potent immunomodulators [12,13]. Moreover spleen of normal and immunodeficient mice displayed significant changes in IFN-γ mRNA expression [14]. Qi et al. reported that, cyclophosphamide treatment induced increase in spleen weight in mice [15].
In this study, cultured primary splenocytes were used for the in vitro study, and cyclophosphamide-induced immunosuppressed rat was used for in vivo analysis. In order to assess the immunostimulating effects of PME the productions of various immunity-related cytokines such as IL-2, IL-12, TNF-α, IFN-γ in in vitro and in vivo models were investigated.
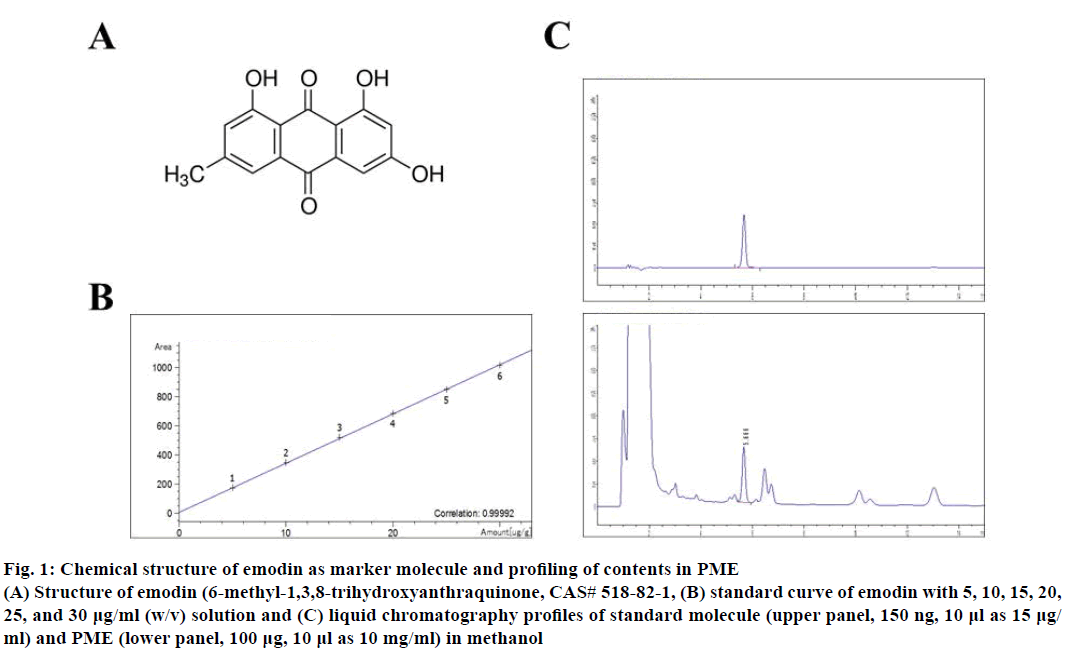
The whole plant of P. multiflorum was purchased from the Jiri Mountain P. multiflorum Co-operative Association in San Chung, Gyungnam Province, Korea. Dried tuberous root of P. multiflorum (1.0 kg) was extracted with 6.0 l of 70 % ethanol-DDW (v/v) at 80° for 3 h. The extracts were centrifuged at 3 000×g for 20 min at 4° and the supernatant was filtered through Whatman No. 3 filter paper and concentrated using a rotary evaporator. The supernatant extract was lyophilized to produce a sticky powder and then aliquots were kept at –80° until use to experiment. The yield of the ethanol extract of P. multiflorum was approximately 21.6 % of whole plants. The contents of total phenol and total flavonoids of extracts were 3205.11±40.32, 595.23±35.52 μg/g. Moreover, primary marker substance of P. multiflorum, emodin was analysed to 156.37±0.179 μg/g (Figure 1) [16]. Microanalysis for liquid chromatography analysis of emodin contents in PME were measured using Agilent Technologies 1260 Infinity LC system (Agilent, Tokyo, Japan). Analysis was performed at the Mokpo Marine Food- Industry Research Center (Mokpo, Korea).
