- *Corresponding Author:
- Jinke Sun
Department of Orthopaedic, Tianjin University Tianjin Hospital, Tianjin 300200,China
E-mail: naturelh2024@163.com
| Date of Received | 25 February 2023 |
| Date of Revision | 07 November 2023 |
| Date of Acceptance | 14 May 2024 |
| Indian J Pharm Sci 2024;86(3):1008-1016 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Using both cellular and animal models, this research assessed emodin effect on human chondrocytes as well as its underlying anti-inflammatory mechanisms. The results demonstrated that emodin had no significant impact on cell viability in normal chondrocytes, but exhibited a dose-dependent protective effect on lipopolysaccharides-induced inflammatory chondrocytes and inhibition of cell apoptosis. Moreover, emodin was found to reduce the levels of inflammatory cytokines induced by lipopolysaccharides. Mechanistically, emodin decreased the levels of reactive oxygen species in lipopolysaccharides-treated TC28a2 human chondrocyte cells and hampered the stimulation of the high mobility group box-1/toll-like receptor 4/nuclear factor kappa B signaling pathway. In a knee osteoarthritis rat model, emodin improved the morphology of cartilage tissue, suppressed cell apoptosis, and restrained the high mobility group box-1/toll-like receptor 4/nuclear factor kappa B signaling pathway and reactive oxygen species accumulation. In summary, emodin exhibited an anti-inflammatory effect, improving cell survival, suppressing apoptosis in chondrocytes, as well as reducing the levels of inflammatory cytokines, which might be achieved by modulating the high mobility group box-1/toll-like receptor 4/nuclear factor kappa B signaling pathway and reactive oxygen species levels. These results indicate that emodin seems to have therapeutic value in the treatment of knee osteoarthritis.
Keywords
Emodin, knee osteoarthritis, high mobility group box-1/toll-like receptor 4, inflammatory factors, cellular apoptosis
Knee Osteoarthritis (KOA) is a widely recognized degenerative joint disease characterized by cartilage degeneration, inflammation, as well as ultimately joint dysfunction[1]. When joint cartilage is damaged, the body releases inflammatory mediators that trigger an inflammatory response. This inflammatory response leads to pathological changes in the tissues surrounding the joints, exacerbates the damage to the articular cartilage, and promotes the progression of KOA[2]. Despite extensive research, effective treatments for KOA remain limited. At present, the therapeutic approaches of KOA encompass the following; conservative treatment, drug administration, physical therapy, and surgical treatment. However, there are shortcomings in these methods, such as conservative treatment may temporarily relieve symptoms but cannot effectively eliminate the symptoms, the effect of physical therapy varies widely among individuals and requires longterm persistence to observe the effect, and Non- Steroidal Anti-Inflammatory Drugs (NSAIDs) are widely applied in drug therapy. Although these drugs have a rapid onset of action and can relieve pain and inflammation, they have large side effects, especially gastrointestinal reactions, and although the success rate of surgical treatment is high, it also has the disadvantages of surgical risk, longterm recovery, and high cost. As a result, there is an urgent need for research and development of new interventions.
Some studies have shown that inhibiting the inflammatory response of patients with KOA can effectively alleviate pain and improve quality of life[3]. Chao et al.[4] used ultrasonography to predict inflammation and assess clinical response in corticosteroid-injected patients with KOA and found a significant correlation between pain subscale scores and the presence of inflammatory response over a longer period of time. Dainese et al.[5] implemented a systematic review of the literature on the interaction between inflammatory markers and suffering in patients with KOA and discovered that signs of joint inflammation and molecular inflammatory factors were linked to pain. It has been found that the level of inflammatory factors in human Osteoarthritis (OA) chondrocytes is also inhibited when Nuclear Factor Kappa B (NF- κB) activation is inhibited[2]. In the progression of KOA, NF-κB activation can be inhibited through a variety of pathways, thereby exerting a therapeutic effect. Zhang et al.[6] concluded that curcumin was capable of reducing the level of inflammation in KOA rats through the Toll-Like Receptor 4 (TLR4)/Myeloid Differentiation Factor 88 (MyD88)/NF-κB signaling pathway. Xu et al.[7] found that lidocaine-containing nd-Acu was effective in relieving pain, reducing inflammation, and slowing the progression of KOA biochemically and histologically, all of which were attributed to in vivo regulation of the High Mobility Group Box-1 (HMGB1)/TLR4 signaling pathway. Therefore, we can find that HMGB1/TLR4 and NF-κB signaling pathways are important signaling pathways in the development of KOA. In addition, Li et al.[8] Forsythoside B attenuated cartilage damage and degeneration via the HMGB1/TLR4/ NF-κB pathway.
