- Corresponding Author:
- R. Panchagnula
Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Sector 67, S. A. S. Nagar (Mohali) - 160 062, India. E-mail: panchagnula@yahoo.com
| Date of Submission | 7 March 2006 |
| Date of Revision | 26 June 2007 |
| Date of Acceptance | 23 July 2007 |
| Indian J Pharm Sci, 2007, 69 (4): 556-561 |
Abstract
Nifedipine, an important therapeutic agent in the management of cardiovascular disorder is recommended to administer as modified release dosage form in order to avoid the fluctuations in blood levels. An in vitro evaluation of modified release formulations, marketed in India was conducted and compared their performance with a novel matrix-based multi particulate system. The results indicate that even though the marketed formulations are found to comply to the definition of modified release formulations and predicted to produce therapeutic blood level for a prolonged period of time, the fluctuations were expected to be found uncontrolled except in the osmotic systems and the matrix-based multi particulate system. Thus, it was concluded that novel matrix-based multi particulate system were found to be superior to any other marketed formulations with respect to the therapeutic advantage as well as manufacturing feasibility.
Approximately one quarter of the total global population is affected by at least any one form of the cardiovascular disease (CVD) [1]. CVD caused 2.3 million deaths in India in 1990, which may double by year 2020, where hypertension alone contribute for 57% of all stroke death and 24% of all coronary heart disease [2]. Thus, management of CVD in particular the hypertension becomes important to improve the heath care systems. Several drugs are prescribed for successful management of CVD in which nifedipine (NFD), a dihydropyridine derivative is effectively used in management of various CVDs like angina, mild to moderate hypertension, myocardial infarction and Raynaud phenomenon [3]. Though NFD is administered as immediate release solid oral dosage forms, a short elimination half-life with significant fluctuation in plasma concentration necessitated it to formulate into modified release dosage forms. Recently, it was reported that smooth plasma profile of NFD by modified release dosage forms decreased morbidity and mortality, prevents myocardial infarction in diabetes mellitus patients and reduces the atherosclerosis in carotid and coronary arteries [4]. Conceiving this in mind earlier in our lab a modified release formulation of NFD based on multiple unit matrix particulate system was developed and evaluated for both in vitro and in vivo performance [5]. A blood level of NFD were found to be with in the therapeutic limits for a prolonged period of time (till 8th hour post dose) for a single dose administration of 40 mg dose of test product (NIPER formulation) and was comparable to that of reference product (Cardules retard manufactured by Nicholas Piramal Ltd., India). The present study focuses on complete in vitro evaluation of marketed nifedipine modified release products and compares their performance with the NIPER formulation through dissolution studies by kinetic modeling and in vivo predictions which gave an insight regarding the performance of the currently marketed NFD modified release formulations in India.
Materials and Methods
NFD was received as gift sample from Unichem Laboratories Ltd., India and the modified release formulations of NFD marketed in India (Table 1) were purchased from local pharmacies. Sodium lauryl sulphate (96% purity) used for dissolution studies was purchased from Loba Chemie, India. All other chemicals were analytical grade.
| Formulation code | Brand name | Manufacturer | Strength (batch no.) |
|---|---|---|---|
| N1 | Adalat retard OROS | Bayer Ltd., India | 30 mg (N103) |
| N2 | Calbloc retard | Unichem Ltd., India | 10 mg (12002); |
| 20 mg (320007) | |||
| N3 | Calcigard retard | Torrent Ltd., India | 20 mg (1202001) |
| N4 | Cardules retard | Nicholas Piramal Ltd., India | 10 mg (P1003); |
| 20 mg (P0012) | |||
| N5 | Depicor SR | E.Merck Ltd., India | 10 mg (A202); |
| 20 mg (Al201) | |||
| N6 | Depin retard | ZydusMedica Ltd., India | 20 mg (MA2595) |
| N7 | Nicardia retard | Unique Ltd., India | 10 mg (02037); |
| 20 mg (03022); | |||
| 30 mg (P300413) | |||
| N8 | Nifedine SR | Sarabhai Piramal Pvt. Ltd., India | 10 mg (0F128); |
| 20 mg (2A112) | |||
| N9 | Nifelet retard | Cipla Ltd., India | 20 mg (DJ2050) |
All the formulations were matrix tablets, except formulation N1 (an osmotic tablet) and N4 (multiunit matrices loaded in a gelatin capsules).