Figure 1: Chemical structure of emodin as marker molecule and profiling of contents in PME
(A) Structure of emodin (6-methyl-1,3,8-trihydroxyanthraquinone, CAS# 518-82-1, (B) standard curve of emodin with 5, 10, 15, 20,
25, and 30 µg/ml (w/v) solution and (C) liquid chromatography profiles of standard molecule (upper panel, 150 ng, 10 µl as 15 µg/
ml) and PME (lower panel, 100 µg, 10 µl as 10 mg/ml) in methanol
Male Sprague Dawley rats (SD; 6-8 w) purchased from Orient Bio (Sungnam, Korea) were acclimated for 7 d. All animals were housed in a controlled environment (22±3°, 12-h light/dark cycle) during acclimation and the experiment and fed ad libitum with normal diet and water. All experiments approved by the Institutional Animal Care and Use Committee at Wonkwang University (Approval No. WKU16-20).
Splenocytes were obtained from SD rats. Briefly, the spleen was removed aseptically, washed thoroughly, diced and spleen cells were released by trituration. The splenocyte preparation was cleaned up by centrifugation and resuspending the pellet in Hank’s buffered salt solution (HBSS, pH 7.4), and the erythrocytes were lysed. The splenocyte preparation was again centrifuged, and splenocytes were washed two more times with HBSS. Cell count and viability were assessed by trypan blue exclusion prior to plating. Cells were resuspended in RPMI containing 10 % fetal bovine serum. Collected splenocytes were seeded on a 96-well plate with 100 μl of 2×105 cells/well. The final volume per well was 100 μl. Cells were incubated in a CO2 incubator (5 % at 37°) under humidified conditions for 24 h. A WST-1 assay kit (ITSBio, Seoul, Korea) or micro-culture tetrazolium assay (MTT) test [17,18] for splenocyte proliferation rate measurement and the results were determined using a Microplate-Reader (Molecular Devices, Sunnyvale, CA, USA).
Seven male rats per group were treated with saline or P. multiflorum oral administration once a day for 28 d, respectively. In every week, the rats are weighed, and their appearances were judged. After administration of samples, animals anesthetized with diethyl ether and whole blood was collected through the abdominal vena cava. Using the collected whole blood, each element of the blood cells, including neutrophils, eosinophils, basophils, and monocytes/macrophages, was measured with a Hemavet950 (Drew Scientific Group, Dallas, TX, USA).
To measure IL-2, IL-12, TNF-α and IFN-γ by ELISA, the splenocytes were stimulated with cyclophosphamide in 96-well plates according to Won et al. [19]. The supernatants harvested at 24 h and then transferred to 96-well ELISA plates. IL-2, IL-4, IL-10, IL-12, TNF-α and IFN-γ concentrations were determined using commercial ELISA kits (R&D system, Minneapolis, MN, USA).
All data are expressed as the mean±SEM. A one-way analysis of variance (ANOVA) test employed followed by Tukey’s multiple range tests to compare each group. Statistical analyses conducted using SPSS for Windows software (Ver 10.0, Chicago, IL, USA) and data with different superscript letters are significantly different when p value is less than 0.05.
The endogenous cytotoxicity of PME on rat splenocyte was evaluated through MTT assay. The multiple concentrations of PME were used and effective doses were calculated from dose-response results at 24 and 48 h. Results of the endogenous cytotoxicity evaluation against splenocytes of the PME are shown in Figure 2. The PME exhibited no significant activity against the splenocyte at ≤300 μg/ml. On the contrary, the high dose of PME exhibited significant cytotoxic effect against the splenocyte with ≥500 μg/ml at 24 and 48 h later. Therefore, optimal dosage of PME was 300 μg/ml was available for in vitro test with rat splenocytes and adjusted administrative dose for in vivo experiments.