Traditional herbal and traditional Chinese herbal formulas have demonstrated efficacy in relieving KOA symptoms but their complex composition and multi-component nature make it difficult to accurately understand their therapeutic mechanisms and pharmacodynamics components[9]. Therefore, the development of Traditional Chinese Medicine (TCM) monomers has become a feasible method. Emodin obviously is one of TCM. Emodin has been shown to protect human Neural Precursor Cells (NPCs) from Interleukin-1 Beta (IL-1β)- induced apoptosis and inflammation by inhibiting Reactive Oxygen Species (ROS)-mediated NF- κB activation[10]. Otoo et al.[11] found that emodin was capable of limiting the HMGB1/TLR4/NF-κB signaling pathway and improving ovarian function in Polycystic Ovary Syndrome (PCOS) mice. From this, we can find that emodin is able to affect HMGB1/TLR4/NF-κB signaling pathways and improve inflammation, which is closely related to the progression of KOA. Furthermore, researchers have also indicated that emodin, as an active ingredient of Erxian Decoction (EXD) (a TCM decoction), may play a prominent role in relieving cartilage damage in KOA[12].
In conclusion, emodin may have a promising therapeutic effect on KOA by modulating the HMGB1/TLR4 signaling pathway to inhibit inflammation and apoptosis. However, according to the available research data, this possible mechanism has not been effectively validated. We will investigate the effects of emodin on KOA inflammatory factors and apoptosis. The research focuses on the HMGB1/TLR4 signaling pathway. This provides a basis for further research on the potential of emodin as a treatment for KOA, and provides a direction for exploring new therapeutic strategies and drug development.
Materials and Methods
Cell culture and treatment:
In a suitable incubator, TC28a2 human chondrocyte cells (SCC042, Merck) were cultured in Dulbecco's Modified Eagle Medium (DMEM) High Glucose (DMEMHG), (Zen-Bio, Inc., Durham, North Carolina, United States of America (USA)) supplemented with 10 % Fetal Bovine Serum (FBS) (MBS2568758, MyBioSource, San Diego, California, USA) and 1 % (Penicillin-Streptomycin (PSF)-1, Zen-Bio, Inc., North Carolina, USA).
The TC28a2 cells were divided into control and Lipopolysaccharides (LPS) groups. The control group did not receive any treatment, while the LPS group was given 10 μg/ml LPS (14011S, Cell Signaling Technology) for 12 h to induce TC28a2 cells inflammation. The experimental group was further divided into LPS and emodin groups. The emodin group was treated with 60 μmol/l emodin (E409173), while the LPS group received treatment with an equal volume of solvent (Dimethyl Sulfoxide (DMSO)). Experiments were conducted on logarithmic phase cells.
Cell viability:
Logarithmic phase TC28a2 human chondrocyte cells were randomly allocated into control and LPS groups and treated according to the aforementioned cell culture methods. In the control group, samples were incubated with 0, 10, 20, 40, 60, and 100 μmol/l of emodin. In the LPS group, medium supplemented with 0, 10, 20, 40, and 60 μmol/l. After preparing single-cell suspensions, cells were counted and seeded to incubate overnight. Then, the Cell Counting Kit-8 (CCK-8) solution was incorporated per well, and the mixture was cultivated at a constant temperature for 1 h. A microplate reader was utilized to measure absorbance values at 450 nm, and the results were recorded for later analysis.
Flow cytometry analysis of cell apoptosis:
TC28a2 cells from each group were collected and resuspended in a culture medium. After seeding, the cells were placed in a sterile incubator for cultivation. Following incubation, the cells were resuspended in 1× binding buffer and transferred to flow cytometry tubes. Then, 5 μl of Annexin V-Fluorescein Isothiocyanate (FITC) (abx290007, Abbexa Ltd.,) was added and mixed thoroughly, followed by the addition of 5 μl of Propidium Iodide (PI) solution (abx290007, Abbexa Ltd.,) and further mixing. The mixture was incubated in the dark for 15 min, and cell apoptosis was assessed using a flow cytometer.
Enzyme-Linked Immunosorbent Assay (ELISA):
Adjust the cell density to 2×105 cells and seed them into 6-well plates. Grouping and treatment were performed according to the methods described in cell culture and treatment. Each group was set up with 3 replicate wells. After 1 h of incubation, collect the cell culture supernatant or treated tissues and follow the instructions of the kit to perform ELISA to detect the levels of IL- 1β (EK0394, BOSTER, Wuhan), IL-6 (EK0410, BOSTER, Wuhan), and Tumour Necrosis Factor Alpha (TNF-α) (EK0527, BOSTER, Wuhan).
Detection of ROS level:
The ROS assay kit (Beyotime, Shanghai, China) was utilized to ascertain the ROS levels present in tissues or cells. Cells were treated to 10 mmol/l Dichlorofluorescein Diacetate (DCFDA) for 20 min. The cells were then cleaned, put back into suspension, filtered, and the DCF was measured.
Western Blotting (WB):
The chondrocytes were split into groups and treated according to the procedures outlined in the cell culture and treatment section. After trypsin digestion, cells from each group were collected and lysed on ice with cell lysis buffer, then centrifuged at 15 000 rpm for 10 min to collect the supernatant. A Bicinchoninic Acid (BCA) protein assay kit was exploited to determine protein concentration. Protein samples were prepared by adding protein loading buffer and loading 30 μg of protein per well. Proteins were separated utilizing electrophoresis on a 12 % polyacrylamide gel (B7021S, New England Biolabs, USA) and then transferred to membranes. The membranes were blocked and then incubated with primary antibodies, which included HMGB1 (1:1000, 6893), TLR4 (1:1000, 38519), NF-κB p65 (1:1000, 8242), and p-NF-κB p65 (1:1000, 3033). All the primary antibodies were from cell signaling technology. Subsequently, the membranes were incubated with secondary antibodies (1:2000, 32935), and then washed with membrane wash buffer three times. Protein bands were visualized using an imaging system after exposure to the developing solution. Protein band grayscale values were analyzed using ImageJ software.
