- *Corresponding Author:
- K. Priya
Aster Medcity, South Chittoor P O, Cheranelloor, Kochi-682 027, India
E-mail: priya.karunakaran@dmhealthcare.com
| Date of Submission | 05 September 2016 |
| Date of Revision | 30 March 2017 |
| Date of Acceptance | 05 October 2017 |
| Indian J Pharm Sci 2017;79(6): 1017-1022 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study was undertaken to evaluate the impact of electronic prescription audit for outpatients in a quaternary care hospital. This reviews the clinical benefits of pharmacist driven electronic prescription audit process in monitoring and detecting prescription errors before it reaches the patient. This prospective study was conducted for one year (August 2015-July 2016) by the Department of Clinical Pharmacy, Aster Medcity. During the audit process, each prescription generated through Computerized Physician Order Entry will appear immediately in an electronic prescription audit tool which is integrated with a clinical decision support system. Pharmacist audits each outpatient prescription for drug interactions, drug allergies, dosing errors, frequency errors and therapeutic duplications. Clinical decision support system integrated with the audit tool provides brand and monograph details of prescribed drugs and automatic alerts for drug interactions and drug allergies. Pharmacist reported 266 interventions during the study period. Out of that 0.08 % (N=140) errors were prevented before it reached the patient and 0.05 % (N=86) interventions were rejected by physicians with proper justifications. Drug interactions were found to be 0.03%, wrong drug frequency errors were found to be 0.04 % and drug allergies (prescriptions with pre-identified allergic drugs) were found to be 0.00 5%. Reported medication errors were categorized according to National Coordinating Council for Medication Error Reporting and Prevention index. Real time audit of outpatient prescriptions using automated prescription audit tool can reduce the risk of harm that arises from prescribing errors, improve the quality of prescriptions, and enhance the safety and quality of the prescribing process.
Keywords
Computerized physician order entry, clinical decision support systems, electronic prescription audit tool, medication errors, outpatient
The dilemma of medication management poses a significant safety risk for patients. Medication errors and adverse drug events (ADEs) most frequently occur at the drug ordering or prescribing stage [1-3]. Outpatient prescription errors are commonly encountered in healthcare settings but most of the time they are unidentified at the point of prescription and dispensing. Prescribing medicines to patients is an important part of medical care. It includes decision-making about the choice of medicines, its communication to pharmacist in the form of prescriptions for dispensing and finally, administration of medicines. The whole process of prescription requires effective communication at various stages. This process involves multiple individuals and prone to produce errors with the potential of jeopardizing patient care. An error can occur at any stage of the prescription process like choosing a medicine, prescription writing, formulation used, dispensing of medication, administering/taking the medicine and monitor therapy. Failing to alter therapy on real time may sometimes account for serious errors [4].
The tendency to use advanced technology in healthcare have put forward the concept of electronic prescription. It is considered as the main solution to overcome the major drawbacks of the paper-based medication prescription [5]. One intervention that has substantial potential for improving the medication ordering process is computerized physician order entry (CPOE), an application in which prescribers write orders online has been shown to decrease medication errors by 55-80 % [6-8]. The system then transmits the order to the appropriate department, or individuals, so that the order can be carried out. The most advanced implementations of such systems also provide real-time clinical decision support such as dosage and alternative medication suggestions, duplicate therapy warnings, and drug-drug and drug-allergy interaction checking [9]. Prescribing errors related to illegible handwriting, drug/allergy interactions, wrong dose formulation, and incomplete orders were judged to be preventable in almost all cases. Prescribing errors due to inaccurate or missing patient medication histories and medication omissions would likely be unpreventable by most currently available CPOE systems [10].
In addition to improving the selection and specification process of transmitting orders, most CPOE systems are equipped with some degree of clinical decision support. Clinical decision support systems (CDSS) provide computerized information helpful to physicians and pharmacists such as clinical practice guidelines, patient allergies, potential drug duplications, and drug interactions. Clinical decision support is provided in a variety of forms. For instance, pop up alerts may be generated about a potentially harmful drug interaction that requires the user to take action before proceeding or links to drug information resources and clinical treatment guidelines may be provided. In summary, clinical decision support provide useful point-of-care references and relevant patient information to assist in medical decision-making [11].