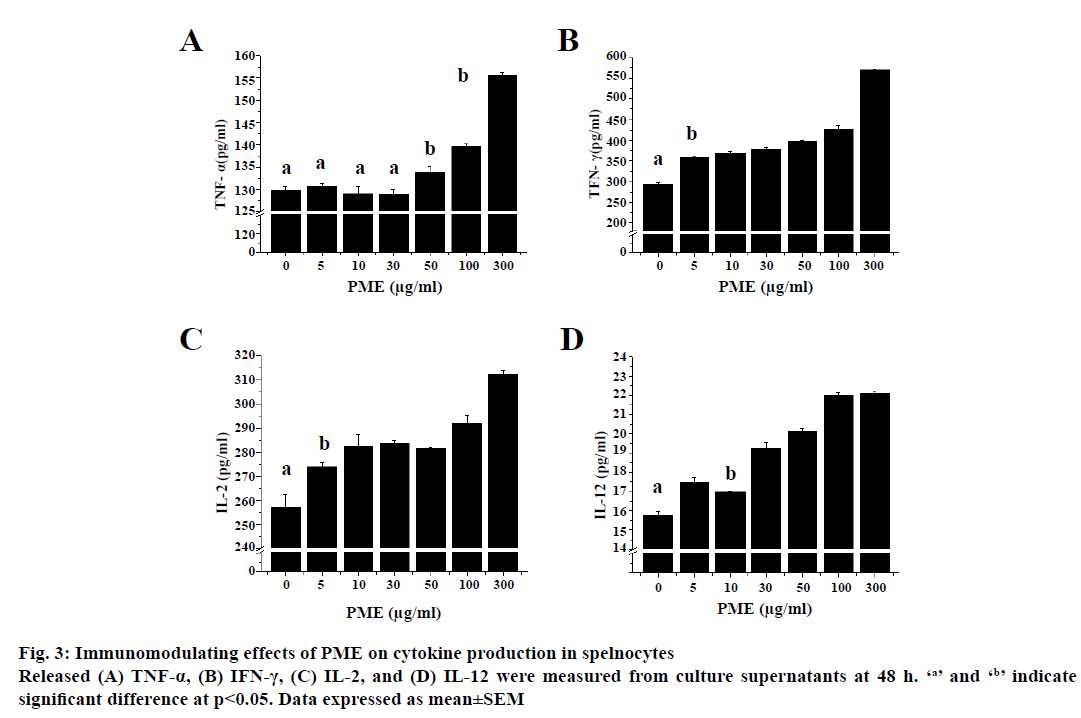
Splenocytes of normal rats released cytokines when treated with various concentration (Figure 3). Low dose (<50 μg/ml) of PME did not induce release of TNF-α from spelnocytes, whereas IFN-γ, IL-2, IL-12 release was increased compared to untreated group in a dosedependent manner. TNF-α release was increased at a relative high dose (≥50 μg/ml) of PME in a dosedependent manner from splenocytes compared to the untreated group.
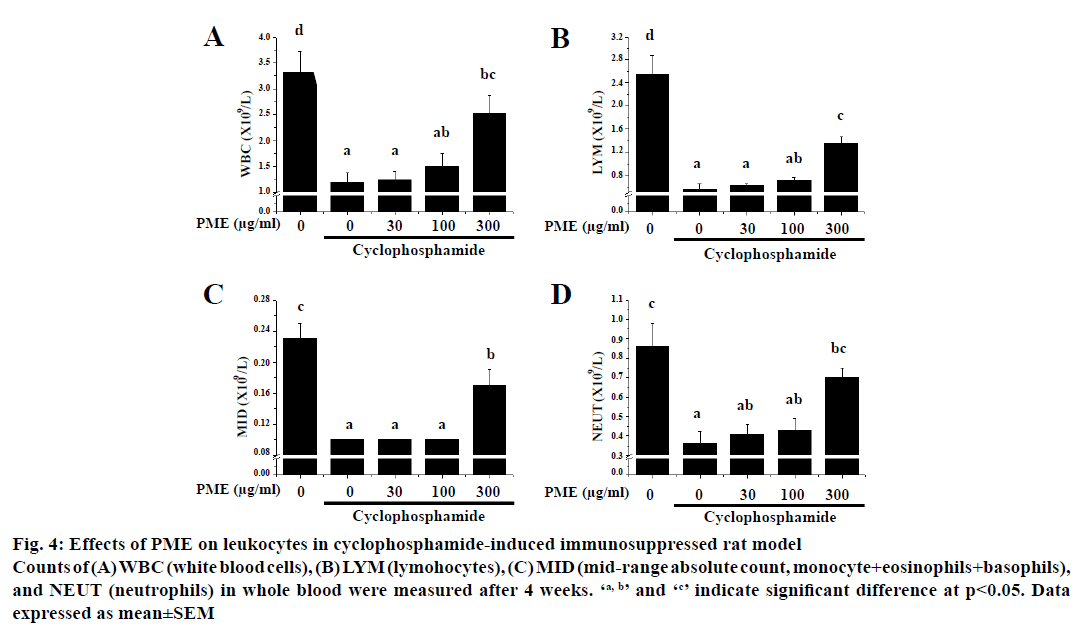
To evaluate the immunopotentiating effect of PME in vivo, cyclophosphamide-induced immunosuppressed rats were administered with PME and the numbers of peripheral leucocytes were measured (Figure 4).
