- *Corresponding Author:
- S. Shruthi
Department of Applied Zoology, Alva’s College, Vidyagiri, Moodbidri-574 227, Karnataka, India
E-mail: shruthisujnan@gmail.com
| Date of Submission | 11 January 2017 |
| Date of Revision | 28 April 2017 |
| Date of Acceptance | 15 December 2017 |
| Indian J Pharm Sci 2018;80(1):150-160 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Gallic acid is a triphenolic acid, widely distributed in fruits, vegetables and plants and is reported to produce antioxidant, antiinflammatory, antifungal, antiviral and antitumor effects. In the present study, immunomoduatory effect of gallic acid was tested against cyclophosphamide and cisplatin; two widely used anticancer agents induced immunosuppression in Swiss albino mice. Cyclophosphamide and cisplatin are known immunosuppressive agents, which elicit variety of immune responses. In recent years much attention is given for the identification of plants or their bioactive compounds as immunomodulators. Three different doses of gallic acid i.e., 100, 200 and 400 mg/kg weight were administered orally for 7 consecutive days. Cyclophosphamide (50 mg/kg) and cisplatin (10 mg/kg) were administered intraperitoneally as single dose. Levamisole 50 mg/kg was used as standard immunomodulatory drug. 0.5 % carboxymethyl cellulose was used as solvent control. Evaluation of immunomodulatory property of gallic acid was done by using haemagglutination antibody titre response and haematological parameters such as white blood cells, red blood cells, platelet counts and haemoglobin levels. Relative weight of thymus an important lymphoid organ was also determined. Augmentation of antibody titre values and haematological end points clearly indicated immunomodulatory effect of gallic acid against cyclophosphamide and cisplatin-induced myelosuppression in Swiss albino mice. Results indicate that, gallic acid could be used as an adjuvant with immunosuppressive drugs to reduce their adverse effects on immune system.
Keywords
Gallic acid, cyclophosphamide, cisplatin, immunomodulatory, Swiss albino mice
The immune system is a major defence mechanism evolved in an organism to protect against the foreign invaders and to eliminate diseases [1]. Immune system involves various types of cells of which some are immunostimulants and others are immunosupperessors in their functions [2]. Immunomodulation is the enhancement of immune reactions by immunostimulant agents. This primarily involves the stimulation of non-specific systems, i.e. granulocytes, macrophages, complement, certain T-lymphocytes and different effector substances. Suppression of the individual elements of the immune system; may allow the pathogenic organisms to surge over the host, which lead to secondary infections [3]. Immunosuppression is the suppression of body’s immune response owing to some environmental or chemotherapeutic influences [4]. Hence it is necessary to evaluate the mechanisms of immunotoxicity induced by a drug, whereas a change in cellular components of the blood is one of the events leading to immunosuppression [5].
In recent years, lot of importance is given for the identification of better immunostimulating agents from natural sources to impart better immune responses [6]. Immunomodulation using medicinal plants is a novel approach in phytomedicine to enhance the host defence mechanism during some autoimmune disorders [1]. In Indian traditional system of medicine many of the plants and their bioactive components are known to possess immunomodulatory properties, hence they are being used as an alternative approach to minimise the irreversible effects of modern drugs like adjuvants, synthetic agents and antibody reagents in the immune system [7,8]. Whole plants or their secondary metabolites are being used as drugs for the treatment of various ailments and in efficiency of immune response. In Ayurveda, rasayana drugs consisting of various plants are used to improve the defence mechanism and immunomodulatory activity [9-11]. Literature indicated that the Indian medicinal plants are rich source of immonomodulators. Also, plant derived components such as proteins, lectins, polysaccharides, alkaloids, flavonoids and phenolic substances have shown immunomodulatory properties along with their antioxidant and antiinflammatory properties [12,13]. Some of the plants with established immunomodulatory properties are Eclipta prostrate, Phyllanthus emblica, Glycyrrhiza glabra, Piper longum, Aloe vera, Allium sativum, Withaia somnifera, Emblica officinalis, Tinospora cordifolia and Ocimum sanctum [8,14,15].
Bone marrow is the primary lymphoid organ where all types of immunocytes originate. Thymus is another primary lymphoid organ where immune-competent T-cells develops and matures, hence; it plays a primary role in adaptive immune responses [16]. Mature cells migrate from lymphoid organs to the periphery to perform their functions. If any damage occurs to two primary lymphoid organs, production of immunocytes may be reduced leading to and induce immune dysfunction [17,18]. Evaluation of haematological parameters, lymphoid organ weight and histopathology are considered as immunological endpoints in subchronic and chronic rodent studies (OCSPP guidelines, 2013) [19]. These endpoints have been used by many investigators to study possible effects on immune function [20-23].
Humoral immunity refers to production of antigen specific antibodies and it plays a vital role in the immune system [24]. Haemagglutination titre assay is one of the simple assays used to measure specific antibody response towards the given antigen and it is commonly used in blood grouping and viral quantification. Antigen-antibody reactions can be visualised with the formation of agglutination [25,26]. There are many of using haemagglutination titre assay as one of the test parameters to study the immunomodulatory effects of plants or their bioactive molecules [1,21,27,28]. In our study haemagglutination titre assay and thymus weight and haematological parameters were used to study the immunomodulatory effects of gallic acid.
Cyclophosphamide is a widely used alkylating drug used in the treatment of various types of cancers such as lymphoma, myeloma and chronic lymphocytic leukaemia [29]. However, it is also effective immunosuppressive agents, which cross links the DNA of actively dividing cells thereby inhibiting the both cellular and humoral response immunity [30,31]. Cyclophosphamide-induced immunosuppression is reported to prompt various types of infections [13,32]. As per researchers, cyclophosphamide can be used as immunosuppressive agent to study the immunomodulatory effects of plant extracts [10,16,22,33].
In contrast, cisplatin is the first platinum based potent chemotherapeutic drug, widely used for the treatment of testicular, ovarian, bladder and other carcinomas [34,35]. A few reports are available on the effects of cisplatin on the cellular components of immune systems, but it might have potential in the control of inflammatory and autoimmune diseases [36]. Cisplatin has shown immunosuppressive and immunotoxic effects by affecting the body fluid components [37,38]. Investigator has been reported the immunosuppressive potential of cisplatin and protective role of plant extracts against it [37].
