- *Corresponding Author:
- Najiah M Alyamani
Department of Biological Sciences, College of Science, University of Jeddah, Jeddah 21493, Saudi Arabia
E-mail: nmalyamani@uj.edu.sa
| Date of Received | 03 October 2025 |
| Date of Revision | 06 October 2025 |
| Date of Accepted | 21 October 2025 |
| Indian J Pharm Sci 2025;87(5):220-232 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The green synthesis of nanoparticles is gaining popularity due to its environmentally friendly, non-toxic and cost-effective properties. The distribution used of microproducts into the world are raised the concern of their implications on public health, in particular, Perfluorooctanoic acid which are variety used in a range of industries and consumer products as surfactants. The current study focused on the effects of biologically synthesized silver nanoparticle extract from Aloe vera (Aloe vera- silver nanoparticles) on genotoxicity induced by Perfluorooctanoic acid. After the treatment period, the rats were euthanized, blood and liver samples were collected for biochemical, histological and genetic analysis. The results indicate a notable increase in oxidative stress markers (glutathione, superoxide dismutase, catalase and lipid peroxidation), associated with a pathological change in the liver cells and deoxyribonucleic acid damage following treatment with Perfluorooctanoic acid. In contrast, treatment with silver nanoparticle extract of Aloe vera gel significantly mitigated oxidative stress, enhanced liver function and decreased deoxyribonucleic acid strand br eaks.
Keywords
Perfluorooctanoic acid, nanoparticles, the green synthesis, eco-friendly, toxicity, antioxidant
Nanotechnology has profoundly impacted numerous facets of human life, sparking significant enthusiasm in life sciences, especially in biomedical devices and biotechnology[1]. The unique attributes of nanomaterials, attributed to their tiny size and extensive surface area compared to their volume, set them apart from more significant substances[2]. These materials exhibit distinct physical, chemical and biological properties that enhance their utility across various applications[3].
This technology focuses on producing tiny nanoparticles, which are essential for the treatment of diseases in both plants and animals, demonstrating remarkable potential for enhancing human health and overall quality of life[4]. Green or biogenic synthesis refers to environmentally friendly methods of producing materials, particularly Silver Nanoparticles (AgNPs), through biological such as plants, fungi, algae and bacteria[5]. These AgNPs exhibit various beneficial properties, including antimicrobial, antiangiogenic, antitumor, anti-inflammatory and antioxidant activities[6]. Consequently, silver Nanotechnology has profoundly impacted numerous facets of human life, sparking significant enthusiasm in life sciences, especially in biomedical devices and biotechnology[1]. The unique attributes of nanomaterials, attributed to their tiny size and extensive surface area compared to their volume, set them apart from more significant substances[2]. These materials exhibit distinct physical, chemical and biological properties that enhance their utility across various applications[3].
This technology focuses on producing tiny nanoparticles, which are essential for the treatment of diseases in both plants and animals, demonstrating remarkable potential for enhancing human health and overall quality of life[4]. Green or biogenic synthesis refers to environmentally friendly methods of producing materials, particularly Silver Nanoparticles (AgNPs), through biological such as plants, fungi, algae and bacteria[5]. These AgNPs exhibit various beneficial properties, including antimicrobial, antiangiogenic, antitumor, anti-inflammatory and antioxidant activities[6]. Consequently, silver Herbal remedies one noteworthy example is Aloe vera (A. vera) which has long been lauded for its health benefits, mainly attributable to its anti-inflammatory and antioxidant characteristics[11]. Studies have demonstrated that A. vera can aid in mitigating liver damage by decreasing oxidative stress and inflammation, which are critical contributors to liver disorders[12]. This plant flourishes in hot, dry climates and can adapt to various environments, such as deserts, grasslands and even coastal or alpine regions[13]. In Saudi Arabia, it is prevalent, particularly in the desert landscape of the Asir area, where it is locally referred to as ?Al-Maguar? or ?Sabar?[14]. Of the over 400 recognized species of Aloe, Aloe barbadensis Miller (also categorized as A. vera Linne and commonly known as A. vera) is the most renowned and extensively utilized. A. vera comprises at least 75 bioactive constituents contributing to various biological effects, including anti-inflammatory, antioxidant and antitumor properties[15]. Research has indicated that A. vera extracts can aid in restoring liver function and improving liver health following chemical-induced damage[16]. Thus, the current research focused on the physiological and histological impact on the liver of male rats by estimating the changes in liver enzymes and oxidative stress parameters induced by PFOA exposure and evaluating the potential effects of green silver nanoparticle extract of A. vera gel. In addition, evaluate the genotoxic effects on the Deoxyribonucleic Acid (DNA) of liver cells by analysing the alterations triggered by PFOA and the possible influence of green silver nanoparticles extracted from A. vera gel.
Materials and Methods
Chemicals:
PFOA: PFOA (95 % purity, 171468-5G) was obtained from Sigma-Aldrich St. Louis, MO, USA).
Extraction and preparation:
Green AgNPs from A. vera gel extract, A. vera (Aloe barbadense Miller) was purchased from the local market in Jeddah city, Saudi Arabia. Green silver nanoparticles from A. vera preparation and fresh A. vera gel were prepared according to Khan et al.[17].
Characterization of AgNPs:
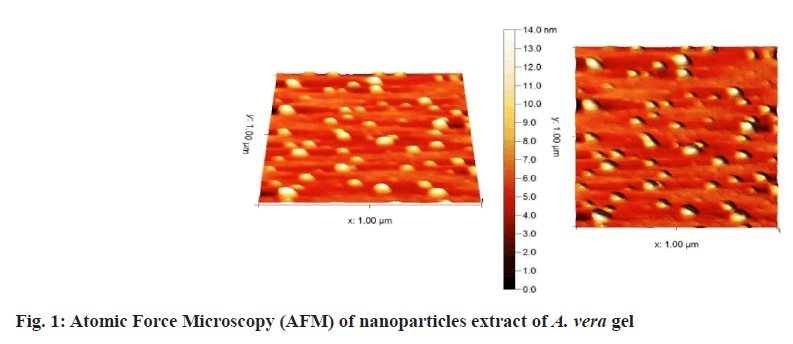
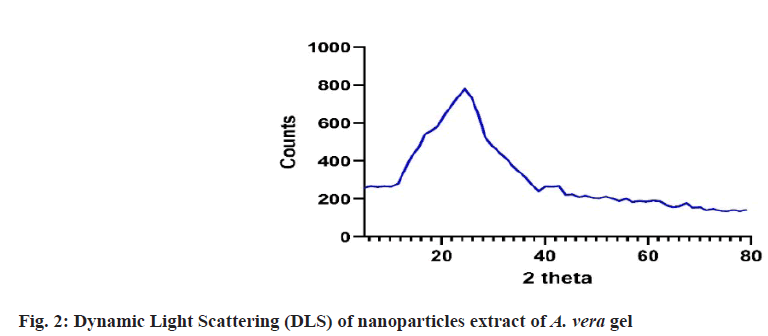
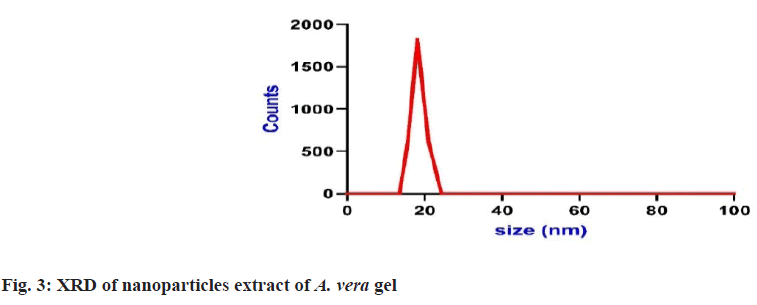
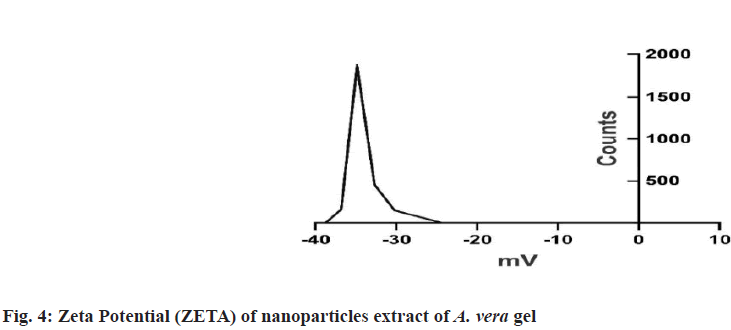
Prepared herbal nanoparticles were confirmed by Atomic Force Microscopy (AFM)[18], Dynamic Light Scattering (DLS)[19], X-Ray Diffraction (XRD)[20] and zeta potential (fig. 1?fig. 4)[21].