Figure 4: Effects of PME on leukocytes in cyclophosphamide-induced immunosuppressed rat model
Counts of (A) WBC (white blood cells), (B) LYM (lymohocytes), (C) MID (mid-range absolute count, monocyte+eosinophils+basophils),
and NEUT (neutrophils) in whole blood were measured after 4 weeks. ‘a, b’ and ‘c’ indicate significant difference at p<0.05. Data
expressed as mean±SEM
Treatment of cyclophosphamide significantly reduced the numbers of total white blood cells (WBC), lymphocytes absolute count (LYM), midrange absolute count (MID), and neutrophil absolute count (NEUT) relative to the normal group after 4 w of cyclophosphamide treatment. Interestingly, a 4 w-treatment with PME reversed the numbers of WBC, LYM, MID, and NEUT in cyclophosphamidetreated rats. Furthermore, PME-treated group showed normal body weight, consumption ratio of food and water as control group. Moreover no severe symptoms such as death, bleeding, and diarrhoea were observed in all animals during these experiments.
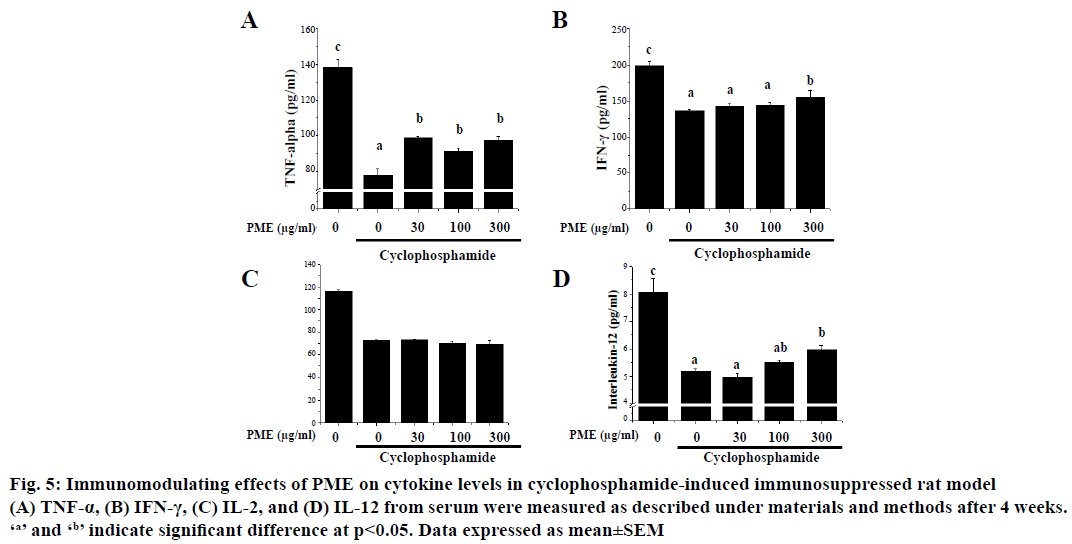
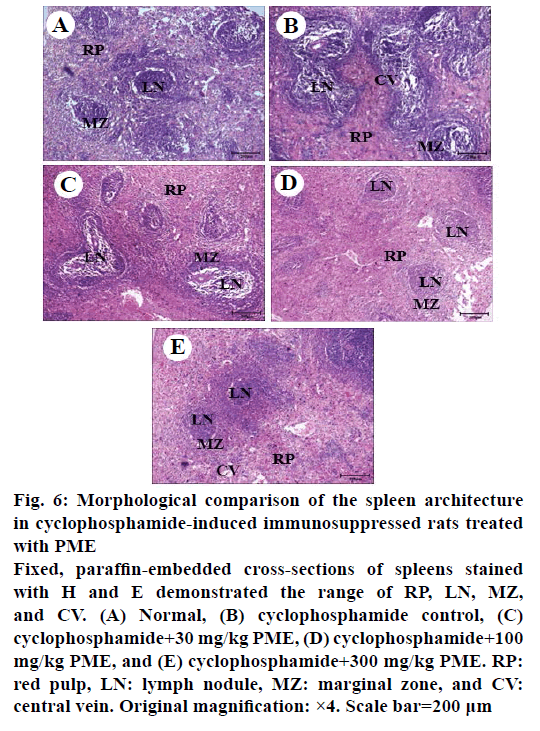
Effect of PME on the expression of immunomodulating cytokines such as IL-2, IL-12, TNF-α and IFN-γ in cyclophosphamide-treated rats was assessed (Figure 5). As shown, treatment of cyclophosphamide significantly reduced the expression of IL-2, IL-12, TNF- α, and IFN-γ. However, PME treatment in cyclophosphamidetreated rat reversed these cytokines expressions than the normal rat in a dose-dependent manner. Cyclophosphamide-induced immunosuppression resulted in dramatically reduced spleen cellularity, both in white and red pulps compared to normal group (Figure 6). The white pulp became hardly identifiable with sparse areas. Only remnants of follicles and marginal zone indicated the original location of the white pulp. Abnormal aggregated cell populations, uneven distribution of haematopoiesis, were observed in red pulp. Whereas, administration of PME ameliorated the disruption of splenic architecture in cyclophosphamideinduced rat model in a dose-dependent manner (Figure 6C-E). In PME-administrated group red pulp, white pulp, and marginal zone were almost re-established and recovered than control group.