Gallic acid, (3,4,5-trihydroxybenzoic acid) found in fruits and plant materials in the form of free acids, esters, catechin derivatives and hydrolysable tannins also it is one of the major bioactive phenolic compounds present in the plants [39,40]. These plant bioactive compounds are considered as effective antioxidants. Even, the antioxidant property of gallic acid as a specific compound or as bioactive compound was well recognised by researchers [41-44]. Reactive oxygen species (ROS) and nitrogen radicals, which are formed naturally in our body, which results in oxidative stress causing deleterious effects on genetic material. Such effects can be minimized by dietary antioxidants containing polyphenol compounds [45]. Balance between oxidation and antioxidation mechanism, which helps to maintain healthy biological system [46]. Consequently, the antioxidant properties of gallic acid facilitated in the modulation of immune function under both in vivo and in vitro conditions as an active component of plants or as an herbal product [47-52]. Also, antiinflammatory properties of gallic acid prevent the expression of inflammatory chemicals including cytokines and histamines and hence it can be used to treat inflammatory allergic diseases [53].
Since there are very few reports are available on the immunomodulatory effects of gallic acid as a pure compound, the present study was undertaken to investigate the in vivo immunostimulatory/ immunomodulatory effects of gallic acid against cyclophosphamide- and cisplatin-induced immunosuppression by using haematological and haemagglutination titre assays as test parameters.
Materials and Methods
Gallic acid (CAS No.: 5995-86-8), Sigma Aldrich (Lot No: MKBP6646V) was used. Levamisole 50 mg/kg (Khandelwal Laboratories Pvt. Ltd., Mumbai) was used as reference standard immunomodulatory drug. Cyclophosphamide (CAS No.-6055-19-2), Endoxan-N Baxter Oncology, Germany (Batch No- JN1045) and cisplatin (CAS: 15663-27-1), Sigma Life Science (Lot No.: MKBN7276V) were used as positive immunosuppressant drugs. All other chemicals were obtained from Merck, SRL and Hi-media, India. Alsevier’s solution was prepared by dissolving the following reagents: dextrose- 2.05 g, sodium citrate- 0.80 g, sodium chloride- 0.42 g, distilled water- 100 ml; pH of the solution was adjusted to 6 by using 0.1 N HCl or NaOH. Sheep red blood cells (SRBC’s) was collected in Alsevier’s solution (1:1), washed in equal volumes of sterile normal saline solution thrice and adjusted to a concentration of 1×108 cells/0.1 ml were used for immunization and challenge.
Experimental animals
Swiss albino mice belonging to Mus musculus species, bred and maintained in the institutional animal house, were used for the experiment. They were housed in polypropylene shoe box type cages, bedded with rice husk and kept in air-conditioned room, at 23° (±2°) and RH 50±5 %, were fed with a pelleted diet (Amruth Feeds, India) and water ad libitum. A 12:12, light:dark cycle was followed. Racks were positioned in a room so as to optimise air exchange. Eight to ten week old animals with average body weight of 25±2 g were used for the experiments. Five animals (3 females+2 males) were used for each treatment and control group. Care and experimental procedures were conducted as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals, India. All groups of animals were kept under an absolute hygienic condition as per the recommended procedures by fulfilling the necessary ethical standards. In vivo animal studies were conducted after obtaining the prior approval from Institutional Animal Ethics Committee (IAEC) of Mangalore University (MU/AZ/99/2013- 14/IAEC dt: 2.04.2013).
Dose and treatment schedule
Scheme of an appropriate dosing schedule and regimen should be based on clinical use, exposure pattern, pharmacokinetics and practical consideration. If the substance is genotoxic, highest dose level used will show the evidence of adverse effects and maximum tolerated dose is normally used to set this dose level. Accordingly, doses of gallic acid selected for this study were 100, 200 and 400 mg/kg. The LD50 value of gallic acid has been reported as 5000 mg/kg in rats (Nair and Nair, 2013) [42]. Experimental animals were immunized by injecting 0.1 ml of 20 % SRBC (1×108 cells) intraperitoneal (i.p.), prepared in normal saline on day 0. Three different doses of 0.2 ml of GA i.e., 100, 200 and 400 mg/kg were administered orally for 7 consecutive days. Cyclophosphamide (50 mg/kg) and cisplatin (10 mg/kg) were dissolved in distilled water and 0.9 % saline, respectively and were administered i.p., in 0.1 ml quantity as a single dose. On the 7th d, 2 h after last treatment, the animals were euthanized and about 1-1.5 ml of blood was collected by heart puncture. Levamisole 50 mg/kg was used as standard immunomodulatory drug. It was centrifuged at 3000 rpm at (–20°) and serum was collected from supernatant fraction to measure the antibody titre value. About 0.5 % carboxymethyl cellulose (CMC), distilled water and 0.9 % saline administered groups were maintained separately, which formed as negative controls for gallic acid, cyclophosphamide and cisplatin, respectively.
Haemagglutination antibody titre assay
Haemagglutination test was performed by following the standard method [54]. Two fold serial dilutions of serum samples were made in 96 well U bottomed haemagglutination microtitre plates containing 100 μl of phosphate-buffered saline. Then, 50 μl of 1 % SRBC suspension is added to each well. The plates were shaken gently and incubated at room temperature for 2 h and examined visually for agglutination. The value of the highest serum dilution causing visible haemagglutination was considered as the antibody titre [1]. Thymus weight was determined immediately after the animals were euthanized. The weight was measured in milligrams and expressed as relative weight using the formula; relative weight= weight of thymus in milligrams/weight of the animals in grams×100.
Haematological studies
Haematological parameters such as total RBC, WBC, platelet and Hb count was found to be vital constituents of the immune system and were analysed using haematology analyser (Unitron Bio-Medicals, India). For differential count of WBCs, Wright’s staining method was followed.
Statistical analysis
Statistical significance of the results was tested by comparing gallic acid and levamisole treated groups with negative control 0.5 % CMC treated groups. Gallic acid combined treatment groups was compared with the respective cyclophosphamide and cisplatin treated groups by employing one way ANOVA and Dunnett’s post hoc tests using GraphPad Prism 5 (GraphPad Software, Inc., CA, USA). Differences with a P-value of 0.05 or lower were considered to be statistically significant.
Results and Discussion
Animals administered with cyclophosphamide and cisplatin showed adverse effects on body weight and reduced food consumption. Whereas gallic acid combined treatment groups increased the food consumption rate thereby increased animal body weight, which is comparable with vehicle treated groups.