Experimental animals:
The animals were 24 adult healthy albino male rats of Wister strain with weights between 140 and 180 g. Animals were obtained from the pharmacy college student?s section at King Abdul-Aziz University (KAU), Jeddah, Saudi Arabia. The experiment period was 6 weeks and completed in the pharmacy college student?s section at KAU.
Experimental design:
Male albino rats (140±180 g) were used in this study and acclimated under standard conditions. Rats were randomly classified into six groups (n=6):
G1 (Negative control group): Served as control and received oral administration of distilled water daily for 6 weeks.
G2 (Positive control group): Rats were treated orally with 25 mg/kg/day PFOA for 6 weeks[22].
G3: Rats were treated orally with 150 mg/kg/day nanoparticles extract of A. vera gel for 6 weeks[23].
G4: Rats were treated with PFOA + nanoparticles extract of A. vera gel daily for 6 weeks.
Biochemical assay:
Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), and bilirubin, as well as liver oxidative stress biomarkers: Glutathione (GSH), Superoxide Dismutase (SOD), Catalase (CAT), and Lipid Peroxidation (LPO), were analyzed using rat Enzyme-Linked Immunosorbent Assay (ELISA) kits obtained from BioSource USA following the procedures in the research[24].
Histological assessment:
The liver samples were fixed in 10 % formalin solution for 48 h, then embedded in paraffin wax. Sections were stained with Ehrlich?s Hematoxylin and Eosin (H&E) for histological study according to Bancroft and Stevens et al.[25].
Genetic procedures:
The comet assay kits (3-well slides) were obtained from (ab238544).
Statistical analysis:
The mean value±standard error was used to express the data by a t-test to determine if the experimental groups had significant differences. A p-value of <0.05 was considered significant. The Statistical Package for Social Sciences (SPSS) software (version 26, SPSS INC., USA) was used for all statistical analyses.
Results and Discussion
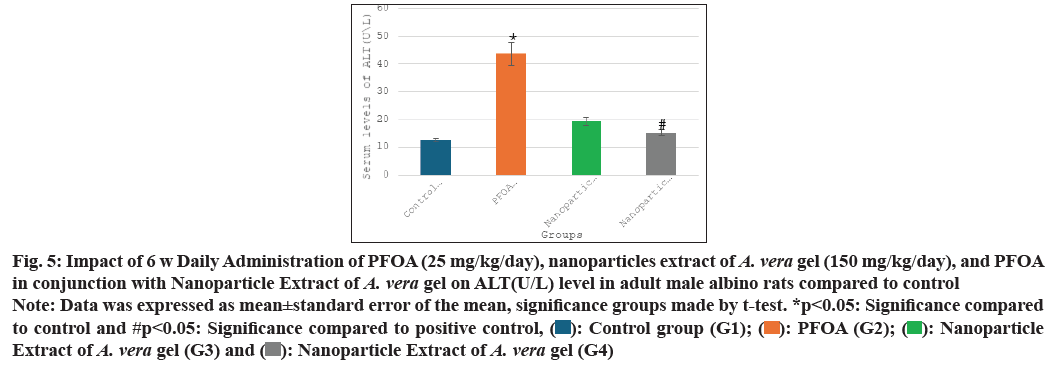
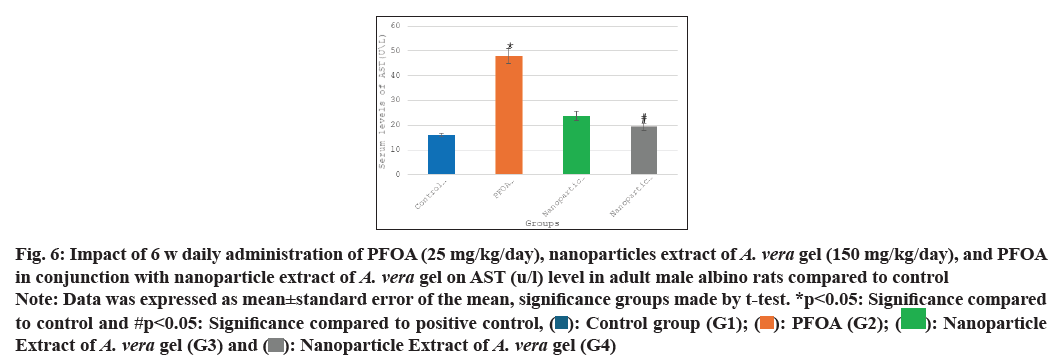
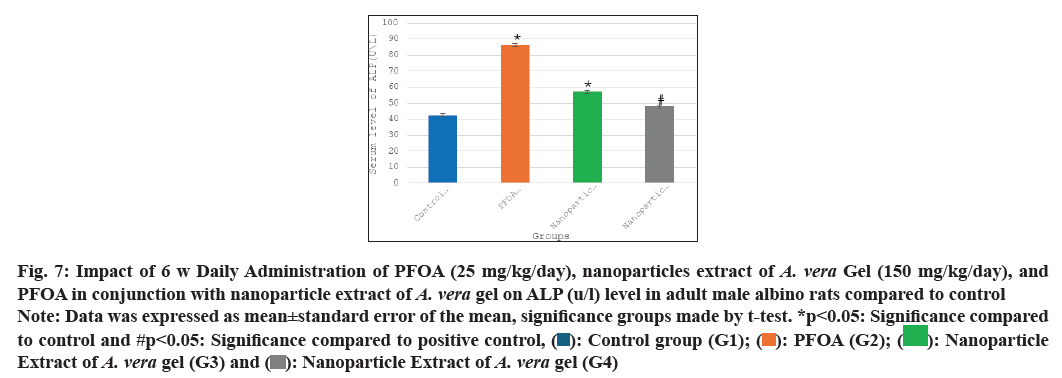
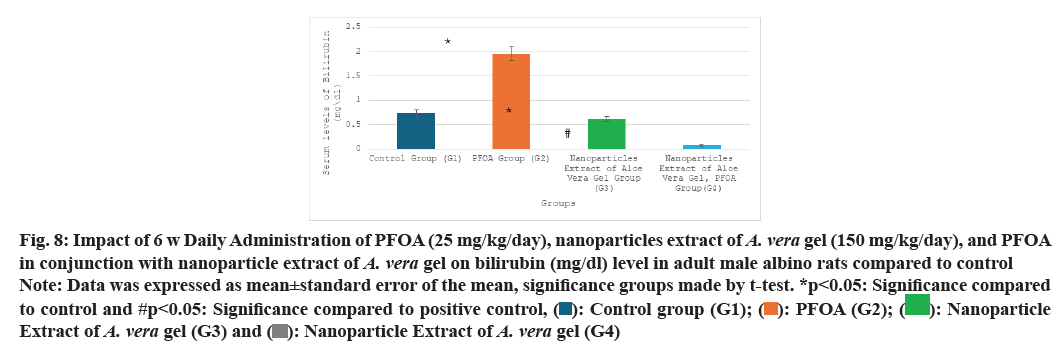
Table 1 and fig. 5-fig. 8 the current study shows the PFOA group (G2) has levels of liver function enzymes, including ALT (43.60±4.05 U/L), AST (47.91±3.02 U/L), ALP (86.25±3.67 U/L), and bilirubin (1.96±0.15 mg/dl) in the PFOA group (G2) compared to the control group (G1) at p<0.05. In contrast, the group receiving a nanoparticle extract of A. vera gel (G3) displays a significant rise in ALP levels, alongside a noteworthy decrease in bilirubin levels relative to the control group (G1) at p<0.05. Furthermore, treatment with the nanoparticle extract of A. vera gel (G4) alongside PFOA results in a significant reduction in liver enzymes: ALT decreases by (15.25±0.99 U/L), AST decreases by (19.41±1.54 U/L), ALP decreases by (47.83±1.92 U/L), and bilirubin decreases by (0.08±0.03 mg/dl) compared to the positive control group (G2) at p<0.05. Suggest that the nanoparticle extract of A. vera gel effectively ameliorates liver enzyme levels impacted by PFOA toxicity.