Prescription Drug Monitoring Programs (PDMPs) are electronic databases used to monitor the prescribing and dispensing of drugs to patients. This information can help to identify patients who would benefit from early interventions. California was the first state in USA to introduce a PDMP in 1939. By 1992, 10 states had operational PDMPs and many more emerging countries were in the process of enacting legislation for the establishment of a PDMP. Automated PDMP can give a prescriber or pharmacist regarding the critical information to prevent medication errors on actual time. Real time prescription audit using electronic prescription audit tool will allow transmission of prescription details from the prescriber to pharmacist. Its review mechanism will check each prescription either for internal inconsistencies such as excessive dosage, wrong frequency or for conflicts with the patient’s known allergies and interactions with other active medications [12].
Study implicates the clinical importance of pharmacist driven electronic PDMP and prevention of medication errors before it reaches the patient. It also provides beneficial information concerning electronic prescription auditing system to a variety of stakeholders. The severity rating of the prescribing error was based on the potential of the error to result in an ADE or inadequate therapeutic response if the order was carried out. Prescribing errors were thus classified into 9 letter-designated categories according to the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) index for categorizing medication errors. These categories medication errors into ascending order of seriousness include category A (error occurred but no potential to cause harm), category B (an error occurred but the error did not reach the patient), category C (an error occurred that reached the patient, but did not cause patient harm), category D (an error occurred that reached the patient and required monitoring to confirm that it resulted in no harm to the patient and/or required intervention to preclude harm), category E (an error occurred that may have contributed to or resulted in temporary harm to the patient and required intervention), category F (an error occurred that may have contributed to or resulted in temporary harm to the patient and required initial or prolonged hospitalization), category G (an error occurred that may have contributed to or resulted in permanent patient harm), category H (an error occurred that required intervention necessary to sustain life), category I (an error occurred that may have contributed to or resulted in patient’s death) [13]. For the present study, these categories were concised into following 3 categories include no harm (A-C), monitoring required (D) and harmful (E-I).
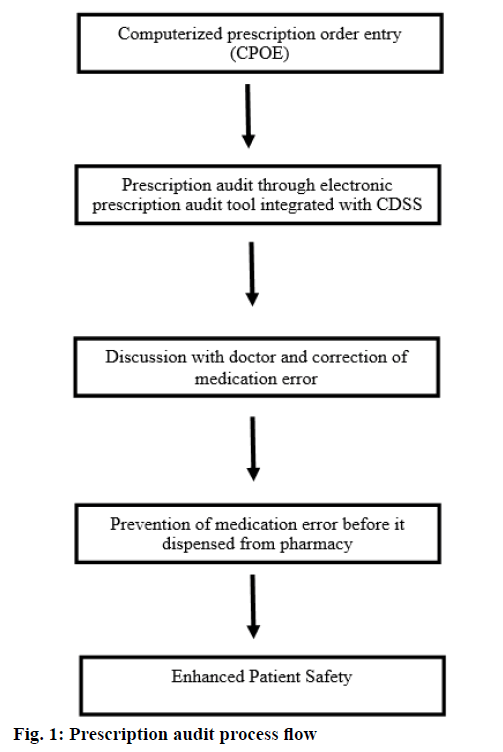
The data were collected and audited from a quaternary care hospital during the study period of one year from August 2015 to July 2016. During this period 23750 prescriptions were audited using electronic prescription audit tool. Inpatient prescriptions were excluded from the study. As shown in Figure 1, the electronic prescription audit tool was integrated with a CDSS and electronic prescription module of hospital information system. Through the audit tool each prescription was made available to pharmacist immediately after generation of e-prescription. CDSS provides different colour coded alerts like orange, sky blue, pink, green and deep blue for brand details, monograph details, drug to drug interaction (if any), drug to health interaction (if any) and drug to allergy interaction (if any), respectively for each drug. Each alert will give specific information as follows. Brand details: dosage form, composition, therapeutic class, manufacturer and packaging prize. Monograph details: dosage, administration, contraindication, special precautions, adverse reactions, mechanism of action and Anatomical Therapeutic Chemical (ATC) classification. Drug to drug interaction: major, moderate and minor drug interactions according to the severity of interaction. Drug to health interaction: any interaction with patient disease condition documented electronically by the prescriber. Drug allergy alerts: prescription of drugs having cross sensitivity reactions to the electronically documented allergy.