In antibody titre assay levamisole induced significant antibody production compared to the 0.5 % CMC control, which indicated the stimulatory activity of the positive immunomodulatory agent (Table 1). The relative weight of thymus was also increased in levamisole treated animals. In gallic acid treated animals, the antibody titre values were increased. These results are comparable with positive control levamisole treated group. Augmentation in antibody titre values in gallic acid treated groups indicates the immunostimulatory activity of gallic acid.
| Treatment (mg/kg) |
Initial body weight in (g) ±SEM | Final body weight in (g) ±SEM | Relative weight of thymus (g) ±SEM | Antibody titre±SEM |
|---|---|---|---|---|
| Dist. water | 25.80±0.20 | 26.66±0.20 | 1.50±0.02 | 7.40±0.20 |
| Saline | 24.72±0.14 | 26.08±0.22 | 1.54±0.01 | 7.40±0.24 |
| CMC (0.5 %) | 25.96±0.04 | 26.98±0.34 | 1.53±0.02 | 7.40±0.24 |
| Levamisole | 25.99±0.12 | 27.07±0.14 | 1.86±0.02 | 16.0±0.31 |
| Gallic acid (100) | 26.86±0.21 | 27.80±0.24 | 1.56±0.02 | 12.6±0.24 |
| Gallic acid (200) | 26.14±0.20 | 27.18±0.25 | 1.55±0.02 | 12.4±0.24 |
| Gallic acid (400) | 26.20±0.33 | 27.02±0.36 | 1.58±0.01 | 11.6±0.24 |
Table 1: Body Weight, Relative Weight of Thymus and Antibody Titre Values in Various Control Groups
Whereas in cyclophosphamide and cisplatin alone treated groups, there was a reduction in the antibody titre values when compared with distilled water and saline controls, respectively. This result indicates the immunosuppressive property of cyclophosphamide and cisplatin resulting in decrease in the antibody production. The production of antibody was increased in levamisole and gallic acid treated groups by increasing the antibody titre values. Gallic acid enhanced antibody titre values showing similar effect to that of positive immunomudulatory drug levamisole. In gallic acid+cyclophosphamide and gallic acid+cisplatin combined treatment groups, there is a significant improvement in the antibody titre values and moreover there was significant increase in relative thymus weight at all three doses tested; compared with cyclophosphamide and cisplatin alone treated groups (Tables 2 and 3). Increase in the thymus weight indicates that there was significant stimulation of immune response with gallic acid treated groups. This may confirm the immunostimulant action of gallic acid is cell mediated immunity. But, the maximum effect of gallic acid was observed at lower dose of 100 mg/kg. At higher doses gallic acid may acts as prooxidant.
| *Treatment (mg/kg) |
Initial body weight (g) ±SEM | Final body weight in (g) ±SEM | Relative weight of thymus (g) ±SEM | Antibody titre±SEM |
|---|---|---|---|---|
| Cyclophosphamide (50) | 26.13±0.18 | 24.33±0.17 | 1.30±0.01 | 3.00±0.00 |
| CMC+cyclophosphamide (50) | 26.34±0.34 | 24.84±0.33 | 1.30±0.01 | 3.20±0.20 |
| Levamisole+cyclophosphamide (50) | 26.46±0.30 | 25.30±0.31 | 1.97±0.02 | 5.60±0.24 |
| Gallic acid (100)+cyclophosphamide (50) | 26.21±0.16 | 24.39±0.29 | 1.96±0.07 | 4.40±0.24 |
| Gallic acid (200)+cyclophosphamide (50) | 26.06±0.38 | 23.90±0.50 | 1.69±0.04a | 4.00±0.00 |
| Gallic acid (400)+cyclophosphamide (50) | 26.00±0.31 | 24.43±0.43 | 1.63±0.04a | 3.60±0.24a |
Table 2: Body Weight, Relative Weight of Thymus and Antibody Titre Values in Groups Treated with Cyclophosphamide and Gallic Acid
| *Treatment (mg/kg) |
Initial body weight (g) ±SEM | Final body weight (g) ±SEM | Relative weight of thymus (g) ±SEM | Antibody titre ±SEM |
|---|---|---|---|---|
| Cisplatin (10) | 26.28±0.15 | 24.02±0.09 | 1.24±0.02 | 2.80±0.20 |
| CMC+cisplatin (10) | 26.50±0.20 | 24.60±0.22 | 1.27±0.01 | 3.00±0.00 |
| Levamisole+cisplatin (10) | 25.70±0.16 | 24.14±0.19 | 1.98±0.04 | 5.60±0.24 |
| Gallic acid (100)+ cisplatin (10) | 26.18±0.08 | 25.34±0.09 | 1.66±0.01a | 6.60±0.24 |
| Gallic acid (200)+cisplatin (10) | 26.42±0.23 | 25.00±0.29 | 1.58±0.03a | 4.20±0.20 |
| Gallic acid (400)+cisplatin (10) | 26.70±0.18 | 25.08±0.09 | 1.50±0.03a | 4.00±0.00 |
Table 3: Body Weight, Relative Weight of Thymus and Antibody Titre Values in Groups Treated with Cisplatin and Gallic Acid
The effect of gallic acid on haematological parameters was studied. The parameters studied in this were differential and total leucocyte count. There was significant reduction in Hb concentration, total RBC, WBC and platelet counts in cyclophosphamide and cisplatin treated groups (Table 2). Levamisole, a standard reference drug, significantly increased all these parameters when compared with negative control groups. Similarly, gallic acid alone treated groups significantly increased WBC counts compared to 0.5 % CMC control group. However, there was no much variation in the RBC and platelet counts and Hb concentration (Table 4).