| Groups\Variables | ALT (U\L) | AST (U\L) | ALP (U\L) | Bilirubin (mg\dl) |
|---|---|---|---|---|
| G1 | 12.59±0.51 | 16.16±0.69 | 42.33±0.84 | 0.73±0.08 |
| G2 | 43.60±4.05* | 47.91±3.02* | 86.25±3.67* | 1.96±0.15* |
| G3 | 19.25±1.43 | 23.79±1.86 | 57.08±5.24* | 0.62±0.05* |
| G4 | 15.25±0.99# | 19.41±1.54# | 47.83±1.92# | 0.08±0.03# |
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control
Table 1: Impact of 6 w daily administration of pfoa (25 mg/kg/day), nanoparticles extract of A. Vera gel (150 mg/kg/day), and PFOA in conjunction with nanoparticle extract of A. Vera gel on alt (u/l), ast (u/l), alp (u/l) and bilirubin (mg/dl) levels in adult male albino rats compared to control
Fig. 5: Impact of 6 w Daily Administration of PFOA (25 mg/kg/day), nanoparticles extract of A. vera gel (150 mg/kg/day), and PFOA in conjunction with Nanoparticle Extract of A. vera gel on ALT(U/L) level in adult male albino rats compared to control
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control, ( ): Control group (G1); (
): Control group (G1); ( ): PFOA (G2); (
): PFOA (G2); ( ): Nanoparticle
Extract of A. vera gel (G3) and (
): Nanoparticle
Extract of A. vera gel (G3) and ( ): Nanoparticle Extract of A. vera gel (G4)
): Nanoparticle Extract of A. vera gel (G4)
Fig. 6: Impact of 6 w daily administration of PFOA (25 mg/kg/day), nanoparticles extract of A. vera gel (150 mg/kg/day), and PFOA in conjunction with nanoparticle extract of A. vera gel on AST (u/l) level in adult male albino rats compared to control
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control, ( ): Control group (G1); (
): Control group (G1); ( ): PFOA (G2); (
): PFOA (G2); ( ): Nanoparticle
Extract of A. vera gel (G3) and (
): Nanoparticle
Extract of A. vera gel (G3) and ( ): Nanoparticle Extract of A. vera gel (G4)
): Nanoparticle Extract of A. vera gel (G4)
Fig. 7: Impact of 6 w Daily Administration of PFOA (25 mg/kg/day), nanoparticles extract of A. vera Gel (150 mg/kg/day), and PFOA in conjunction with nanoparticle extract of A. vera gel on ALP (u/l) level in adult male albino rats compared to control
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control, ( ): Control group (G1); (
): Control group (G1); ( ): PFOA (G2); (
): PFOA (G2); ( ): Nanoparticle
Extract of A. vera gel (G3) and (
): Nanoparticle
Extract of A. vera gel (G3) and ( ): Nanoparticle Extract of A. vera gel (G4)
): Nanoparticle Extract of A. vera gel (G4)
Fig. 8: Impact of 6 w Daily Administration of PFOA (25 mg/kg/day), nanoparticles extract of A. vera gel (150 mg/kg/day), and PFOA in conjunction with nanoparticle extract of A. vera gel on bilirubin (mg/dl) level in adult male albino rats compared to control
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control, ( ): Control group (G1); (
): Control group (G1); ( ): PFOA (G2); (
): PFOA (G2); ( ): Nanoparticle
Extract of A. vera gel (G3) and (
): Nanoparticle
Extract of A. vera gel (G3) and ( ): Nanoparticle Extract of A. vera gel (G4)
): Nanoparticle Extract of A. vera gel (G4)
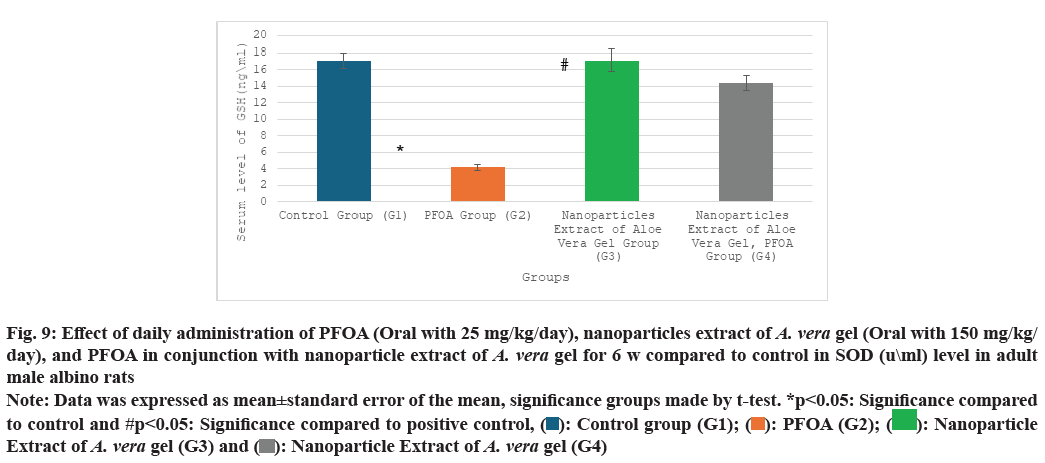
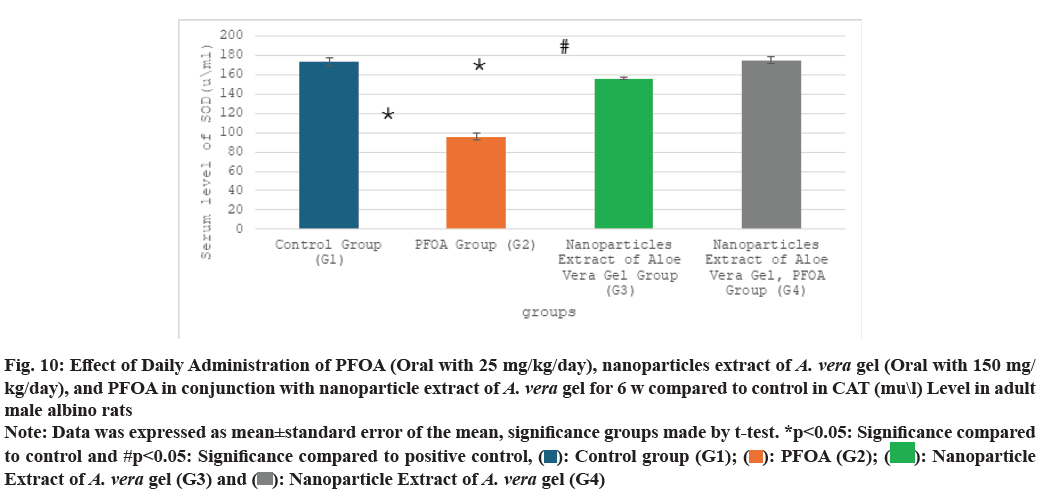
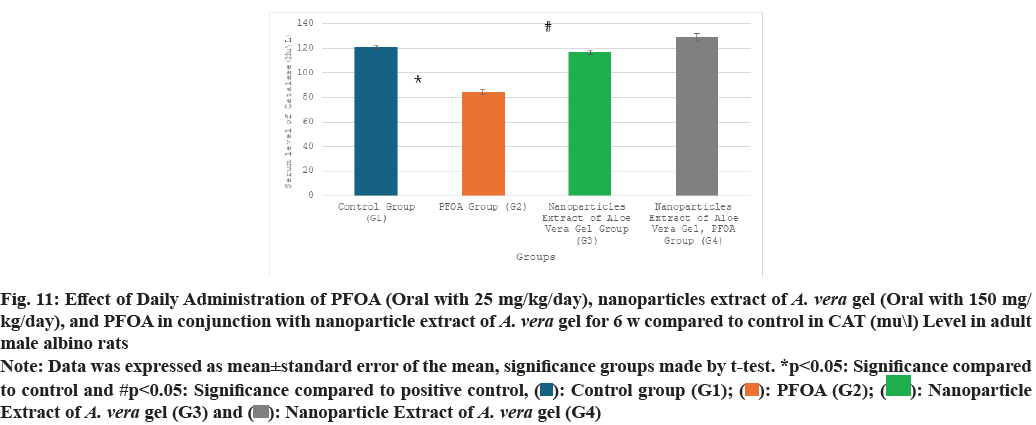
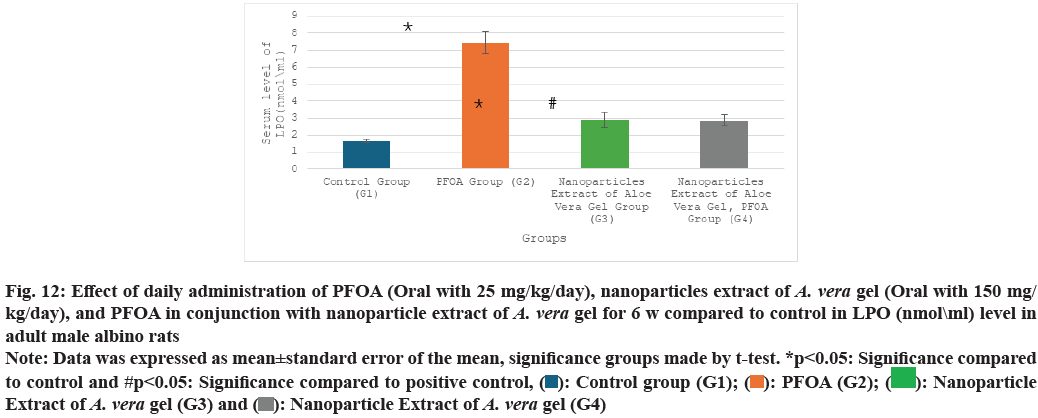
Table 2 and fig. 9-fig. 12 the liver oxidative stress analysis results across different study groups reveal the following findings: In the PFOA treatment group (G2), there is a significant decrease in levels of GSH, measured at (4.18±0.39 ng/ml), as well as SOD at (95.75±3.56 u/ml) and CAT at (84.50±2.48 U/ml). Also, there is a marked increase in LPO, recorded at (7.45±0.63 nmol/ml) compared to the control group (G1), with statistical significance noted (p<0.05). In the group treated with the nanoparticle extract of A. vera gel (G3), there is a significant decrease in oxidative stress as indicated by SOD levels at (156.17±1.34 ?/ml) and a notable improvement in LPO, measured at (2.88±0.46 nmol/ml), compared to the control group (G1) (p<0.05). Meanwhile, in the group receiving both PFOA and the nanoparticle extract of A. vera gel (G4), there is a significant decrease in LPO at (2.87±0.31 nmol/ ml), along with substantial increases in CAT levels at (129.16±2.94 U/ml), GSH at (14.36±0.92 ng/ml) and SOD at (175±3.65 u/ml) compared to the PFOA positive control group (G2) (p<0.05). It suggests that the nanoparticle extract of A. vera gel effectively ameliorates. Specifically, the extract enhances the activity of key antioxidant enzymes impacted by PFOA toxicity.