Figure 5: Immunomodulating effects of PME on cytokine levels in cyclophosphamide-induced immunosuppressed rat model
(A) TNF-α, (B) IFN-γ, (C) IL-2, and (D) IL-12 from serum were measured as described under materials and methods after 4 weeks.
‘a’ and ‘b’ indicate significant difference at p<0.05. Data expressed as mean±SEM
Figure 6: Morphological comparison of the spleen architecture
in cyclophosphamide-induced immunosuppressed rats treated
with PME
Fixed, paraffin-embedded cross-sections of spleens stained
with H and E demonstrated the range of RP, LN, MZ,
and CV. (A) Normal, (B) cyclophosphamide control, (C)
cyclophosphamide+30 mg/kg PME, (D) cyclophosphamide+100
mg/kg PME, and (E) cyclophosphamide+300 mg/kg PME. RP:
red pulp, LN: lymph nodule, MZ: marginal zone, and CV:
central vein. Original magnification: ×4. Scale bar=200 μm
Cyclophosphamide is generally used for immune suppressed model by typical cytotoxity and increased the apoptotic cells in immune organ and tumor [9,20,21]. In this report, the cyclophosphamideinduced immunosuppression was verified by means of cytokines production both in vitro and in vivo. In other report, cyclophosphamide-induced immune modulation changes T and B lymphocyte subsets and increases cytokines release by Th1/Th2 lymphocytes in mice model [13]. After treatment with PME, rats had better body and organs weight compared to cyclophosphamide-treated group. Therefore, PME can protect spleen and other immune systems against cyclophosphamide-mediated immune impairment. However, cyclophosphamide treatment caused release of many other cytokines and caspases, the intracellular death signal. In some report, cyclophosphamide treatment was reported to induce apoptosisdependent [22], and/or apoptosis-independent [23] tumour regression in vivo and in vitro. However in this report, the focus was mainly on immunomodulation in animal model and positive effects were observed after the administration of PME. Furthermore this property of PME could be extended in other disease models for further studies.