| *Treatment (mg/kg) |
Hb concentration (g/dl) | Total WBC×103/mm3 | RBC×106/mm3 | Platelet× 104 mm3 |
|---|---|---|---|---|
| Distilled water | 13.42±0.05 | 4.72±0.20 | 9.01±0.07 | 2.48±0.58 |
| CMC | 13.32±0.10 | 4.70±0.23 | 9.25±0.08 | 2.42±0.05 |
| Levamisole | 14.64±0.10 | 6.57±0.11 | 9.80±0.22 | 2.86±0.07 |
| Gallic acid (100) | 13.40±0.07 | 5.26±0.05 | 9.38±0.12 | 2.47±0.03 |
| Gallic acid (200) | 13.16±0.10 | 5.34±0.13 | 9.14±0.12 | 2.36±0.03 |
| Gallic acid (400) | 13.26±0.25 | 5.18±0.12 | 8.95±0.06 | 2.35±0.02 |
| Cyclophosphamide (50) | 9.96±0.27 | 3.56±0.10 | 6.64±0.11 | 2.16±0.03 |
| CMC+cyclophosphamide (50) |
10.24±0.14 | 3.60±0.10 | 6.74±0.08 | 2.20±0.04 |
| Levamisole+cyclophosphamide (50) | 13.99±0.11 | 6.61±0.20 | 8.99±0.12 | 2.87±0.01 |
| Gallic acid (100)+ cyclophosphamide (50) | 13.96±0.08 | 6.70±0.31 | 8.38±0.12 | 2.38±0.02 |
| Gallic acid (200)+ cyclophosphamide (50) | 12.54±0.08 | 6.70±0.23 | 8.23±0.12 | 2.33±0.11 |
| Gallic acid (400)+ cyclophosphamide (50) | 12.08±0.10 | 6.40±0.13 | 7.64±0.39 | 2.33±0.02 |
| Cisplatin (10) | 9.82±0.10 | 3.44±0.10 | 6.38±0.05 | 2.15±0.00 |
| CMC+cisplatin (10) | 10.04±0.05 | 3.56±0.08 | 6.36±0.02 | 2.15±0.01 |
| Levamisole+cisplatin (10) | 14.22±0.27 | 6.00±0.20 | 9.19±0.14 | 2.65±0.02 |
| Gallic acid (100)+cisplatin (10) | 13.62±0.08 | 5.50±0.15 | 7.65±0.16 | 2.81±0.01 |
| Gallic acid (200)+cisplatin (10) | 13.26±0.13 | 5.00±0.10 | 7.01±0.06 | 2.27±0.02 |
| Gallic acid (400)+cisplatin (10) | 13.06±0.09 | 4.68±0.11a | 6.70±0.03a | 2.26±0.00 |
Table 4: Haematological Parameters of Various Treatment and Control Groups
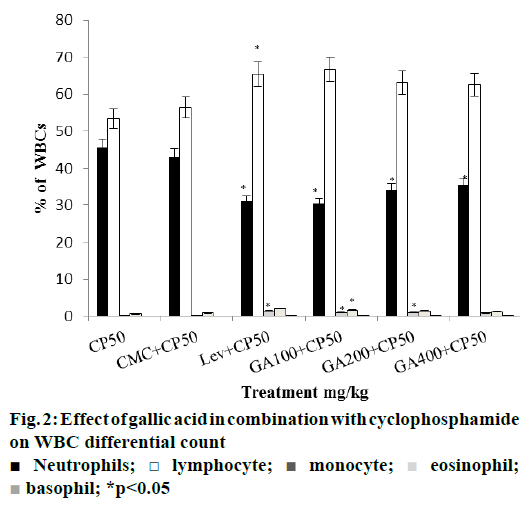
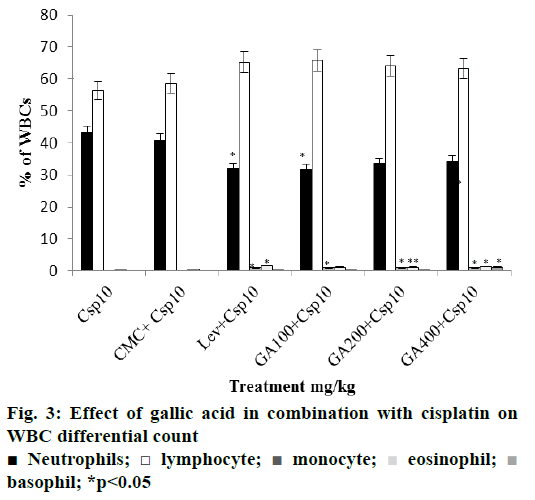
Further, in both combined treatment groups, levamisole increased all the above mentioned blood parameters significantly. Similarly, all three doses of gallic acid tested improved the blood parameters. Among them, lower dose of gallic acid (100 mg/kg) was found to be more effective in ameliorating the haematosuppressive effect induced by cyclophosphamide and cisplatin (Figures. 2 and 3).
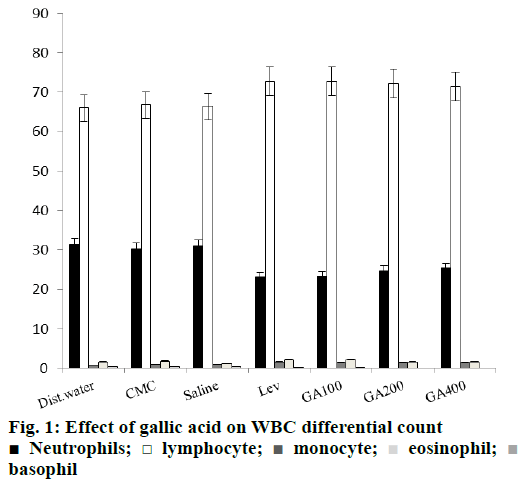
There was significant reduction in lymphocytes and monocytes percent in both cyclophosphamide and cisplatin treated groups, compared with distilled water and saline control groups. Levamisole and gallic acid alone treated groups significantly enhanced these values (Figure 1). In combined treatment groups levamisole and gallic acid resulted in increase in the percent lymphocytes and monocytes significantly. The effect of gallic acid at a dose of 100 mg/kg in combination of cyclophosphamide and cisplatin was almost with that of standard drug levamisole (Figures. 2 and 3).
In this study we observed that, there was a proliferation of total leucocyte and lymphocyte count with all the doses tested when compared with positive control levamisole treated groups. The proliferation of leucocyte and lymphocyte shows the increased immune response of the host [55]. Increase in the WBC counts indicates the immunostimulant activity. Macrophages are phagocytic in function and play a fundamental role in the cellular nonspecific defence mechanism. Gallic acid is immunostimulatory in function by enhancing the phagocytes and lymphocytes, the major innate immune cells.
Cyclophosphamide and cisplatin are potent chemotherapeutic drugs, which supresses the growth of cancerous cells by their alkylating action. In addition to their chemotherapeutic properties most chemotherapeutic drugs are known to cause toxic effects on normal cells by damaging the respiratory, cardiovascular, renal, hepatic, nervous, gastrointestinal and haematopoietic systems [56]. Cyclophosphamide and cisplatin were considered as most effective immunosuppressive drugs [57,58].