| Groups\Variables | GSH (ng\ml) | SOD (u\ml) | CAT (mu\l) | LPO (nmol\ml) |
|---|---|---|---|---|
| G1 | 17.10±0.9 | 173.50±3.90 | 121.10±1.1 | 1.65±0.1 |
| G2 | 4.18±0.39* | 95.75±3.56* | 84.50±2.48* | 7.45±0.63* |
| G3 | 17.08±1.34 | 156.17±1.34* | 116.76±1.42 | 2.88±0.46* |
| G4 | 14.36±0.92# | 175±3.65# | 129.16±2.94# | 2.87±0.31# |
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control
Table 2: Effect of daily administration of PFOA (oral with 25 mg/kg/day), nanoparticles extract of A. Vera gel (oral with 150 mg/kg/day), and PFOA in conjunction with nanoparticle extract of A. Vera gel for 6 w compared to control in gsh (ng\ml), sod (u\ml), cat (mu\l), and lpo (nmol\ml) levels in adult male albino rats
Fig. 9: Effect of daily administration of PFOA (Oral with 25 mg/kg/day), nanoparticles extract of A. vera gel (Oral with 150 mg/kg/day), and PFOA in conjunction with nanoparticle extract of A. vera gel for 6 w compared to control in SOD (u\ml) level in adult
male albino rats
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control, ( ): Control group (G1); (
): Control group (G1); ( ): PFOA (G2); (
): PFOA (G2); ( ): Nanoparticle
Extract of A. vera gel (G3) and (
): Nanoparticle
Extract of A. vera gel (G3) and ( ): Nanoparticle Extract of A. vera gel (G4)
): Nanoparticle Extract of A. vera gel (G4)
Fig. 10: Effect of Daily Administration of PFOA (Oral with 25 mg/kg/day), nanoparticles extract of A. vera gel (Oral with 150 mg/
kg/day), and PFOA in conjunction with nanoparticle extract of A. vera gel for 6 w compared to control in CAT (mu\l) Level in adult
male albino rats
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control, ( ): Control group (G1); (
): Control group (G1); ( ): PFOA (G2); (
): PFOA (G2); ( ): Nanoparticle
Extract of A. vera gel (G3) and (
): Nanoparticle
Extract of A. vera gel (G3) and ( ): Nanoparticle Extract of A. vera gel (G4)
): Nanoparticle Extract of A. vera gel (G4)
Fig. 11: Effect of Daily Administration of PFOA (Oral with 25 mg/kg/day), nanoparticles extract of A. vera gel (Oral with 150 mg/
kg/day), and PFOA in conjunction with nanoparticle extract of A. vera gel for 6 w compared to control in CAT (mu\l) Level in adult
male albino rats
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control, ( ): Control group (G1); (
): Control group (G1); ( ): PFOA (G2); (
): PFOA (G2); ( ): Nanoparticle
Extract of A. vera gel (G3) and (
): Nanoparticle
Extract of A. vera gel (G3) and ( ): Nanoparticle Extract of A. vera gel (G4)
): Nanoparticle Extract of A. vera gel (G4)
Fig. 12: Effect of daily administration of PFOA (Oral with 25 mg/kg/day), nanoparticles extract of A. vera gel (Oral with 150 mg/
kg/day), and PFOA in conjunction with nanoparticle extract of A. vera gel for 6 w compared to control in LPO (nmol\ml) level in
adult male albino rats
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control, ( ): Control group (G1); (
): Control group (G1); ( ): PFOA (G2); (
): PFOA (G2); ( ): Nanoparticle
Extract of A. vera gel (G3) and (
): Nanoparticle
Extract of A. vera gel (G3) and ( ): Nanoparticle Extract of A. vera gel (G4)
): Nanoparticle Extract of A. vera gel (G4)
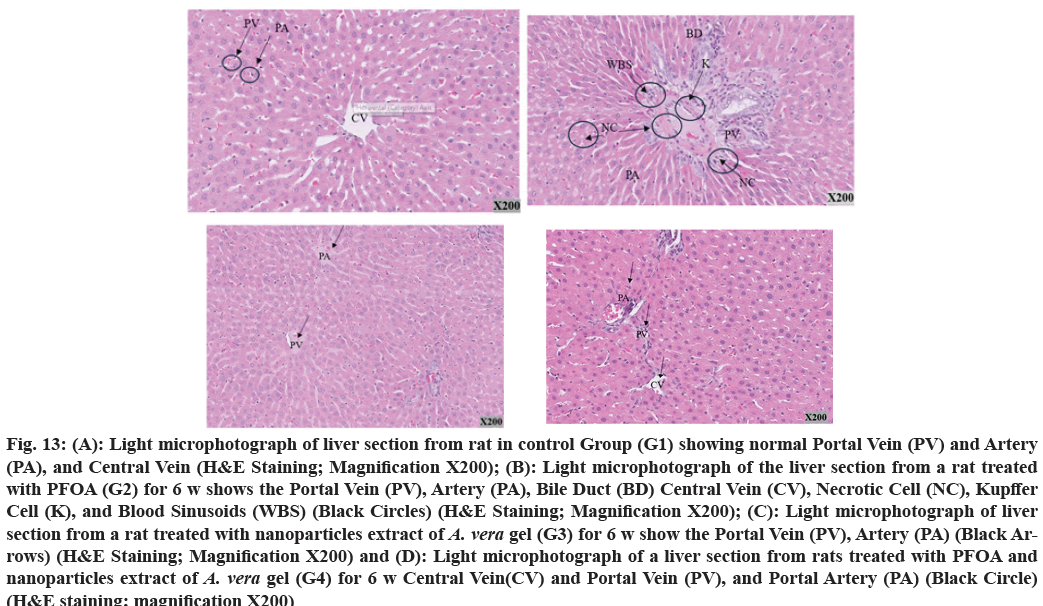
Histological analysis of liver sections from the control group (G1) present a typical structure (fig. 13A). In contrast, examination of liver tissue treated with PFOA at 25 mg/kg/day shows notable alterations in the portal tract, including the infiltration of inflammatory cells in the surrounding area. Furthermore, changes in the Bile Ducts (BD) present as inflammation, cellular necrosis, increased arterial wall thickness, and blood clots. Additionally, the Central Veins (CV) appear constricted and compact in shape (fig. 13B).