All medications were checked by pharmacist for errors in dosing, dosage interval, pharmaceutical form, therapeutic duplications, drug to drug interactions, drug to health interaction and known allergy to the prescribed drug. Relevant interventions were identified and communicated to doctors on time to make necessary changes in prescription and prevent patient harm. Medication errors were classified according to NCC MERP guidelines. In addition to pharmacist own professional knowledge other clinical guidelines like UpToDate and PubMed journals were also used. Automatic alerts helped the pharmacist to reduce the prescription monitoring time and act immediately upon medication errors.
Audited data were subjected to analysis using Minitab statistical tool. One sample t-test and Pareto chart were used for acceptance of interventions and percentage of errors prevented, respectively.
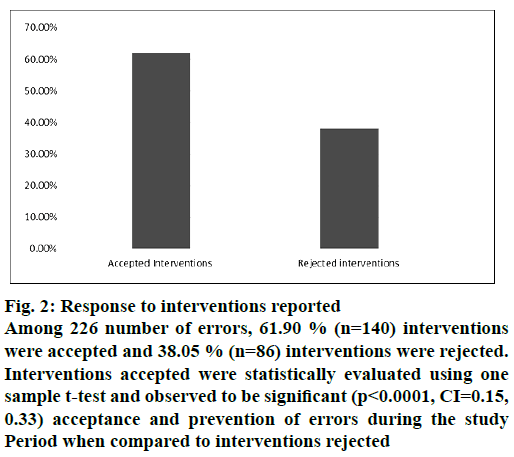
During the study period, pharmacist audited 23 750 prescriptions using electronic prescription audit tool and reported 0.13 % (n=226) number of errors. Among 226 errors, 61.90 % (n=140) interventions were accepted and 38.05 % (n=86) interventions were rejected. Interventions accepted were statistically evaluated using one sample t-test and observed significant (p<0.0001, CI=0.15, 0.33) acceptance and of errors during the study period when compared to interventions rejected (Figure 2).
Figure 2: Response to interventions reported
Among 226 number of errors, 61.90 % (n=140) interventions were accepted and 38.05 % (n=86) interventions were rejected. Interventions accepted were statistically evaluated using one sample t-test and observed to be significant (p<0.0001, CI=0.15, 0.33) acceptance and prevention of errors during the study Period when compared to interventions rejected
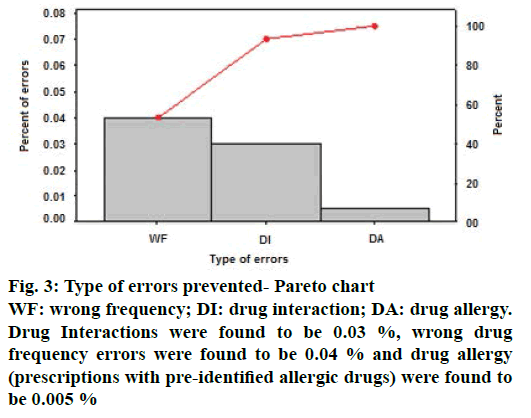
From the Pareto chart, as shown in Figure 3 it is observed that, out of 140 errors prevented among 23 750 prescriptions, 93.3 % of errors were drug interactions and wrong drug frequency. Drug interactions were found to be 0.03 %, wrong drug frequency errors were found to be 0.04 % and drug allergy (prescriptions with pre-identified allergic drugs) were found to be 0.005 % Out of the 86 interventions rejected, reasons contributed for rejection include benefit overweighs risk (0.02 %, n=42), observed clinical relevance for the patient (0.01 %, n=25), not significant due to noncompliance to treatment (0.007 %, n=12) and patient resistant to change (0.004 %, n=7, Table 1). Rejected interventions include the prescription of psychiatric and palliative care patients where chances of treatment noncompliance were high.