In this study, we evaluated the immunomodulating effects of gallic acid against cyclophosphamide and cisplatin-induced immunosuppression in Swiss albino mice. Immunosuppressive/chemotherapeutic agents, cyclophosphamide and cisplatin were administered to experimental animals through intraperitoneal route. Whereas, i.p. route is an alternative route to the most conventional routes used for chemotherapy and used to deliver the drugs to target site within peritoneal cavity [59]. Bioavailability of i.p. route is almost similar to intravenous (i.v.) and is greater than the oral and this is the most commonly used route in small laboratory animals as it is difficult to trace the vein. Moreover, i.p. route of cyclophosphamide and cisplatin administration is to avoid the drugs which are degraded by gastric juices and acids, if given by oral. Also, cyclophosphamide requires metabolic activation by oxidase enzyme, which is present in the liver. By contrast, compounds absorbed from the mouth diffuse into the bloodstream and are not transported directly to the liver and their biotransformation is thereby delayed [59].
Gallic acid is the major bioactive phenolic compounds present in the plants [39,60]. Dietary polyphenols are derived from plants and are consumed in the forms of fruits, vegetables, species and herbs. Polyphenols are agents targeted for disease prevention and have oral bioavailability. Gallic acid is nontoxic to mammals at pharmacological doses is generally absorbed in the intestine [42]. Oral route of administration is the only viable route and hence gallic acid was administered to animals through oral route [61].
Among various naturally occurring polyphenols, gallic acid is one of the triphenolic compounds found in a variety of foods and herbs, which has been considered as effective antioxidant agent [62]. Antioxidants were deliberated as major immunomodulators, which results in the increase or decrease in the immune cells by playing a dual role on the immune system. Higher concentrations of antioxidants were found in immune cells than the other cells; hence, immune cells provide protection against various oxidative stress [63]. In this point, we have selected gallic acid an antioxidant as a test compound, to study the in vivo immunostimulating effects on cyclophosphamide and cisplatin-induced immunosuppression in mice. In view of evaluating the possible protective effects of gallic acid on immune system, thymus weight was considered as one of the study parameters. Also, we focused on variation in the haematological parameters, if any variation in this may indicates the modulation in the immune responses. Therefore, the assessment of haematological parameters is also useful to determine the extent of deleterious/favourable effects of foreign compounds on immune response related cells. A rise or fall in the haematological parameters may vary the immune constitution of the body, thereby affecting the health [64,65]. Differential count of WBCs; total count of WBCs and erythrocytes and also platelet counts were done from the blood samples collected from various control and treated groups. The level of Hb was also determined.
In the present study, pre-treatment with gallic acid has effectively reduced the immune suppression induced by two anticancer drugs i.e cyclophosphamide and cisplatin in Swiss albino mice. Further, gallic acid improved the antibody titer effects indicating that, it facilitates humoral immune response.
Several investigators have reported the immunomodulatory properties of some plants containing gallic acid as major bioactive molecule. Pretreatment with alcoholic extract of Terminalia chebula shown increase in counts of neutrophils and lymphocytes and further it significantly ameliorated the damage on immune cells induced by cyclophosphamide by increasing the phagocytic activity in male Wistar rats [48]. Experimental evidences has demonstrated the immunomodulatory and wound healing properties of methanolic extract of Schinus terebinthifolius against cyclophosphamide-induced damage in male Swiss albino mice [51]. These immunostimulating properties of this extract is due to the presence of phenolic compounds; where gallic acid is also one of the major phytochemical constituents [50], reported the immunomodulatory properties of ethanolic extract of Luffa acutangula, using carbon clearance and neutrophil adhesion test in vivo. Concurrently they stated that this effect is due to the presence of plant phenolics such as gallic acid and p-hydroxybenzoic acid as major polyphenolics. In Swiss albino mice, pre-treatment with hydroalcoholic extraction of Dendrophthoe falcata (L.f) Ettingsh stimulated the macrophages for phagocytosis and also increased the humoral immune response in a dosedependent manner. Further this study demonstrated the presence of gallic acid as one of the chemical constituents in this plant is responsible to attribute the immunostimulatory effect [28]. Similarly, triphala, an Ayurvedic herbal product a rich source of vitamin C, ellagic acid, gallic acid, chebulinic acid, bellericanin, sitosterol and flavonoids has been reported as good immunomodulator [47,52].
In the present study, gallic acid improved the antibody titre effects indicating that, it may facilitate humoral immune response. The humoral immunity involves interaction of B cells with the antigen and their subsequent proliferation and differentiation into antibody-secreting plasma cells. Antibody functions as the effector of the humoral response by binding to antigen and neutralizing it or facilitating its elimination by cross-linking to form clusters that are more readily ingested by phagocytic cells [53,66]. In our study, anticancer drugs cyclophosphamide and cisplatininduced significant inhibition of antibody titre response whereas; gallic acid counteracted the suppression of humoral response induced by cyclophosphamide and cisplatin. Furthermore, gallic acid significantly increased the weight of thymus a primary lymphoid organ, which involved in the adaptive immune response in both gallic acid alone and combined treatment groups. This observation shows its direct effect on the immune cells as well as its immunoprotective effects when administered along with immunosuppressive agents. Thereby, gallic acid ameliorated the immune suppressive effects induced by cyclophosphamide and cisplatin efficiently. Cyclophosphamide and cisplatin also induced significant decrease in total leukocyte count, RBC count and Hb level. Whereas, administration with gallic acid improved blood parameters, which is suppressed by anticancer drugs cyclophosphamide and cisplatin. Results indicate that gallic acid restored effects induced by immunosuppressive drugs. These results revealed the role of gallic acid in the immunomodulation process. The results are correlating with the antibody titre assays.
In this study, levamisole was used as a standard reference immunomodulatory drug. Experimental evidences have been proved levamisole as an good immunomodulator in both human and animals [67] and it has been used as standard immunomodulatory drug [35,68,69-72]. The results obtained in the present study are comparable with positive control levamisole treated groups indicating the immunostimulatory effects of gallic acid.
Although there are some reports available on the immunomodulatory effects of gallic acid as a constituent or as a bioactive molecule of several plants or herbal products, but there is no much reports available on the individual immunomodulatory properties of gallic acid as a pure compound. Hence, in this study we evaluated the immunomodulatory effect of gallic acid as a pure compound against two widely used anticancer, immunosuppressive drugs, cyclophosphamide and cisplatin in Swiss albino mice. In the present study, the positive test agents cyclophosphamide and cisplatin significantly reduced WBC and RBC counts and also Hb concentration compared to the distilled water and saline controls. Pre-treatment with gallic acid at three different doses significantly increased the values of all the above mentioned parameters, indicating its protective effects on the blood cells.