Fig. 13: (A): Light microphotograph of liver section from rat in control Group (G1) showing normal Portal Vein (PV) and Artery
(PA), and Central Vein (H&E Staining; Magnification X200); (B): Light microphotograph of the liver section from a rat treated
with PFOA (G2) for 6 w shows the Portal Vein (PV), Artery (PA), Bile Duct (BD) Central Vein (CV), Necrotic Cell (NC), Kupffer
Cell (K), and Blood Sinusoids (WBS) (Black Circles) (H&E Staining; Magnification X200); (C): Light microphotograph of liver
section from a rat treated with nanoparticles extract of A. vera gel (G3) for 6 w show the Portal Vein (PV), Artery (PA) (Black Arrows)
(H&E Staining; Magnification X200) and (D): Light microphotograph of a liver section from rats treated with PFOA and
nanoparticles extract of A. vera gel (G4) for 6 w Central Vein(CV) and Portal Vein (PV), and Portal Artery (PA) (Black Circle)
(H&E staining; magnification X200)
The liver section treated with a nanoparticle extract of A. vera gel (G3) (150 mg/kg/day) shows no changes, as illustrated in fig. 13C. Upon examination of the liver section, significant alterations are noted due to treatment with PFOA and the nanoparticle extract of the A. vera gel group (G4). Mild inflammation is detected, particularly in the portal tract, containing inflammatory cells. The CV exhibits a few inflammatory cells and mild fibrosis, as depicted in fig. 13D.
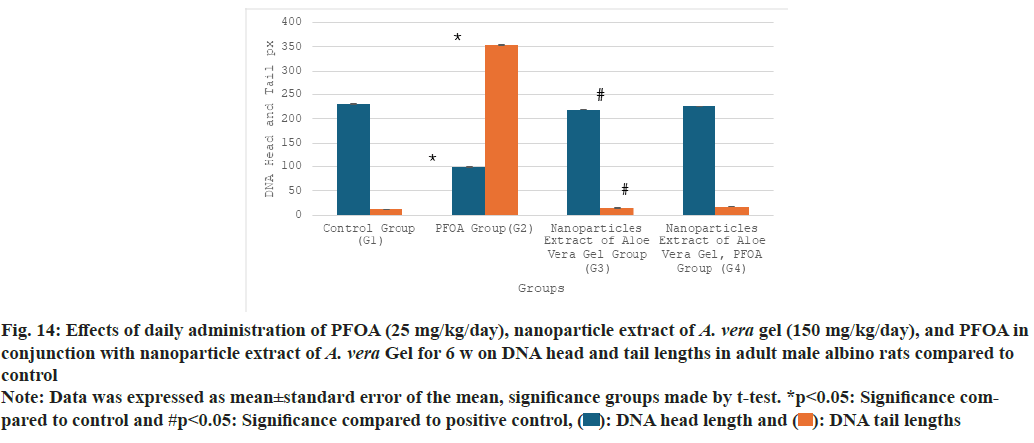
Table 3 and fig. 14 illustrate the effects of PFOA, nanoparticle extracts of A. vera gel, and PFOA combined with nanoparticle extracts from A. vera gel on DNA head and tail lengths. The results show that the PFOA group (G2) demonstrates a significant reduction in head length (99.46±0.29) and a substantial increase in tail length (354.40±1.7) compared to the control group (G1), with statistical significance at p<0.05.
| Group | DNA head length Mean±SE | DNA tail length Mean±SE |
|---|---|---|
| G1 | 230.15±0.82 | 11.76±0.14 |
| G2 | 99.46±0.29* | 354.40±1.7* |
| G3 | 218.66±0.20* | 14.80±0.32* |
| G4 | 225.53±0.3# | 17.06±0.12# |
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared to control and #p<0.05: Significance compared to positive control
Table 3: Effects of daily administration of PFOA (25 mg/kg/day), nanoparticle extract of A. Vera gel (150 mg/kg/day), and pfoa in conjunction with nanoparticle extract of A. Vera gel for 6 w on dna head and tail lengths in adult male albino rats compared to control
Fig. 14: Effects of daily administration of PFOA (25 mg/kg/day), nanoparticle extract of A. vera gel (150 mg/kg/day), and PFOA in
conjunction with nanoparticle extract of A. vera Gel for 6 w on DNA head and tail lengths in adult male albino rats compared to
control
Note: Data was expressed as mean±standard error of the mean, significance groups made by t-test. *p<0.05: Significance compared
to control and #p<0.05: Significance compared to positive control, ( ): DNA head length and (
): DNA head length and ( ): DNA tail lengths
): DNA tail lengths
The group treated with nanoparticle extracts of A. vera gel (G3) exhibits a notable decrease in head length (218.66±0.20) and a minor increase in tail length (14.80±0.32) compared to the control group, also at p<0.05.
Furthermore, the group receiving both PFOA and nanoparticle extracts from A. vera gel (G4) shows a significant increase in head length (225.53±0.3) along with a decrease in tail length (17.06±0.12) compared to the PFOA-Positive group (G2), with statistical significance at p<0.05. The nanoparticle extracts of A. vera gel suggest a considerable reduction in DNA strand breaks or damage.
PFOA is a persistent environmental pollutant widely used in various industrial applications. Evidence has shown that PFOA exposure disrupts enzymatic functions, promotes oxidative stress, and induces tissue and genetic damage, ultimately contributing to chronic health risks, particularly hepatic dysfunction[26]. After ingestion, PFOA is transported through the bloodstream to the liver, which undergoes metabolic processing and excreted via bile[27,28]. This hepatic burden frequently results in hepatocellular injury and impaired liver function.
Serum levels of liver enzymes such as ALT, AST, ALP, and bilirubin serve as essential biomarkers for liver damage. Elevated levels indicate hepatocyte membrane disruption or bile duct obstruction[29,30]. In agreement with previous studies[31,32], our findings demonstrate significant liver enzyme (ALT, AST, ALP, and Bilirubin) elevations following oral administration of PFOA (25 mg/kg/day for 6 w), confirming its hepatotoxic effects.
Study of Yang et al.[33] highlights the links between PFOA exposure and hepatic toxicity in animal models, underscoring how oxidative stress can lead to LPO and subsequent hepatocyte injury this body of work emphasizes the need for continued monitoring and research into the long-term health effects of PFOA exposure and the mechanisms by which it exerts its toxic effects on the liver Oxidative stress mechanisms demonstrate that elevated levels of stress-related Endoplasmic Reticulum (ER) proteins in response to PFOA exposure lead to ER stress. Additionally, PFOA impairs mitochondrial function within liver tissue, further exacerbating liver damage. This is particularly concerning due to the crucial relationship between the ER and mitochondria in regulating cellular metabolism, signalling pathways, and maintaining overall homeostasis[34].