| Reasons for rejecting Interventions | Interventions rejected in Percentage |
|---|---|
| Benefitoverweighs risk | 0.02% (n=42) |
| observed clinical relevance for the patient | 0.01% (n=25) |
| Not significant due to noncompliance to treatment | 0.007% (n=12) |
| Patient resistant to change | 0.004% (n=7) |
Table 1: Reasons for Rejecting the Interventions
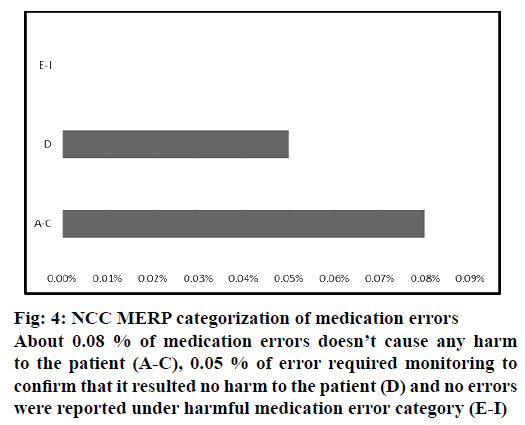
The severity of reported medication errors were categorized according to NCC MERP category (Figure 4). About 0.08 % of medication errors did not cause any harm to the patient (A-C) , 0.05 % of error required monitoring to confirm that it resulted no harm to the patient (D) and no errors were reported under harmful medication error category (E-I).
In India, over the last 40 y advances in drug therapy has improved patient care but also led to a noticeable increase in the incidence of drug related problems. Studies conducted in developed countries showed that approximately 5 % of all hospital admissions were drug related and 50 % of those were avoidable [14]. Pharmacist driven electronic PDMP in outpatient setting helps to identify and notify drug related problems to physicians and rectify the errors within short period of time. The majority of data on technological interventions is focused on the inpatient hospital setting. But this study at Aster Medcity is contrary, as it was carried out among outpatients because most of the errors in the outpatient setting gets often unidentified. Studies have shown that in outpatient setting between 18 % and 25 % of patients may have an ADE [15,16]. Our study varies in several ways. To the best of our knowledge, none have conducted and published studies on CPOE and CDSS yet in India. This is the first work that shows the effect of real time prescription audit in an outpatient setting.
Medication errors can occur due to various causes, such as illegible handwritten prescriptions and improperly written prescriptions that could be misinterpreted. In various published studies to date, investigators reported that computerized prescribing resulted in substantially fewer prescribing errors than at similar without CPOE [17]. CPOE systems minimize errors by providing very drug-specific information that can clarify potential confusion due to drug names that sound and look alike [18]. Among the 226 interventions, 140 (61.9 %) errors including drug interaction, wrong drug frequency and known allergy were accepted and prevented at the prescription level itself. In the ADE prevention study, carried out by Bates et al., CPOE prevented up to 84 % of medication errors among patients hospitalized at two academic medical centers. Evans et al. found that implementation of computerized ADE surveillance, coupled with alerts to pharmacists about drug allergies, standardization of antibiotic administration rates, and physician notification about ADEs, reduced ADE rates [19]. Also, Steele et al. investigated the use of CPOE with CDS in a medical outpatient clinic for reducing medication errors and ADEs that demonstrated a non-statistically significant reduction from twelve ADEs in the baseline period to two in the intervention period [20].