Animals and KOA model:
A random drawing method was utilized to divide Sprague-Dawley (SD) rats (A12100, genetmed) into two groups; the control group and KOA group. After anesthesia of the SD rats, a longitudinal incision was made on the medial side of the knee joint, the anterior cruciate ligament was cut, avoiding the articular cartilage surface, and the incision was closed layer by layer. Postoperatively, each rat received intramuscular injections of antibiotics for 3 consecutive days to prevent infection, followed by free activity in the cage. The KOA model was established 6 w after surgery. Rats successfully modeled were randomly assigned to the KOA group and the emodin group using the drawing method. In the control group and KOA group, equal volumes of solvent were administered orally to the rats daily, while the emodin group received oral administration of 40 mg/(kg·d) emodin. Administration was performed once daily for 12 consecutive weeks, after which the rats were euthanized, and cartilage tissues were collected.
Hematoxylin and Eosin (H&E) staining:
After 48 h of fixation of the rat knee joints, they were placed in a decalcification solution for decalcification. The decalcification solution was changed every (5-7) d, and after 45 d, the decalcification status of the bone tissue was assessed. If the bone could be cut into slices with a knife, it indicated that decalcification was completed; otherwise, it needed to be continued in the decalcification solution. After decalcification, the bone tissues were washed with tap water for 24 h, followed by routine dehydration, embedding in paraffin, and sectioning at a thickness of 3 μm. And then oven-dry the slides. Tissue slides were stained using the H&E staining method (C0105S, Beyotime), and images of relevant sections were captured.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL):
The cartilage tissues from each group of rats were embedded in paraffin, sectioned at 5 μm thickness, and treated with 3 % Hydrogen peroxide (H2O2) and proteinase K. TUNEL assay was performed by adding TUNEL reagent and incubated, followed by 3,3′-Diaminobenzidine (DAB) staining and counterstaining with hematoxylin. Apoptotic positive cells were identified by brown-yellow granules in the cell nuclei. Five random fields were observed to count the TUNEL-positive cells (%).
Immunohistochemistry (IHC):
The knee joint sections were dewaxed in xylene, followed by blocking with 10 % goat serum. Subsequently, they were incubated with primary antibody HMGB1 (1:100, 6893, cell signaling technology) and secondary antibody sequentially. After DAB and hematoxylin staining, the sections were dehydrated, cleared, and mounted for microscopy. Samples from relevant areas were photographed. Hematoxylin stained the cell nuclei blue, while DAB-positive staining appeared brown-yellow.