Oxidative stress is central to PFOA-induced liver damage. PFOA increases Reactive Oxygen Species (ROS) production in hepatocytes, leading to LPO and DNA damage[35]. PFOA exposure impairs Mitochondria, primary ROS sources, which exacerbate hepatotoxic effects[36,37]. Antioxidants like GSH, SOD, and CAT protect cells by neutralizing ROS[38]. However, PFOA depletes these antioxidants, impairing mitochondrial function and increasing oxidative stress markers such as LPO [39,40]. Our results corroborate these findings, showing significant reductions in GSH, SOD, and CAT levels alongside increased LPO after PFOA treatment (p<0.05).
Moreover, high doses of PFOA increase LPO and decrease vital antioxidant enzymes like SOD and GSH Peroxidase (GSH-PX), which are crucial for free radical scavenging[41]. In our study, liver samples from PFOA-treated rats showed significant reductions in SOD and GSH-PX compared to controls. Maintaining the oxidant-antioxidant balance is essential for regulating key cellular processes, including proliferation and apoptosis[42,43]. Overall, our findings align well with previous research, supporting PFOA?s established hepatotoxic and oxidative stress-related effects.
Our study shows that oral PFOA treatment (25 mg/ kg/day for 6 w) causes notable liver histopathological changes, such as portal tract inflammation, bile duct necrosis, arterial wall thickening with clots, and central vein narrowing. These alterations are in line with the findings of[33], suggesting that inflammatory responses, possibly driven by hepatocyte-released cytokines, play a significant role in liver injury and may sensitize the tissue to other toxins.
Recent studies further associate PFOA with hepatocyte apoptosis, altered bile acid metabolism, and increased adipogenesis[44,45]. PFOA also increases ROS levels, promoting oxidative stress and apoptosis[46].
Our results demonstrate that oral administration of PFOA at 25 mg/kg/day resulted in a notable decrease in head length and a significant increase in tail length of the DNA compared to the control group at p<0.05. This aligns with findings in HepG2 cells, where PFOA increased DNA strand breaks in a dose-dependent manner[47,48]. Further, PFOA-induced oxidative stress, driven by elevated ROS, contributes to DNA damage, mitochondrial dysfunction, and LPO[49,50]. Increased micronuclei frequency and reduced antioxidant capacity confirm its genotoxicity[49]. Animal studies also report similar DNA damage in liver tissue at high PFOA doses[51,52].
These results confirm that PFOA induces hepatotoxicity primarily through oxidative stress, elevated liver enzymes, decreased antioxidant defenses, and DNA damage. These molecular disruptions impair liver function and cause tissue alterations, driven by elevated LPO and decreased antioxidant capacity in rat liver. Ongoing research highlights the serious risks PFOA poses to liver health.
Research highlights the effectiveness of plant extracts in eliminating ROS, with nanoparticles exhibiting enhanced antioxidant capacity due to their larger surface area, which helps minimize DNA damage[53,54].
In parallel, increasing interest has been directed toward plant-derived antioxidants, including nanoparticle formulations, for mitigating oxidative stress. A. vera gel, rich in phytochemicals such as flavonoids, anthraquinones, and vitamins, has demonstrated potent antioxidant, anti-inflammatory, and hepatoprotective effects[55,56]. Nanoparticle extracts of A. vera gel enhance these effects due to their higher surface area and improved bioavailability[57,58].
Oral administration of nanoparticles extract of A. vera gel at 150 mg/kg/day significantly enhances liver enzyme levels in PFOA-treated groups compared to the control group (p<0.05).
This aligns with previous research reporting the protective effects of A. vera against chemically induced liver damage, including CCl4 toxicity[23]. Silver nanoparticles synthesized using A. vera have also demonstrated hepatoprotective effects, potentially through antioxidant-rich compounds such as anthraquinones and flavonoids[59,60]. These nanoparticles promote parenchymal cell healing and neutralize ROS, which may account for the observed enzyme normalization in our study.
Current research demonstrates a reduction in GSH activity, alongside increased activities of CAT and SOD, and decreased LPO in liver tissues after oral treatment with A. vera gel nanoparticles at a dosage of 150 mg/kg/day. This suggests that A. vera has a significant impact on alleviating oxidative stress in the liver.
Research suggests that nanoparticles extract of A. vera gel may enhance the activities of key antioxidant enzymes, including SOD and GPX. Notably, in cases of chemical-induced toxicity, there is a marked decrease in the activities of SOD, CAT, and GSH in liver tissues, but treatment with silver nanoparticles (AV-AgNPs) has been shown to reverse this decline[61,62].
Recent findings by Nauroze et al.[23] highlight the added advantages of employing silver nanoparticles synthesized with A. vera, as the plant not only aids in reducing silver ions to form nanoparticles but also inhibits the release of these ions through its phytochemical properties, providing improved defense against oxidative stress and toxicity. Seif et al.[63] reports that the normalization of liver enzyme levels after AV-AgNP treatment suggests the recovery and regeneration of hepatocytes within the liver tissue.
Biochemical and histological analyses show that AVAgNPs effectively protect against oxidative stress by reducing LPO and restoring liver function. Their antioxidant and free radical scavenging properties help normalize liver parameters, making AV-AgNPs promising for treating hepatotoxicity. Plant-derived silver nanoparticles also exhibit enhanced synergistic antioxidant effects[64].
Compared to the control group, our study demonstrates the effects of orally administered nanoparticle extracts of A. vera gel (at a dose of 150 mg/kg/day) on liver damage caused by PFOA exposure. The findings revealed that these nanoparticles significantly reduced liver damage, as evidenced by mild inflammation and limited tissue damage. Histopathological analysis showed that hepatic tissue exhibited minimal fibrosis and only a few inflammatory cells surrounding the portal tract.
Previous studies support the hepatoprotective effects of A. vera, especially in nanoparticle form, through antioxidant and anti-inflammatory actions that reduce LPO and oxidative damage[65,12]. A. vera nanoparticles also limit hepatocyte proliferation and fibrosis, improving liver histology under toxic conditions[66]. As evidenced by our findings, A. vera improved liver structure and reduced lipid accumulation, as Abubakar et al.[67] observed.
The current study illustrates the effect of administering nanoparticle extract of A. vera gel orally (150 mg/ kg/day). The results revealed a significant increase in head length and a reduction in tail length of DNA compared to the control group (p<0.050), indicating reduced DNA fragmentation. Studies confirm A. vera Geno protective effects through its antioxidant components, which reduce DNA damage and maintain genomic stability under oxidative stress[68,69]. Its nanoparticles neutralize ROS due to antioxidants like vitamins A, C, GSH peroxidase, and SOD, helping preserve DNA integrity. A. vera reduced AFB1-induced DNA fragmentation in mice, like the genotoxicity caused by PFOA. These findings are consistent with our results, which showed that A. vera nanoparticles significantly reduced DNA damage in PFOA-treated groups, supporting its role as a protective agent against oxidative DNA injury.
In conclusion, oral administration of nanoparticle extract of A. vera gel ignificantly mitigated PFOAinduced hepatotoxicity by reducing liver enzyme levels, restoring antioxidant defenses, improving histological architecture, and protecting DNA integrity. These findings underscore the therapeutic potential of A. vera nanoparticles as a natural, plantbased strategy for preventing oxidative liver injury. Future research should further explore the long-term safety, dose optimization, and pharmacodynamics of A. vera nanoparticles in various toxicological models. Investigating their use with other antioxidants or standard hepatoprotective drugs may offer synergistic benefits. Moreover, preclinical trials and molecular studies are warranted to validate these findings and support the clinical translation of A. vera nanoparticle-based therapies.
Ethical approval:
The experimental animals were used rigidly with the rules and principles established by the Research Ethics Committee at King Abdul-Aziz University (KAU). Ethics committee protocol approval No: p115-2023.