Implementation of PDMPs and effective communication of auditing pharmacist with doctors, dispensing pharmacist and patients prevented the errors before it reached the patient. The use of automated prescription monitoring system is recommended as an effective tool to reduce medication errors on real time. Introducing electronic prescription in healthcare settings will provide a wide opportunity to integrate information technologies to improve the quality, safety and health care efficiency. Outpatient prescription auditing by pharmacist using integrated drug information database will help to monitor, identify, select, and communicate only significant drug related problems to physicians within a short time. Strengthening and supporting real time outpatient drug monitoring program may also decrease the individual health care cost by avoiding drug related problems. It requires the collaboration between all system stakeholders and healthcare information infrastructure, which leads to better health care for every person in the society.
We conclude that a CPOE system integrated with CDSS will decrease the number of medication errors with a potential for harm by more than half. Pharmacist leadership and involvement in the medication management process are key to improve patient safety, which leads to better health care for every person of the society.
Acknowledgement
This short research project was conducted in Aster Medcity, Kochi. The authors would like to thank all the physicians and pharmacists working in Aster Medcity for their timely response and support to prevent the errors.
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support and sponsorship
Nil.
References
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995;274(1):29-34.
- Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med 1993;8(6):289-94.
- Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA 2001;285(16):2114-20.
- Mohan P, Sharma AK, Panwar SS. Identification and quantification of prescription errors. Med J Armed Forces India 2014;70(2):149-53.
- Samadbeik M, Ahmadi M, Asanjan SMH. A theoretical approach to electronic prescription system: lesson learned from literature review. Iran Red Crescent Med J 2013;15(10):8436.
- Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA 1998;280(15):1311-16
- Bates DW, Teich JM, Lee J, Seger D, Kuperman GJ, Ma'Luf N, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc 1999;6(4):313-21
- Kuperman GJ, Teich JM, Gandhi TK, Bates DW. Patient safety and computerized medication ordering at Brigham and Women's Hospital. JtComm J QualImprov 2001;27(10):509-21
- Osheroff JA, Pifer EA, Teich JM, Sittig DF, Jenders RA. Improving Outcomes with Clinical Decision Suppport: An Implementer's Guide. 1st ed. New York: Productivity Press publishers; 2005.
- Bobb A, Gleason K, Husch M, Feinglass J, Yarnold PR, Noskin GA. The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med 2004;164(7):785-92.
- Minesh P, Isha P, Jongwha C, Rachel R, Jatine S, Akram A, et al. Computerized physician order entry (CPOE) Systems- An introduction. J Pharm Res 2012;5:4962-7.
- Chen YF, Neil KE, Avery AJ, Dewey ME, Johnson C. Prescribing errors and other problems reported by community pharmacists. TherClin Risk Manag 2005;1(4):333-42.
- Ferracini FT, Marra AR, Schvartsman C, Dos Santos OF, Victor Eda S, Negrini NM, et al. Using Positive Deviance to reduce medication errors in a tertiary care hospital. BMC Pharmacol Toxicol 2016;17(1):36.
- Sonal Sekhar M, Adheena Mary C, Anju PG, Hamsa NA. Study on drug related hospital admissions in a tertiary care hospital in South India. Saudi Pharm J 2011;19(4):273-8.
- Gandhi TK, Burstin HR, Cook EF, Puopolo AL, Haas JS, Brennan TA, et al. Drug complications in outpatients. J Gen Intern Med 2000;15(3):149-54.
- Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, et al. Adverse drug events in ambulatory care. N Engl J Med 2003;348(16):1556-64.
- Weingart SN, Toth M, Sands DZ, Aronson MD, Davis RB, Phillips RS. Physicians' decisions to override computerized drug alerts in primary care. Arch Intern Med 2003;163(21):2625-31.
- Bobb A, Gleason K, Husch M, Feinglass J, Yarnold PR, Noskin GA. The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med 2004;164(7):785-92.
- Evans RS, Pestotnik SL, Classen DC, Horn SD, Bass SB, Burke JP. Preventing adverse drug events in hospitalized patients. Ann Pharmacother 1994;28(4):523-7
- Steele AW, Eisert S, Witter J, Lyons P, Jones MA, Gabow P, et al. The effect of automated alerts on provider ordering behavior in an outpatient setting. PLoS Med 2005;2(9):e255.