There are several reports on the immunomodulatory effects of plants constituents against cyclophosphamide and cisplatin-induced myelosuppression in Swiss albino mice. The possible immunomodulatory effects of these plants may be due to the presence of flavonoids, phenols, saponins, phenols, proteins, terpenes, alkaloids and tannins as their major constituents. In view of this [73] immunomodulatory properties of ethanolic extract of Glycosmis pentaphylla were reported. In their study, cyclophosphamide has shown its immunosuppressive effect by decreasing the antibody titre values whereas this plant extract stimulated the both the specific and nonspecific immune responses by enhancing the responsiveness of macrophages, T and B lymphocytes. Administration of albino mice with Abutilon indicum extract significantly ameliorated both the primary and secondary haemagglutination titre suppression induced by cyclophosphamide and also potentiated the delayed type of hypersensitivity and phagocytic reactions [66]. Methanolic extract of Sesbania grandiflora flowers, enhanced the production of antibody in a dosedependent manner against cyclophosphamide-induced myelosuppression in mice by potentiating the humoral as well as cell mediated immunity [74]. In previous studies, effective immunomodulatory properties of Dalbergia latifolia extract against cyclophosphamide-induced myelosuppression were reported [22]. Pretreatment with plant extract elevated the depleted levels of RBC, WBC counts and percent Hb and neutrophils in Swiss albino mice.
Some authors have correlated the immunomodulatory effects with the antioxidant status [75] reported that higher antioxidant capacity of E. malaccensis L. and E. uniflora L. helped to reduce the inflammatory response in in vivo. Plant extracts with antioxidant activity could also have immunomodulatory ability [76]. Antioxidants commonly present in our diet improves different immune function exhibiting protection against various infectious agents [77]. Brambilla et al. [78] reviewed the beneficiary role of dietary antioxidant in the regulation of immune response. This type of mechanism can also be expected in the present study with gallic acid because it is one of the phenolic compounds with high antioxidant activity and hence, it may show immunomodulatory effects. Though all three doses of gallic acid tested, showed immunomodulatory effect but the highest effect was observed at lower dose (100 mg/kg). This may be due to under certain circumstances at higher dose some antioxidants may acts as prooxidant [79,80]. High level of antioxidant supplements may disturb normal physiological balance between the ROS formation and neutralization [81]. Gallic acid naturally occurring flavonoid in fruits, vegetables and other plant parts and they have many favourable biological effects due to their antioxidant and free radical scavenging abilities. Investigation on bioactive polyphenolics shows the correlation between the antioxidant/prooxidant effects [82]. According to this study, the most effective antioxidants are also the most cytotoxic and effective antiproliferative agents, may be due to the dual antioxidant/prooxidant effect of polyphenols.
In conclusion, our study revealed the immunostimulatory potential of gallic acid against two potent anticancer drugs, cyclophosphamide and cisplatin-induced immunosuppression in Swiss albino mice. Immunostimulants can be used as adjuvants to heighten the specific immune response. Hence, gallic acid can be used as an immunoadjuvant with immunosuppressive drugs to reduce their contrary effects on immune system.
Acknowledgement
Authors are grateful to the University Grants Commission for providing the Basic Science Research Fellowship to carry out this work.
Conflict of interest
No conflict of interest.
Financial support and sponsorship
Nil.
References
- Mazumder PM, Pattnayak S, Parvani H, Sasmal D, Rathinavelusamy P. Evaluation of immunomodulatory activity ofGlycyrhiza glabra L roots in combination with zing.Asian Pac J Trop Biomed 2012:15-20.
- Haque MR, Ansari SH, Rashikh A. Coffea arabica seed extract stimulate the cellular immune function and cyclophosphamide-induced immunosuppression in mice. Iranian J Pharm Res 2013;12:101-08.
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defences. Clin Microbiol Rev 2009:240-73.
- Choudhary GP. Immunomodulatory activity of alcoholic extract of Tinospora cordifolia. Int J Pharm Chem Sci 2015;4:357-59.
- Al-Fararjeh MA, Jaber MH, Abdelrahman YS. Evaluation of immunomodulatory effects of antiepileptic drug phenytoin. Jordan J Biol Sci 2013;6:328-33.
- Naved T, Siddiqui JI, Ansari SH, Ansari AA, Mukhtar HM. Immunomodulatory activity of Mangifera indicaL. fruits (cv Neelam). J Nat Remedies 2005;5:137-40.
- Gokhale AB, Damre AS, Saraf MN. Investigations into the immunomodulatory activity of Argyreia speciosa. J Ethnopharmacol 2003;84:109-114.
- Mukherjee PK, Nema NK, Bhadra S, Mukherjee D, Braga FC, Matsabisa MG. Immunomodulatory leads from medicinal plants. Indian J Tradit Knowledge 2014;13:235-56.
- Shukla S, Mehta A, Johna J, Mehta P, Vyas SP, Shukla S. Immunomodulatory activities of the ethanolic extract of Caesalpinia bonducellaseeds. J Ethnopharmacol 2009;125:252-6.
- Balekar N, Bodhankar S, Mohan V, Thakurdesai PA. Modulatory activity of a polyphenolic fraction of Cinnamomum zeylanicumL.bark on multiple arms of immunity in normal and immunocompromised mice. J App Pharm Sci 2014;4:114-122.
- Archana, Jatawa S, Paul R, Tiwari A. Indian medicinal plants: a rich source of natural immunomodulator. Int J Pharmacol 2011;7:198-205.
- Saroja M, Santhi R, Annapoorani S. Studies on immunomodulatory activity of flavonoid fractions of Terminalia catappain Swiss Albino mice. Int J Pharma Bio Sci 2012;3:B418-B22.
- Kajaria D, Tripathi JS, Tiwari SK, Pandey BL. Immunomodulatory effect of ethanolic extract of Shirishadi compound. Ayu 2013;34:322-6.
- Rachh P, Dhabaliya F, Rachh M, Lakhani K, Kanani A, Limbani D. Immunomodulatory medicinal plants: A review. Ph Tech Med 2014;3:435-40.
- Nagarathna PKM, Reena K, Reddy S, Wesley J. Review on immunomodulation and immunomodulatory activity of some herbal plants. Int J Pharm Sci Rev Res 2013;22:223-30.