Emodin, a well-known marker of P. multiflorum can also be isolated form many herbs such as Aloe vera, and Cassia obtusifolia [24]. Previous reports summarized various medicinal properties of emodin with antiviral, antibacterial, antiallergic, antiosteoporotic, antidiabetic, immunosuppressive, neuroprotective and hepatoprotective activities [25] with many mechanisms [26,27]. Although, it is possible for PME to have various components including carbohydrates, lipids, proteins, vitamins and minerals, therefore further studies are required. Finally, the data presented here showed that PME treatment could ameliorate immunosuppression and could be used as a therapeutic agent to treat such drug-induced problems.
Acknowledgements
This work was supported by "Food Functionality Evaluation program" under the Ministry of Agriculture, Food and Rural Affairs and National Research Council of Science & Technology (NST) grant by the Korea government (MSIP; No. CAP-16-07-KIOM) and Korea Food Research Institute. K-H-P is recipient of research supporting grant of Nambu University (2017).
Conflicts of interest
The authors declare that they have no competing interests.
References
- Bounda GA, Feng YU. Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacognosy Res 2015;7:225-36.
- Lee CC, Lee YL, Wang CN, Tsai HC, Chiu CL, Liu LF, et al. Polygonum multiflorum Decreases Airway Allergic Symptoms in a Murine Model of Asthma. Am J Chin Med 2016;44:133-47.
- Ahn SM, Kim YR, Kim HN, Shin HK, Choi BT. Beneficial Effects of Polygonum multiflorum on Hippocampal Neuronal Cells and Mouse Focal Cerebral Ischemia. Am J Chin Med 2015;43:637-51.
- Wang M, Zhao R, Wang W, Mao X, Yu J. Lipid regulation effects of Polygoni Multiflori Radix, its processed products and its major substances on steatosis human liver cell line L02. J Ethnopharmacol 2012;139:287-93.
- Um MY, Choi WH, Aan JY, Kim SR, Ha TY. Protective effect of Polygonum multiflorum Thunb on amyloid beta-peptide 25-35 induced cognitive deficits in mice. J Ethnopharmacol 2006;104:144-8.
- lI YM, Li RY, Niu M, Li CY, Bai ZF, Feng WW, et al. Influence of specification on chemical composition of dissolution and hepatocytes toxicity of Polygonum multiflorum. Zhongguo Zhong Yao Za Zhi 2016;41(6):1033-9
- Yu C, Chai X, Yu L, Chen S, Zeng S. Identification of novel pregnane X receptor activators from traditional Chinese medicines. J Ethnopharmacol. 2011;136:137-43.
- Wei X, Zhang J, Li J, Chen S. Astragalus mongholicus and Polygonum multiflorum's protective function against cyclophosphamide inhibitory effect on thymus. Am J Chin Med 2004;32:669-80.
- Wexler EJ, Gravallese EM, Czerniak PM, Devenny JJ, Longtine J, Wong MKK, et al. Tumor biology: use of tiled images in conjunction with measurements of cellular proliferation and death in response to drug treatments. Clin Cancer Res 2000;6:3361.
- Casteels K, Waer M, Bouillon R, Depovere J, Valckx D, Laureys J et al. 1,25-Dihydroxyvitamin D3 restores sensitivity to cyclophosphamide-induced apoptosis in non-obese diabetic (NOD) mice and protects against diabetes. Clin Exp Immunol 1998;112:181-7.
- Li W, Gao S, Wang G, Cai L. Cyclophosphamide induces a Fas/FasL-dependent apoptotic cell death in rat thymus. J Immunol 2000:16:315.
- Jang SE, Joh EH, Ahn YT, Huh CS, Han MJ, Kim DH. Lactobacillus casei HY7213 ameliorates cyclophosphamide-induced immunosuppression in mice by activating NK, cytotoxic T cells and macrophages. Immunopharmacol Immunotoxicol 2013;35:396-402.
- Lis M, Obminska-Mrukowicz B. Modulatory effects of bestatin on T and B lymphocyte subsets and the concentration of cytokines released by Th1/Th2 lymphocytes in cyclophosphamide-treated mice. Cent Eur J Immunol 2013;38:42-53.