Table 1: Details of modified release formulations of nifedipine procured from indian market and subjected for dissolution studies.
Formulations were subjected to various pharmacopeial (weight variation, friability, DT, assay) and nonpharmacopeial (hardness) tests in order to qualify for the dissolution studies. Assay was performed as per the method developed earlier in our laboratory [5], where the drug was extracted with methanol, suitably diluted using phosphate buffer (pH 6.8) containing 1% w/v SLS and analyzed by UV/Vis spectrophotometer (DU 640i, Beckman) at 236 nm. Further, content uniformity was assessed for five individual units by an appropriately modified procedure mentioned above for assay. Dissolution studies were conducted in phosphate buffer (pH 6.8 contained 1% SLS; 900 ml) maintained at 37±0.5o using USP apparatus II (programmable dissolution tester TDT-0P, Electrolab) at a rotational speed of 50 rpm. Samples (5 ml) were withdrawn at different time intervals over a 24 h period, filtered, suitably diluted and analyzed at 236 nm. Further to study the influence of pH on dissolution properties of formulations, studies were also conducted at three different pH levels (pH 2.0, 5.0 and 7.4). Both dissolution studies and assay were performed in subdued light or low actinic sodium vapor monochromatic light.
Dissolution profiles were compared by fit factors (similarity and dissimilarity factors) using dissolution profile of NIPER formulation as reference [5]. Further, drug release data were subjected to regression analysis and fitting to various kinetic models (zero-order, first order, Higuchi, Hixson-Crowell, Baker-Lonsdale and Peppas models) [6]. In vitro drug release parameters (R0 = rate of input and tdel= time of drug release) of most similar release profile (based on f2 value) to that of reference product were fitted to predict in vivo drug concentrations using reported pharmacokinetic properties from single dose [7]. Method of superposition was used for steady state concentration predictions. Values of Cssmax and Cssmin thus obtained were compared to that of actual plasma level profile of reference formulation. The goodness of modified release formulations was evaluated from calculated dosage form index, DI (Eqn. 1) and % fluctuation (Eqn. 2) of formulations [8], where DI= Cssmax/Cssmin (Eqn. 1) and fluctuations (%)= (Cssmax -Cssmin)×100/Cssav(Eqn. 2), in which Cssmax and Cssmin are maximum and minimum blood drug concentrations at steady state, respectively and Cssav is average blood drug concentration at steady state.
Results and Discussion
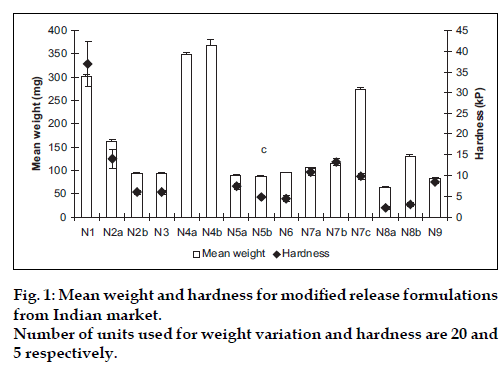
Various pharmacopeial and non-pharmacopeial tests indicated the good quality of selected marketed formulations with respect to crushing strength (fig. 1), friability and assay (data not shown). Weight variation within the same formulation was found less than 0.1%, however large difference in average weight among different formulations was observed which in turn indicated variation in total amount of excipient used by different manufacturer. Crushing strength of different formulations ranged from 5-40 kilopascal (kP) that may be due to difference in technology (wet or dry granulation, or direct compression) used to manufacture. N1 and N4 are osmotic and multiunit matrix system, respectively, while rest of the formulations is single unit matrix system.