- Attia AA, Ghoneam HEM, El-Twessy MY, Hammed NS. Histological and immunohistochemical studies on the role of α-lipoic acid on cyclophosphamide-induced immunosuppressive effect in mice. Int J Adv Res 2014;2:631-42.
- Zhao J, Wang H, Tian EA, Dong F, Zhou B. Toxic effects of fluoride on primary lymphoid organs and white blood cells in female mice. Res Rep Fluoride 2014;47:227-34.
- Gerpe LD, Mendez MR. Alterations induced by chronic stress in lymphocyte subsets of blood and primary and secondary immune organs of mice. BMC Immunol 2001;2:7.
- https://www.epa.gov/sites/production/files/documents/immunotoxicity-retro-analysis.pdf.
- Gupta A, Gautam MK, Singh RK, Kumar MV, Rao CV, Goel RK, et al. Immunomodulatory effect of Moringa oleifera Lam. extract on cyclophosphamide induced toxicity in mice. Indian J Exp Biol 2010;48:1157-60.
- Ketema T, Yohannes M, Alemayehu E, Ketema AA. Evaluation of immunomodulatory activities of methanolic extract of Khat (Catha edulis, Forsk) and cathinone in Swiss albino mice. BMC Immunol 2015;16:1-11.
- Yadav SK, Nagarathna PKM, Yadav CK. Research article of evaluation of immunomodulatory activity of Dalbergialatifolia on Swiss albino mice. J Pharm Biol Sci 2015;10:58-64.
- Ramachandran S, Latha VM, Charan SN, Dhanaraju MD. Immunomodulatory activity of methonolic extract of Murraya koeingiileaves against azathioprine induced immunosuppression in laboratory animals. Int Res J Pharm 2015;6:658-62.
- Qureshi S, Saxena HM. Estimation of titers of antibody against Pasteurella multocidain cattle vaccinated with haemorrhagic septicemia alum precipitated vaccine. Vet World 2014;7:224-28.
- Costabile M. Determining the reactivity and titre of serum using a haemagglutination assay. J Vis Exp 2010:1-2.
- Descotes J. Introduction to immunotoxicology. London: Taylor & Francis Ltd.; 2003. p. 183.
- Pattanayak SP, Mazumder PM. Immunomodulatory activities of Dendrophthoe falcata(L.f) ettingsh in experimental animals: in vitroand in vivoinvestigations. J Sci Res 2011;3:619-30.
- Sumalatha, Bhat RP, Ballal SR, Acharya S. Studies on immunomodulatory effects of Salacia chinensisL. on albino rats. J App Pharm Sci 2012;2:98-107.
- Sultana R, Khanam S, Devi K. Evaluation of immunomodulatory activity of Solanum xanthocarpumfruits aqueous extract. Der Pharmacia Lettre 2011;3(1):247-53.
- Winkelstein A. Mechanisms of immunosuppression: effects cyclophosphamide on cellular immunity. Blood 1973;41:273-84.
- Oger J. Immunosuppression: promises and failures. J Neuro Sci 2007;259:74-8.
- Hamrita B, Rouissi K, Kouidhi S, Jaouadi B, Elgaaied AB. Do diosgenin ameliorate urinary bladder toxic effect of cyclophosphamide and buthionine sulfoximine in experimental animal models? Afr J Biotechnol 2012;11:2146-53.
- Sibi P, Varghese. Evaluation of in vivoimmunomodulatory activity of Punica crantumLinn. Int J Res Ayurveda Pharm 2014;5:175-8.
- Attia SM. The impact of quercetin on cisplatin-induced clastogenesis and apoptosis in murine marrow cells. Mutagenesis 2010;25:281-8.
- Wiltshaw E. Cisplatin in the treatment of cancer: The first metal antitumour drug. Platin Met Rev 1979;23:90-8.
- Li XB, Schluesener HJ. Therapeutic effects of cisplatin on rat experimental autoimmune encephalomyelitis. Arch Immunol Ther Exp 2006;54:51-3.
- Nasr AY. Protective effect of aged garlic extract against the oxidative stress induced by cisplatin on blood cells parameters andhepatic antioxidant enzymes in rats. Toxicol Rep 2014;1:682-91.
- Craciun C, Pasca C. Structural and ultrastructural data on side effects of cisplatin in spleen, kidney and liver of rats. Acta Metallomica 2014;10:9-22.
- Rasool MK, Sabina EP, Ramya SR, Preety P, Patel S, Mandal N, et al. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J Pharm Pharmacother 2010;62:638-43.
- Karamae M, Kosinska A, Pegg RB. Content of gallic acid in selected plant extracts. Pol J Food Nutr Sci 2006;15/56:55-8.
- Locatelli C, Monteiro FBF, Centa A, Creczinsky-Pasa TB. Antioxidant, antitumoral and antiinflammatory activities of gallic acid. Chapter 2013:1-16.
- Nair GG, Nair CKK. Radioprotective effects of gallic acid in mice. Bio Med Res Int 2013;1-13.
- Pandya NB, Tigari P, Dupadahalli K, Kamurthy H, Nadendla RR. Antitumor and antioxidant status of Terminalia catappaagainst ehrlich ascites carcinoma in Swiss Albino mice. Indian J Pharmacol 2013;45:464-9.
- Olayinka ET, Ore A, Ola OS, Adeyemo OA. Ameliorative effect of gallic acid on cyclophosphamide-induced oxidative injury and hepatic dysfunction in rats. Med Sci 2015;3:78-92.
- Ferrari CK, Torres EA. Biochemical pharmacology of functional foods and prevention of chronic diseases of aging. Biomed Pharmacother 2003;57(5-6):251-60.
- Bouayed J, Bohn T. Exogenous antioxidants-Double-edged swords in cellular redox state Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 2010;3(4):228-37.
- Sabina EP, Rasool MK, Mathew L.In vivoand in vitroimmunomodulatory effects of Indian ayurvedic herbal formulation Triphala on experimental induced inflammation. Pharmacologyonline 2009;2:840-9.
- Aher VD, Kumar A, Wahi. Immunomodulatory effect of alcoholic extract of Terminalia chebularipe fruits. J Pharm Sci Res 2010;2:539-44.
- Nastiti K, Sudarsono, Nugroho AE. Evaluation of in vitroimmunomodulatory effect of fractions of Ficus septicaBurm. f. and their total flavonoid and phenolic contents. Int J Food Res 2014;21:1981-7.