Statistical analysis:
The mean standard deviation is displayed following data analysis utilizing the GraphPad Prism program. To identify significant differences between groups for multiple groups, one-way Analysis of Variance (ANOVA) was applied; for two groups, t-tests were utilized. To evaluate the histology examination, ImageJ was also deployed.
Results and Discussion
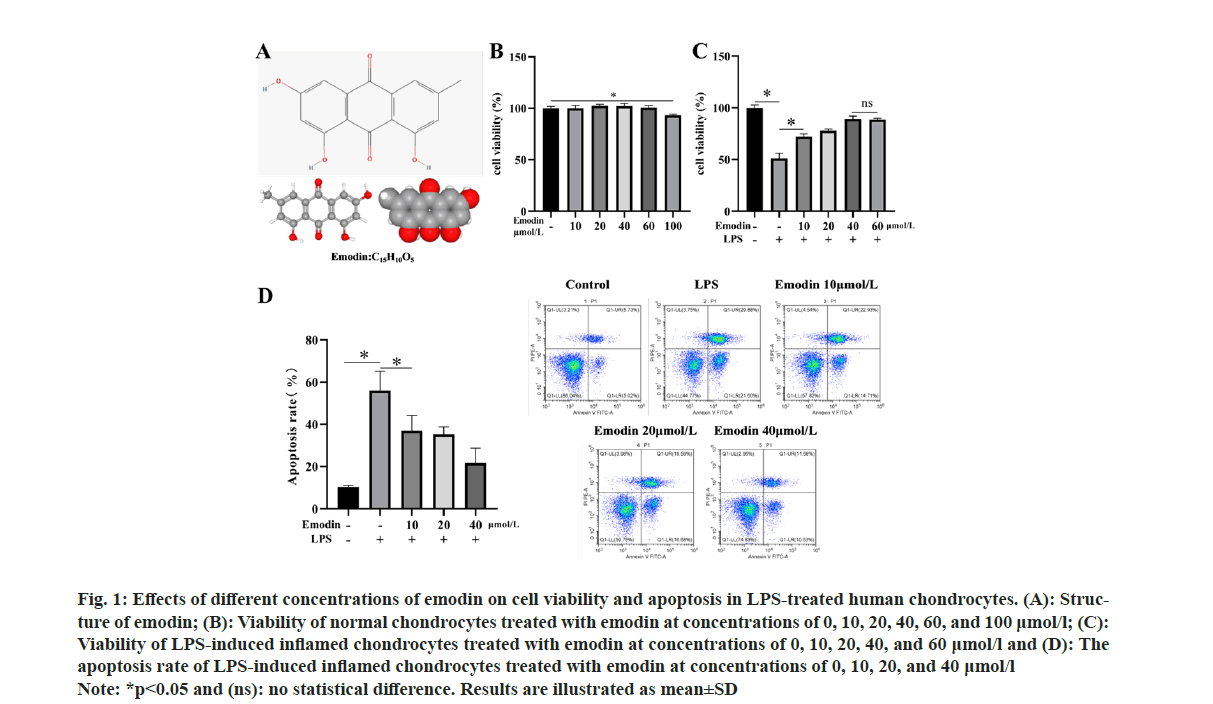
First, we purchased human chondrocytes and treated them with 0, 10, 20, 40, 60, and 100 μmol/l of emodin. The results are shown in fig. 1A and fig. 1B. We observed that emodin treatment at concentrations below 60 μmol/l did not significantly affect the viability of normal chondrocytes. Next, we treated human chondrocytes with 10 μg/ml of LPS for 12 h to induce an inflammatory cell model. We then applied emodin at concentrations of 0, 10, 20, 40, and 60 μmol/l to the inflamed cells to assess the impact of emodin treatment on LPSinduced inflammatory chondrocytes. Our research findings demonstrated a significant decrease in chondrocyte viability after LPS treatment. However, after administering emodin treatment, cell viability significantly increased and exhibited a positive correlation with the concentration (fig. 1C). Notably, the effects of emodin treatment did not exhibit further enhancement when the concentration reached 60 μmol/l. Similarly, the results of flow cytometry analysis revealed a significant increase in apoptosis in LPS-treated chondrocytes. Nevertheless, emodin treatment effectively suppressed chondrocyte apoptosis (fig. 1D).
Fig 1: Effects of different concentrations of emodin on cell viability and apoptosis in LPS-treated human chondrocytes. (A): Structure
of emodin; (B): Viability of normal chondrocytes treated with emodin at concentrations of 0, 10, 20, 40, 60, and 100 μmol/l; (C):
Viability of LPS-induced inflamed chondrocytes treated with emodin at concentrations of 0, 10, 20, 40, and 60 μmol/l and (D): The
apoptosis rate of LPS-induced inflamed chondrocytes treated with emodin at concentrations of 0, 10, 20, and 40 μmol/l
Note: *p<0.05 and (ns): no statistical difference. Results are illustrated as mean±SD
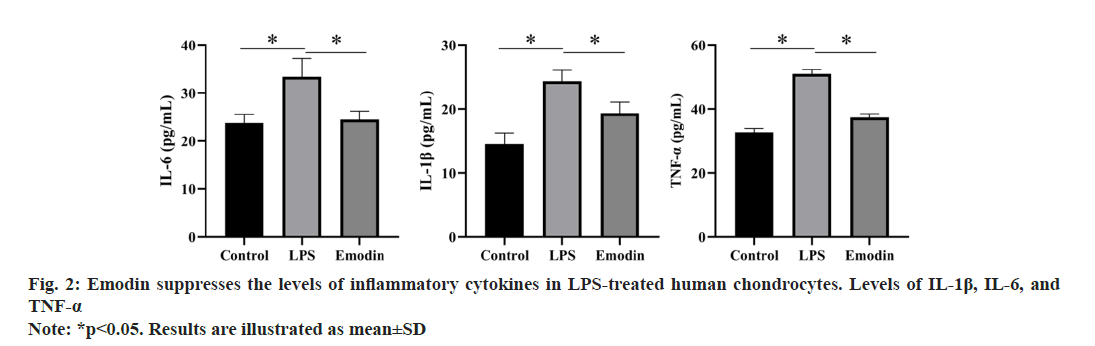
Based on the aforementioned results, we selected a concentration of 40 μmol/l emodin as the treatment for the emodin group to investigate its impact on inflammation in LPS-treated chondrocytes. As illustrated in fig. 2, after LPS treatment, the levels of inflammatory cytokines in chondrocytes showed a significant upward trend. However, upon administration of emodin, this trend was significantly reversed.