- Hasegawa T, Kimura Y, Hiromatsu K, Kobayashi N, Yamada A, Makino M, et al. Effect of hot water extract of Chlorella vulgaris on cytokine expression patterns in mice with murine acquired immunodeficiency syndrome after infection with Listeria monocytogenes. Immunopharmacology 1997;35:273-82.
- Qi L, Song Y, Wang W, Cui W, Zhang X, Liu Z, et al. Comparison of immunosuppression induced by different doses of cyclophosphamide in normal mice. Wei Sheng Yan Jiu 2010;39:313-5, 325.
- Kim MO, Park YS, Nho YH, Yun SK, Kim Y, Jung E, et al. Emodin isolated from Polygoni Multiflori Ramulus inhibits melanogenesis through the liver X receptor-mediated pathway. Chem Biol Interact 2016;250:78-84.
- Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 2005;11:127-52.
- Choi HG, Xie GH, Lee MK, Park SH, Choi SE, Hwang KT,et al. Effects of Allium Hookeri Root Extracts on Oxidative Stress-induced Damage in Rat Cardiomyocyte Cells. Indian J Pharm Sci 2016;78:291-3.
- Won TJ, Kim B, Oh ES, Bang JS, Lee YJ, Yoo JS, et al. Immunomodulatory activity of Lactobacillus strains isolated from fermented vegetables and infant stool. Can J Physiol Pharmacol 2000;89:429-34.
- Pette M, Gold R, Pette DF, Hartung HP, Toyka KV. Mafosfamide induces DNA fragmentation and apoptosis in human T-lymphocytes: a possible mechanism of its immunosuppressive action. Immunopharmacology 1995;30:59-69.
- Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod 1997;56:1490-7.
- Schwartz PS, Waxman DJ. Cyclophosphamide induces caspase 9-dependent apoptosis in 9L tumor cells. Mol Pharmacol 2001;60:1268-79.
- Guerriero JL, Ditsworth D, Fan Y, Zhao F, Crawford HC, Zong WX. Chemotherapy induces tumor clearance independent of apoptosis. Cancer Res 2008;68:9595-600.
- Dong X, Fu J, Yin X, Cao S, Li X, Lin L, et al. A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytother Res 2016;30:1207-18.
- Matsuda Y, Yokohira M, Suzuki S, Hosokawa K, Yamakawa K, Zeng Y, et al. One-year chronic toxicity study of Aloe arborescens Miller var. natalensis Berger in Wistar Hannover rats. A pilot study. Food Chem Toxicol 2008;46:733-9.
- Park SJ, Jin ML, An HK, Kim KS, Ko MJ, Kim CM, et al. Emodin induces neurite outgrowth through PI3K/Akt/GSK-3β-mediated signalling pathways in Neuro2a cells. Neurosci Lett 2015;588:101-7.
- Lo YH, Chen YJ, Chung TY, Lin NH, Chen WY, Chen CY, et al. Emoghrelin, a unique emodin derivative in Heshouwu, stimulates growth hormone secretion via activation of the ghrelin receptor. J Ethnopharmacol 2015;159:1-8.
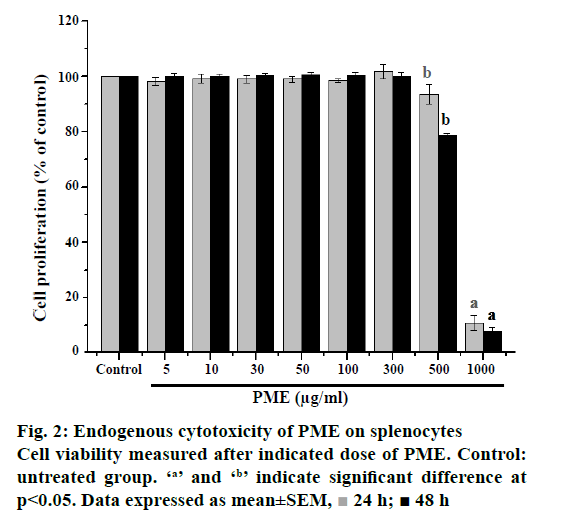
 24 h;
24 h;  48 h
48 h