Conflict of interests:
The authors declared no conflict of interests.
References
- Pandit C, Roy A, Ghotekar S, Khusro A, Islam MN, Emran TB, et al. Biological agents for synthesis of nanoparticles and their applications. J King Saud Univ Sci 2022;34(3):101869.
- Eker F, Duman H, Akdaşçi E, Bolat E, Sarıtaş S, Karav S, et al. A comprehensive review of nanoparticles: From classification to application and toxicity. Molecules 2024;29(15):3482.
[Crossref] [Google Scholar] [PubMed]
- Ahmadi F, Lackner M. Green synthesis of silver nanoparticles from Cannabis sativa: Properties, synthesis, mechanistic aspects, and applications. Chem Eng 2024;8(4):64.
- Nguyen DD, Lai JY. Synthesis, bioactive properties, and biomedical applications of intrinsically therapeutic nanoparticles for disease treatment. Chem Eng J 2022;435(2):134970.
- Shukla AK, Iravani S. Green synthesis, characterization and applications of nanoparticles. Elsevier; 2018.
- Reshi MS, Uthra C, Yadav D, Sharma S, Singh A, Sharma A, et al. Silver nanoparticles protect acetaminophen induced acute hepatotoxicity: A biochemical and histopathological approach. Regulatory Toxicol Pharmacol 2017;90:36-41.
[Crossref] [Google Scholar] [PubMed]
- Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020;10(20):8996.
- Soren S, Chakroborty S, Pal K. Enhanced in tunning of photochemical and electrochemical responses of inorganic metal oxide nanoparticles via rGO frameworks (MO/rGO): A comprehensive review. Mater Sci Eng B 2022;278:115632.
- Glüge J, Scheringer M, Cousins IT, DeWitt JC, Goldenman G, Herzke D, et al. An overview of the uses of per-and Polyfluoroalkyl Substances (PFAS). Environ Sci 2020;22(12):2345-73.
- Ali AM, Higgins CP, Alarif WM, Al-Lihaibi SS, Ghandourah M, Kallenborn R. Per-and polyfluoroalkyl substances (PFASs) in contaminated coastal marine waters of the Saudi Arabian Red Sea: A baseline study. Environ Sci Pollut Res 2021;28(3):2791-803.
[Crossref] [Google Scholar] [PubMed]
- Balkrishna A, Sharma N, Srivastava D, Kukreti A, Srivastava S, Arya V. Exploring the safety, efficacy, and bioactivity of herbal medicines: Bridging traditional wisdom and modern science in healthcare. Future Integr Med 2024;3(1):35-49.
- Cui Y, Ye Q, Wang H, Li Y, Yao W, Qian H. Hepatoprotective potential of Aloe vera polysaccharides against chronic alcohol‐induced hepatotoxicity in mice. J Sci Food Agric 2014;94(9):1764-71.
[Crossref] [Google Scholar] [PubMed]
- Davis UC. The genus Aloe. Botanical Notes; 2009. p. 1-10.
- Almoshari Y. Medicinal plants used for dermatological disorders among the people of the kingdom of Saudi Arabia: A narrative review. Saudi J Biol Sci 2022;29(6):103303.
[Crossref] [Google Scholar] [PubMed]
- Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP. Pharmacological update properties of Aloe vera and its major active constituents. Molecules 2020;25(6):1324.
[Crossref] [Google Scholar] [PubMed]
- Samuhasaneetoa S, Kajornvuthidejb S. Negative effects of Aloe vera gel on paracetamol-induced liver injury in rats. Sci Asia 2014;40(1):42.
- Moosa AA, Ridha AM, Al-Kaser M. Process parameters for green synthesis of silver nanoparticles using leaves extract of Aloe vera plant. Int J Multi Curr Res. 2015;3:966-75.
- Panigrahi T. Synthesis and characterization of silver nanoparticles using leaf extract of Azadirachta indica (Doctoral dissertation). 2013.
- Anandalakshmi K, Venugobal J, Ramasamy VJ. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci 2016;6(3):399-408.
- Ahmadi O, Jafarizadeh-Malmiri H, Jodeiri N. Eco-friendly microwave-enhanced green synthesis of silver nanoparticles using Aloe vera leaf extract and their physico-chemical and antibacterial studies. Green Proc Synth 2018;7(3):231-40.
- Wang L, Zhao F, Kan M, Wen Z, Zhou Y, Yu L, Liu H. Effects of perfluorooctanoic acid on oxidative stress and PPARα and its related CYP4A1 gene expression in rat liver. Wei sheng yan Jiu 2017;46(5):802-6.
- Nauroze T, Ali S, Andleeb S, Ara C, Liaqat I, Mushtaq H, et al. Therapeutic potential of Aloe vera and Aloe vera–conjugated silver nanoparticles on mice exposed to Hexavalent Chromium. Biol Trace Elem Res 2024;202(12):5580-95.
[Crossref] [Google Scholar] [PubMed]
- Fawcett J, Scott J. A rapid and precise method for the determination of urea. J Clin Pathol 1960;13(2):156-9.
[Crossref] [Google Scholar] [PubMed]
- Bancroft JD, Stevens A. 3rd edition. Theory and practice of histological Technique, Churchill Livingston, Edinburgh, London; 1990.
- Zhang Y, Li Y, Gao N, Gong Y, Shi W, Wang X. Transcriptome and metabolome analyses reveal perfluorooctanoic acid-induced kidney injury by interfering with PPAR signaling pathway. Int J Mol Sci 2023;24(14):11503.
[Crossref] [Google Scholar] [PubMed]
- Kudo N, Kawashima Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci 2003;28(2):49-57.
[Crossref] [Google Scholar] [PubMed]
- Yang M, Su W, Li H, Li L, An Z, Xiao F, et al. Association of per-and polyfluoroalkyl substances with hepatic steatosis and metabolic dysfunction-associated fatty liver disease among patients with acute coronary syndrome. Ecotoxicol Environ Safety 2023;264:115473.
- Delemos AS, Ghabril M, Rockey DC, Gu J, Barnhart HX, Fontana RJ, et al. Amoxicillin–clavulanate-induced liver injury. Digestive Dis Sci 2016;61(8):2406-16.
- Han NK, Jung MG, Jeong YJ, Son Y, Han SC, Park S, et al. Plasma fibrinogen-like 1 as a potential biomarker for radiation-induced liver injury. Cells 2019;8(9):1042.
[Crossref] [Google Scholar] [PubMed]
- Gleason JA, Post GB, Fagliano JA. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environ Res 2015;136:8-14.
[Crossref] [Google Scholar] [PubMed]
- Costello E, Rock S, Stratakis N, Eckel SP, Walker DI, Valvi D, et al. Exposure to per-and polyfluoroalkyl substances and markers of liver injury: A systematic review and meta-analysis. Environ Health Perspect 2022;130(4):046001.
[Crossref] [Google Scholar] [PubMed]
- Yang B, Zou W, Hu Z, Liu F, Zhou L, Yang S, et al. Involvement of oxidative stress and inflammation in liver injury caused by perfluorooctanoic acid exposure in mice. Bio Med Res Int 2014;2014(1):409837.
[Crossref] [Google Scholar] [PubMed]
- Xin Y, Wan B, Yang Y, Cui XJ, Xie YC, Guo LH. Perfluoroalkyl acid exposure induces protective mitochondrial and endoplasmic reticulum autophagy in lung cells. Arch Toxicol 2018;92(10):3131-47.
[Crossref] [Google Scholar] [PubMed]
- Liu C, Yu K, Shi X, Wang J, Lam PK, Wu RS, et al. Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquatic Toxicol 2007;82(2):135-43.
[Crossref] [Google Scholar] [PubMed]
- Kelly KA, Havrilla CM, Brady TC, Abramo KH, Levin ED. Oxidative stress in toxicology: established mammalian and emerging piscine model systems. Environ Health Perspect 1998;106(7):375-84.
[Crossref] [Google Scholar] [PubMed]
- Kelly KA, Havrilla CM, Brady TC, Abramo KH, Levin ED. Oxidative stress in toxicology: Established mammalian and emerging piscine model systems. Environ Health Perspect 1998;106(7):375-84.