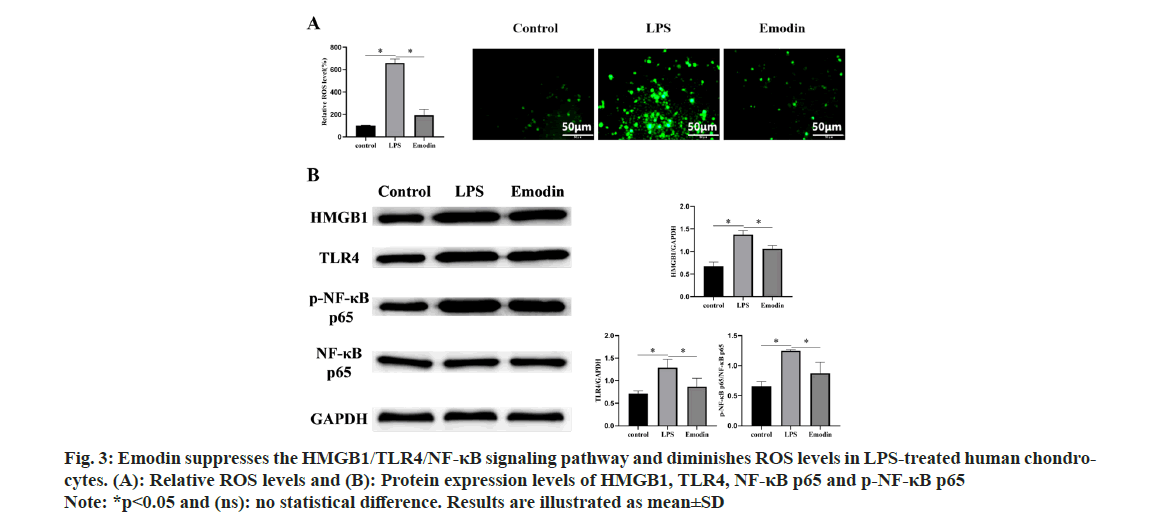
To deeply explore the anti-inflammatory mechanisms of emodin, we conducted subsequent experiments. As shown in fig. 3A and fig. 3B, we observed a significant increase in ROS levels in chondrocytes induced by LPS, while treatment with emodin resulted in a decrease in ROS levels. Similarly, we observed activation of the HMGB1/ TLR4/NF-κB signaling pathway in the LPS group, as evidenced by a noticeable increase in the expression of related proteins. However, in contrast to the LPS group, the expression of HMGB1 and TLR4 proteins markedly decreased in the emodin group, as well as the ratio of p-NF-κB p65/NF-κB p65.
Fig 3: Emodin suppresses the HMGB1/TLR4/NF-κB signaling pathway and diminishes ROS levels in LPS-treated human chondrocytes.
(A): Relative ROS levels and (B): Protein expression levels of HMGB1, TLR4, NF-κB p65 and p-NF-κB p65
Note: *p<0.05 and (ns): no statistical difference. Results are illustrated as mean±SD
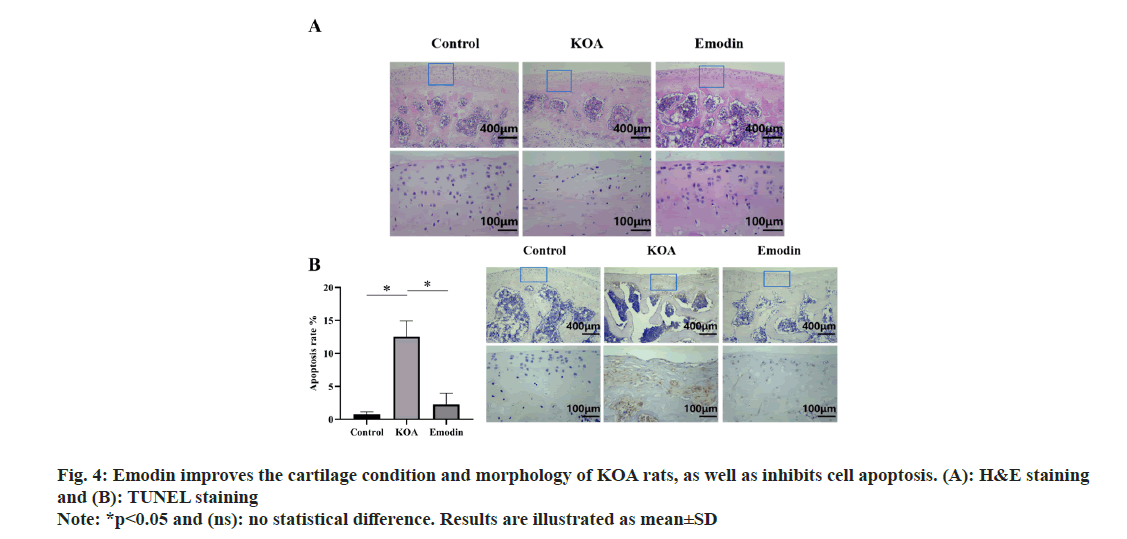
To further investigate the therapeutic effect of emodin on KOA, we established a rat model of KOA to evaluate the in vivo efficacy of emodin treatment. As depicted in fig. 4A, the articular cartilage structure in the control group rats appeared normal, with clear and organized layers of cartilage cells. In contrast, the articular cartilage structure in the KOA group rats was disrupted, characterized by thinning and roughening of the cartilage layer, disorganized arrangement of cartilage cells, and significant infiltration of inflammatory cells. Comparatively, the articular cartilage structure in the emodin group rats appeared relatively intact and clear, with relatively organized arrangement of cartilage cells and a noticeable reduction in inflammatory cell infiltration compared to the KOA group. Additionally, we observed a significant increase in cell apoptosis in the articular cartilage of the KOA group compared to the control group, which was effectively inhibited by emodin treatment, as observed through TUNEL staining (fig. 4B).