- Mohan KG, Sanjay SJ. Free radical scavenging, immunomodulatory activity and chemical composition of Luffa acutangulavar. Amara (Cucurbitaceae) pericarp. J Chil Chem Soc 2014;59:2299-302.
- Lis ES, Fedel-Miyasato, Candida AL Kassuya, Sarah A Auharek, Anelis SN Formagio, Claudia AL, et al. Evaluation of antiinflammatory, immunomodulatory, chemopreventive and wound healing potentials from Schinus terebinthifoliusmethanolic extract. Rev Bras Farmacogn 2014;24:565-75.
- Saraphanchotiwitthaya A, Sripalakit P, Immunomodulatory effect of different proportions of the herbal mixture in triphala on human T- lymphocytes (molt-4). Int J Pharmacol Pharmacother 2015;7:282-8.
- Kim S, Jun C, Suk K, Choi B, Lim H, Park S, et al. Gallic acid inhibits histamine releaseand pro-inflammatory cytokine production in mast cells. Toxicol Sci 2006;91(1):123-31.
- Mediratta PK, Sharma KK, Singh S. Evaluation of immunomodulatory potential of Ocimum sanctumseed oil and its possible mechanism of action. J Ethnopharmacol 2002; 80:15-20.
- Mukherjee KR, Nema KN, Bhadra S, Mukherjee D, Braga CF, Matsabisa GM. Immunomodulatory leads from medicinal plants. Indian J Trad Know 2014;13(2):235-56.
- Allan N, Siller C, Breen A. Anaesthetic implications of chemotherapy. Continuing education in anaesthesia. Anaesth Crit Care Pain J 2011;1-5.
- Balow JE, Hurley DL, Fauci AS. Cyclophosphamide suppression of established cell-mediated immunity: Quantitative vs. Qualitative changes in lymphocyte populations. J Clin Invest 1975;56:65-70.
- Bennett PN. Drugs and Human Lactation. 2nd ed. Amsterdam, Netherlands: Elsevier Science; 1996. p. 712.
- Chaudary K, Haddadin S, Nistala R, Papageorgio C. Intraperitoneal drug therapy: an advantage. Curr Clin Pharmacol 2010;5(2):82-8.
- Karamac M, Kosinska A, Pegg RB. Content of gallic acid in selected plant extracts. Pol J Food Nutr Sci 2006;1:55-8.
- Hu M. Commentary: Bioavailability of Flavonoids and Polyphenols: Call to Arms . Mol Pharm 2007;4(6):803-06.
- Badhani B, Sharma N, Kakkar R. Gallic acid: a versatile antioxidant with promising therapeutic and industrial applications. Res Adv 2015;5:27540-57.
- Watson RR, Preedy VR. Bioactive food as dietary interventions for arthritis and related inflammatory. Cambridge, Massachusetts: Academic Press; 2012. p. 654.
- Ajibade TO, Soetan KO. Evaluation of hematological and plasma biochemical effects of aqueous extracts of Parkia biglobosa seeds in rats. Afr J Biotechnol 2012;11:15446-50.
- Choudhury S, Sinha MP. Effect of aqueous extract of Murraya koenigiion haematological, hormonal and lipid profile of albino rats. J Coastal Life Med 2015;3:901-05.
- Dashputre NL, Naikwade NS. Immunomodulatory activity of Abutilon indicumlinn on Albino mice. Int J Pharm Sci Res 2010;1:178-84.
- Brunner CJ. Muscoplat CC. Immunomodulatory effects of levamisole. J Am Vet Med Assoc 1980;176:1159-162.
- Singh S, Yadav AK. Evaluation of immunomodulatory activity of Grewia asiaticain laboratory animals. J Chem Pharm Res 2014;6:2820-6.
- Sunitha K, Mohan K. Screening of Limonia acidissimafruit pulp for immunomodulatory activity. Res J Pharm Biol Chem Sci 2013;4:439-44.
- Rinki S, Mishra RN. Immunomodulatory activity of triphala megaext. Int J Res Pharm Biomed Sci 2011;2:575-8.
- Gaikwad SB, Mohan GK. Immunomodulatory activity of methanolic extract of Thespesia populnealeaves in Wistar Albino rats. Asian Pharm Clin Res 2011;4:99-101.
- Gaikwad SB, Mohan GK, Reddy KJ. Moringa oleiferaleaves: immunomodulation in Wistar Albino rats. Int J Pharm Pharm Sc 2011;3:426-30.
- Chitra V, Janaki PS, Raju D, Rao VP. Evaluation of immunomodulatory activity of ethanolic extract of leaves of Glycosmis pentaphyllain Swiss albino mice. Int J Pharm Pharm Sci 2013;5:110-13.
- Arunabha M, Satish N. Evaluation of immunomodulatory activity of Sesbania grandiflora flowers extract in mice. Indonesian J Pharm 2014;25:277-83.
- Figueiroa EDO, Silva LCND, Melo CMLD, Neves JKDAL, Silva NHD, Pereira VRA, et al. Evaluation of antioxidant, immunomodulatory and cytotoxic action of fractions from Eugenia unifloraL. and Eugenia malaccensisL.: correlation with polyphenol and flavanoid content. Sci World J 2013;1-7.
- Awah FM, Uzoegwu PN. Free radical scavenging activity and immunomodulatory effect of Stachytarphetaangustifolialeaf extract. Food Chem 2010;119:1409-16.
- Puertollano MA, Puertollano E, de Cienfuegos GA, de Pablo MA. Dietary antioxidants: immunity and host defense.Curr Top Med Chem 2011;11:1752-66.
- Brambilla D, Mancuso C, Scuderi MR, Bosco P, Cantarella G, Lempereur L, et al. The role of antioxidant supplements in immune system, neoplastic and neurodegenerative disorders: a point of view for an assessment of the risk/benefit profile. Nutr J 2008;7:2-9.
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, pro-oxidants and antioxidants: the interplay. Biomed Res Int 2014;1-19.
- Bouayed J, Bohn T. Exogenous antioxidants double edged swords in cellular redox state health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 2010;3:4:228-37.
- Poljsak B, Suput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013;1-11.
- Tourino S, Lizarraga D, Carreas A, Matito C, Ugartondo V, Centelles JJ, et al. Antioxidant/proxidant effects of bioactive polyphenolics. EJEAFChe 2008;7(8):3348-52.
 Neutrophils;
Neutrophils;  lymphocyte;
lymphocyte;  monocyte;
monocyte;  eosinophil;
eosinophil;  basophil
basophil