[Crossref] [Google Scholar] [PubMed]
- Panaretakis T, Shabalina IG, Grandér D, Shoshan MC, DePierre JW. Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma HepG2 cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol Appl Pharmacol 2001;173(1):56-64.
[Crossref] [Google Scholar] [PubMed]
- Fridovich I. Superoxide anion radical (O.2-), superoxide dismutases, and related matters. J Biol Chem 1997;272(30):18515-7.
[Crossref] [Google Scholar] [PubMed]
- Bresciani G, da Cruz IB, González-Gallego J. Manganese superoxide dismutase and oxidative stress modulation. Adv Clin Chem 2015;68:87-130.
[Crossref] [Google Scholar] [PubMed]
- Safhi MM, Khuwaja G, Alam MF, Hussain S, Siddiqui MH, Islam F, et al. Cadmium-induced nephrotoxicity via oxidative stress in male Wistar rats and capsaicin protects its toxicity. Bull Environ Pharmacol Life Sci 2016;5:5-11.
- Tsikas D. Assessment of lipid peroxidation by measuring Malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem 2017;524:13-30.
[Crossref] [Google Scholar] [PubMed]
- Ha TV, Kim S, Choi Y, Kwak HS, Lee SJ, Wen J, et al. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem 2015;178:115-21.
[Crossref] [Google Scholar] [PubMed]
- Eryılmaz O, Ateş PS, Ünal İ, Üstündağ ÜV, Bay S, Alturfan AA, et al. Evaluation of the interaction between proliferation, oxidant–antioxidant status, Wnt pathway, and apoptosis in zebrafish embryos exposed to silver nanoparticles used in textile industry. J Biochem Mol Toxicol 2018;32(1):e22015.
[Crossref] [Google Scholar] [PubMed]
- Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. Per‐and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ Toxicol Chem 2021;40(3):606-30.
[Crossref] [Google Scholar] [PubMed]
- Wang P, Liu D, Yan S, Cui J, Liang Y, Ren S. Adverse effects of perfluorooctane sulfonate on the liver and relevant mechanisms. Toxics 2022;10(5):265.
[Crossref] [Google Scholar] [PubMed]
- Liu S, Yang R, Yin N, Faiola F. The short-chain perfluorinated compounds PFBS, PFHxS, PFBA and PFHxA, disrupt human mesenchymal stem cell self-renewal and adipogenic differentiation. J Environ Sci 2020;88:187-99.
[Crossref] [Google Scholar] [PubMed]
- Yao X, Zhong L. Genotoxic risk and oxidative DNA damage in HepG2 cells exposed to perfluorooctanoic acid. Mutat Res 2005;587(1-2):38-44.
[Crossref] [Google Scholar] [PubMed]
- Eriksen KT, Raaschou-Nielsen O, Sørensen M, Roursgaard M, Loft S, Møller P. Genotoxic potential of the perfluorinated chemicals PFOA, PFOS, PFBS, PFNA and PFHxA in human HepG2 cells. Mutat Res 2010;700(1-2):39-43.
[Crossref] [Google Scholar] [PubMed]
- Wielsøe M, Long M, Ghisari M, Bonefeld-Jørgensen EC. Perfluoroalkylated Substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015;129:239-45.
[Crossref] [Google Scholar] [PubMed]
- Crebelli R, Caiola S, Conti L, Cordelli E, De Luca G, Dellatte E, et al. Can sustained exposure to PFAS trigger a genotoxic response? A comprehensive genotoxicity assessment in mice after subacute oral administration of PFOA and PFBA. Regul Toxicol Pharmacol 2019;106:169-77.
[Crossref] [Google Scholar] [PubMed]
- Kawamoto K, Oashi T, Oami K, Liu W, Jin Y, Saito N, et al. Perfluorooctanoic Acid (PFOA) but not Perfluorooctane Sulfonate (PFOS) showed DNA damage in comet assay on Paramecium caudatum. J Toxicol Sci 2010;35(6):835-41.
[Crossref] [Google Scholar] [PubMed]
- Nakamura M, Takahashi T, Izumi T, Miura M, Kawaguchi S, Yamamoto A, et al. Peroxisome proliferator activated receptor-mediated genotoxicity of perfluoroalkyl acids using human lymphoblastoid cells. Fundam Toxic Sci 2016;3(4):143-50.
- Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 2001;49(11):5165-70.
[Crossref] [Google Scholar] [PubMed]
- Sohal JK, Saraf A, Shukla K, Shrivastava M. Determination of antioxidant potential of biochemically synthesized silver nanoparticles using Aloe vera gel extract. Plant Sci Today 2019;6(2):208-17.
- Langmead L, Makins RJ, Rampton DS. Anti‐inflammatory effects of Aloe vera gel in human colorectal mucosa in vitro. Aliment Pharmacol Ther 2004;19(5):521-7.
[Crossref] [Google Scholar] [PubMed]
- Hu Q, Hu Y, Xu J. Free radical-scavenging activity of Aloe vera (Aloe barbadensis Miller) extracts by supercritical carbon dioxide extraction. Food Chem 2005;91(1):85-90.
- Phull AR, Abbas Q, Ali A, Raza H, Zia M, Haq IU. Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Futur J Pharm Sci 2016;2(1):31-6.
- Otunola GA, Afolayan AJ. In vitro antibacterial, antioxidant and toxicity profile of silver nanoparticles green-synthesized and characterized from aqueous extract of a spice blend formulation. Biotechnol Biotechnol Equip 2018;32(3):724-33.
- Chatterjee S, Dey A, Dutta R, Dey S, Nacharya K. Hepatoprotective effect of the ethanolic extract of Calocybe indica on mice with CCl₄ hepatic intoxication. Int J PharmTech Res 2011;3(4):2162-8.
- Tabassum A, Mahboob T. Role of peroxisome proliferator–activated receptor-gamma activation on visfatin, advanced glycation end products, and renal oxidative stress in obesity-induced type 2 diabetes mellitus. Hum Exp Toxicol 2018;37(11):1187-98.
[Crossref] [Google Scholar] [PubMed]
- Shayesteh TH, Khajavi F, Ghasemi H, Zijoud SM, Ranjbar A. Effects of silver nanoparticle (AgNP) on oxidative stress, liver function in rat: Hepatotoxic or hepatoprotective?. Issues Bio Sci Pharm Res 2014;2(5):40-4.
- Afifi M, Abdelazim AM. Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats. Asian Pac J Trop Biomed 2015;5(10):874-7.
- Abou Seif HS. Physiological changes due to hepatotoxicity and the protective role of some medicinal plants. Beni-Suef Univ J Basic and Appl Sci 2016;5(2):134-46.
- Kumar H, Bhardwaj K, Nepovimova E, Kuča K, Singh Dhanjal D, Bhardwaj S, et al. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials 2020;10(7):1334.
[Crossref] [Google Scholar] [PubMed]
- Bautista-Pérez R, Segura-Cobos D, Vázquez-Cruz B. In vitro antibradykinin activity of Aloe barbadensis gel. J Ethnopharmacol 2004;93(1):89-92.
[Crossref] [Google Scholar] [PubMed]
- BEGUM Q, Mahboob T. Silver nanoparticles protect streptozotocin-induced hepatotoxicity: A biochemical and histopathological approach. Int J Nano Res 2021;4(1):1-9.
- Abubakar AM, Dibal NI, Attah MO, Chiroma SM. Exploring the antioxidant effects of Aloe vera: Potential role in controlling liver function and lipid profile in high fat and fructose diet (HFFD) fed mice. Pharmacol Res Mod Chin Med 2022;4:100150.
- Nahar T, Uddin B, Hossain S, Sikder AM, Ahmed S. Aloe vera gel protects liver from oxidative stress-induced damage in experimental rat model. J Complement Integr Med 2013;10(1):47-53.
[Crossref] [Google Scholar] [PubMed]
- Cui Y, Cheng Y, Guo Y, Xie Y, Yao W, Zhang W, et al. Evaluating the hepatoprotective efficacy of Aloe vera polysaccharides against subchronic exposure of aflatoxins B1. J Taiwan Inst Chem Eng 2017;76:10-7.