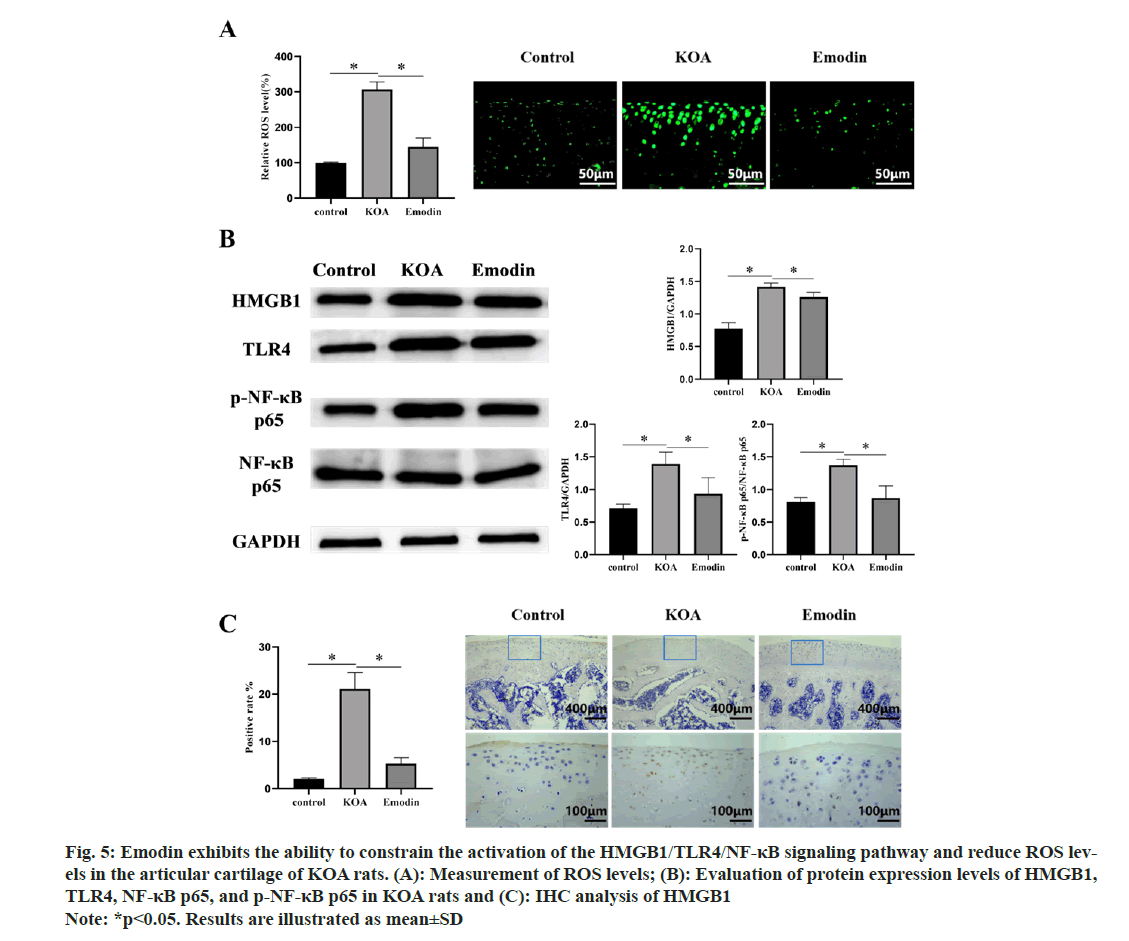
Through in vitro cellular experiments, we obtained preliminary insights into the potential molecular mechanisms underlying emodin’s treatment of KOA. However, these mechanisms remained unvalidated in vivo. Consequently, further validation was conducted through in vivo experiments. As illustrated in fig. 5A-fig. 5C, we observed increased ROS accumulation and activation of the HMGB1/ TLR4/NF-κB signaling pathway in the articular cartilage of KOA rats, presenting a clear contrast to the control group. Simultaneously, emodin significantly suppressed the aforementioned adverse effects induced by KOA, showing consistency with the results obtained from cellular experiments.
Fig 5: Emodin exhibits the ability to constrain the activation of the HMGB1/TLR4/NF-κB signaling pathway and reduce ROS levels
in the articular cartilage of KOA rats. (A): Measurement of ROS levels; (B): Evaluation of protein expression levels of HMGB1,
TLR4, NF-κB p65, and p-NF-κB p65 in KOA rats and (C): IHC analysis of HMGB1
Note: *p<0.05. Results are illustrated as mean±SD
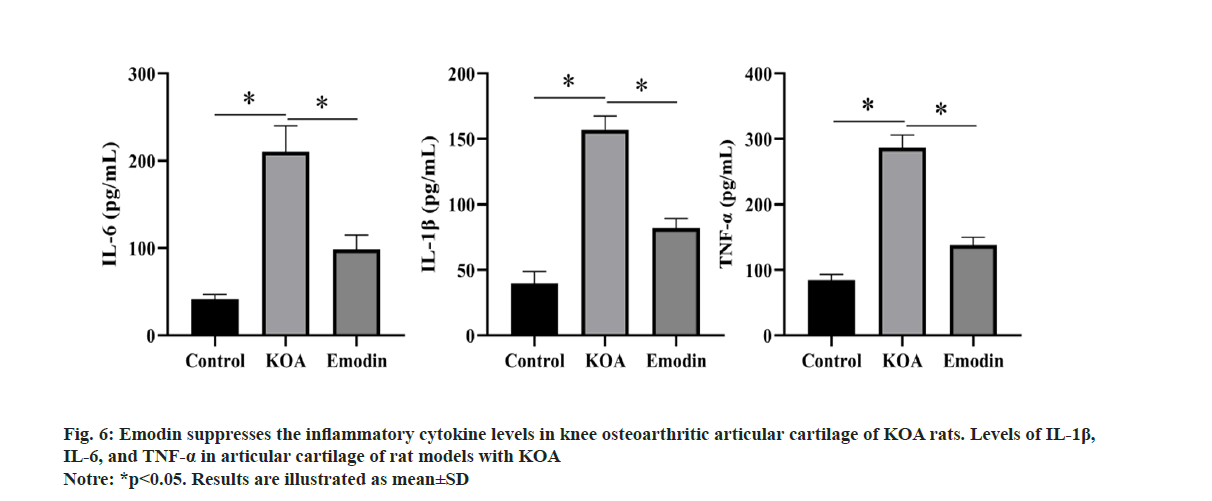
To further investigate the influence of emodin on the levels of inflammatory cytokines in rat models of KOA, in vivo experiments were conducted to assess the levels of inflammatory cytokines. As illustrated in fig. 6, the levels of inflammatory cytokines in the KOA group exhibited a significant enhancement in contrast to the control group. However, the emodin group markedly inhibited this elevation.
The pathogenesis of KOA involves complex interactions among apoptosis, inflammatory mediators, and oxidative stress. In this study, we initially investigated the effect of emodin on cell viability using the CCK-8 assay and found that emodin treatment at concentrations below 60 μmol/l exerted no cytotoxic effects on chondrocytes. Emodin treatment significantly improved the cell viability of LPS-induced inflamed chondrocytes and suppressed cellular apoptosis. Furthermore, we observed a concentration-dependent protective effect, with optimal results achieved at a concentration of 40 μmol/l of emodin, thereby minimizing the drug dosage. In KOA rats, treatment with emodin demonstrated significant improvement in cartilage morphology and structure, as well as a reduction in cellular apoptosis within the joint tissue, thus supporting the potential therapeutic efficacy of emodin in treating KOA. In addition, treatment with emodin effectively suppressed the elevation of inflammatory cytokines in LPSinduced inflamed chondrocytes. HMGB1 can be released by immune cells on their own initiative or passively released from injured cells reciprocating various inflammatory stimulations including infection, injury, or stress[13]. The active release of HMGB1 promotes the recruitment of neutrophils and the release of pro-inflammatory cytokines by macrophages[14]. Wenzhao et al.[15] discovered that HMGB1 plays a vital role in the early-stage inflammation of LPS-induced chondrocytes, where cytoplasmic HMGB1 attenuates the inflammatory response via promoting cellular autophagy and restricting cell apoptosis. Conversely, in the later stage, extracellular HMGB1 spurs the inflammatory response, thereby promoting cartilage damage in OA. The activation of TLRs is a host defense mechanism against infection and tissue injury. It initiates a signaling cascade that continues to the release of numerous inflammatory cytokines as well as the activation of immune cells[16,17]. After indirect binding between LPS and TLR4, a dimer is formed, triggering intracellular signal transduction and subsequently activating downstream signaling pathways, such as the MyD88-dependent pathway. This activation accounts for the activation of the NF-κB transcription factor and promotes the generation of inflammatory cytokines. Excessive activation of TLR4 disrupts immune homeostasis, thereby resulting in the occurrence and progression of OA disease[18]. After specific binding of HMGB1 to TLR4/MD-2, nuclear translocation of NF-κB is induced, contributing to the release of pro-inflammatory cytokines[19]. This study demonstrates that treatment with emodin downregulates the expression of HMGB1 and TLR4, thereby attenuating the activation of the HMGB1/TLR4 signaling pathway. Additionally, it has been shown that some drugs can alleviate inflammation and cellular senescence in mouse OA by inhibiting the accumulation of ROS[20]. Emodin also alleviates the increased levels of ROS induced by LPS, highlighting its antioxidant properties in KOA. Moreover, these findings in KOA rats are consistent with the in vitro results.
In conclusion, this study emphasizes the therapeutic potential of emodin as a promising intervention targeting the HMGB1/TLR4 signaling pathway in the treatment of KOA. By modulating the inflammatory response and cellular apoptosis, emodin presents a multifaceted approach to alleviate joint inflammation and maintain cartilage integrity. However, it is critical to recognize some limitations in this research. Firstly, the exact mechanisms by which emodin acts on the HMGB1/TLR4 signaling pathway need to be further elucidated, such as through the addition of relevant pathway inhibitors for more in-depth investigation. Furthermore, the long-term efficacy and safety of emodin treatment warrant comprehensive evaluation through clinical trials.
Conflict of interests:
The authors declared no conflict of interests.
References
- Wang L, Xu H, Li X, Chen H, Zhang H, Zhu X, et al. Cucurbitacin E reduces IL-1β induced inflammation and cartilage degeneration by inhibiting the PI3K/Akt pathway in osteoarthritic chondrocytes. J Transl Med 2023;21(1):880.
[Crossref] [Google Scholar] [PubMed]
- Chen P, Ruan A, Zhou J, Huang L, Zhang X, Ma Y, et al. Cinnamic aldehyde inhibits lipopolysaccharide-induced chondrocyte inflammation and reduces cartilage degeneration by blocking the nuclear factor-kappa B signaling pathway. Front Pharmacol 2020;11:949.
[Crossref] [Google Scholar] [PubMed]
- Chahal J, Gómez-Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, et al. Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med 2019;8(8):746-57.
[Crossref] [Google Scholar] [PubMed]
- Chao J, Wu C, Sun B, Hose MK, Quan A, Hughes TH, et al. Inflammatory characteristics on ultrasound predict poorer longterm response to intraarticular corticosteroid injections in knee osteoarthritis. J Rheumatol 2010;37(3):650-5.
[Crossref] [Google Scholar] [PubMed]
- Dainese P, Wyngaert KV, de Mits S, Wittoek R, van Ginckel A, Calders P. Association between knee inflammation and knee pain in patients with knee osteoarthritis: A systematic review. Osteoarthr Cartil 2024;30(4):516-34.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Zeng Y. Curcumin reduces inflammation in knee osteoarthritis rats through blocking TLR4/MyD88/NF‐κB signal pathway. Drug Dev Res 2018;80(3):353-9.
[Crossref] [Google Scholar] [PubMed]
- Xu W, Xiao Y, Zhao M, Zhu J, Wang Y, Wang W, et al. Effective treatment of knee osteoarthritis using a Nano‐enabled drug acupuncture technology in mice. Adv Sci 2023;10(28):e2302586.
[Crossref] [Google Scholar] [PubMed]
- Li S, Li Y, Hou L, Tang L, Gao F. Forsythoside B alleviates osteoarthritis through the HMGB1/TLR4/NF-κB and Keap1/Nrf2/HO-1 pathways. J Biochem Mol Toxicol 2024;38(1):e23569.
[Crossref] [Google Scholar] [PubMed]
- Guo J, Peng C, Hu Z, Guo L, Dai R, Li Y. Effect of Wu Qin Xi exercises on pain and function in people with knee osteoarthritis: A systematic review and meta-analysis. Front Med 2022;9:979207.
[Crossref] [Google Scholar] [PubMed]
- Zhu X, Guo S, Zhang M, Bai X. Emodin protects against apoptosis and inflammation by regulating reactive oxygen species-mediated NF-κ B signaling in interleukin-1β stimulated human nucleus pulposus cells. Hum Exp Toxicol 2023;42:9603271221138552.
[Crossref] [Google Scholar] [PubMed]
- Otoo A, Czika A, Lamptey J, Yang JP, Feng Q, Wang MJ, et al. Emodin improves glucose metabolism and ovarian function in PCOS mice via the HMGB1/TLR4/NF-κB molecular pathway. Reproduction 2023;166(5):323-36.
[Crossref] [Google Scholar] [PubMed]
- Ma Z, Wei Y, Zhang L, Shi X, Xing R, Liao T, et al. GCTOF-MS combined LC-QTRAP-MS/MS reveals metabolic difference between osteoarthritis and osteoporotic osteoarthritis and the intervention effect of erxian decoction. Front Endocrinol 2022;13:905507.
[Crossref] [Google Scholar] [PubMed]
- Yuan S, Liu Z, Xu Z, Liu J, Zhang J. High Mobility Group Box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J Hematol Oncol 2020;13(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, et al. The grateful dead: Damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev 2007;220(1):60-81.
[Crossref] [Google Scholar] [PubMed]
- Wenzhao L, Jiangdong N, Deye S, Muliang D, Junjie W, Xianzhe H, et al. Dual regulatory roles of HMGB1 in inflammatory reaction of chondrocyte cells and mice. Cell Cycle 2019;18(18):2268-80.
- Ozato K, Tsujimura H, Tamura T. Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. Biotechniques 2002;33:S66-75.
[Crossref] [Google Scholar] [PubMed]
- Kim HJ, Kim H, Lee JH, Hwangbo C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun Ageing 2023;20(1):67.
[Crossref] [Google Scholar] [PubMed]
- Gómez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nat Rev Rheumatol 2015;11(3):159-70.
[Crossref] [Google Scholar] [PubMed]
- He M, Bianchi ME, Coleman TR, Tracey KJ, Al-Abed Y. Exploring the biological functional mechanism of the HMGB1/TLR4/MD-2 complex by surface plasmon resonance. Mol Med 2018;24(1):21.
[Crossref] [Google Scholar] [PubMed]
- Lin C, Ge L, Tang L, He Y, Moqbel SA, Xu K, et al. Nitidine chloride alleviates inflammation and cellular senescence in murine osteoarthritis through scavenging ROS. Front Pharmacol 2022;13:919940.
[Crossref] [Google Scholar] [PubMed]