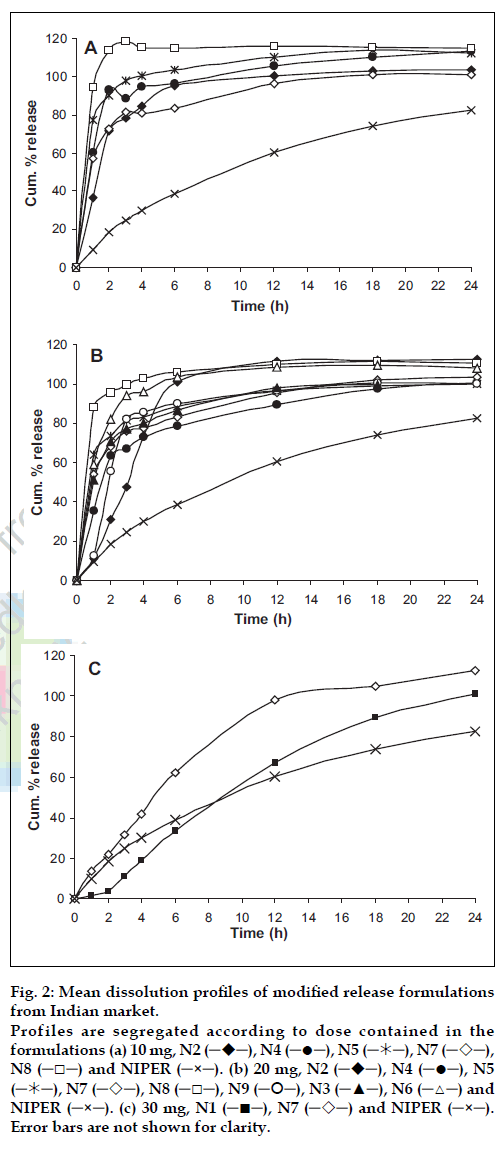
In drug release studies, initial dissolution testing was performed in the previously optimized dissolution medium of 1% w/v SLS in phosphate buffer pH 6.8. Addition of surfactant in dissolution medium was used to provide sink condition, which simulated the physiological environment as well more closely than other approaches [9]. Mean dissolution profiles of NIPER formulation with tested marketed formulations are shown in fig. 2. N1 is an osmotic tablet showed a lag time of 2 h for initiation of release and released almost at zero-order release rate. However an ideal modified release formulation should release loading dosage in first few hours and remaining maintenance dose at a constant rate. On the other hand formulations N6 and N8 released more than 80% of the drug in 1 h, where an immediate burst effect and a sustained release was observed for formulations N3, N4, N5, N7, and N9. Interestingly a significant difference in the release pattern was observed for N7 at different doses (fig. 2b and 2c). This may be due to the higher amount of polymer used in the formulation with dose 30 mg (fig. 1), however similar trend was not seen in N8 (10 and 20 mg dose) though there is a double the quantity of excipient added in 20 mg dosed formulation.
Figure 2: Mean dissolution profiles of modified release formulations from Indian market. Profiles are segregated according to dose contained in the formulations (a) 10 mg, N2 (─!─), N4 (─●─), N5 (─"─), N7 (─#─), N8 (─□─) and NIPER (─×─). (b) 20 mg, N2 (─!─), N4 (─●─), N5 (─"─), N7 (─#─), N8 (─□─), N9 (─$─), N3 (─▲─), N6 (─△─) and NIPER (─×─). (c) 30 mg, N1 (─■─), N7 (─#─) and NIPER (─×─).Error bars are not shown for clarity.
Earlier in our laboratory Cardules retard (20 mg) (in the present study N4) was used as reference product during demonstration of bioequivalence of NIPER formulation [5]. A Cmax of 35.54 ng/ml was observed to achieve for a 40 mg dose by N4 and characterized with a drug plasma profile of an immediate release dosage form though plasma levels are with in the therapeutic range for at least 24 h. On the other hand, NIPER formulation a flattened profile was observed which sustained for minimum of 24 h. This study demonstrated the feasibility of achieving an exact controlled release dosage form from a simple multiunit matrix system [10]. Based on these results in the present study, the formulations, which were performing similar to N4 in vitro, were predicted to behave in a similar manner in vivo also to that of N4. Thus only few formulations (N1 and N7) were selected to proceed further based on the fit factor values.
Fit factors are calculated (f2 and f2) for comparison of drug release profiles from different formulations and/or at different conditions from mean dissolution data [11]. For a dissolution profile to be considered similar value of f2 should lie between 50 and 100, on the other hand, dissimilarity factor f1 was also calculated to approximate the percentage error between two profiles. The value of f1 is zero when test and reference formulation profiles are identical and increases proportionally with the dissimilarity between two profiles. Calculated f1 and f2 values are shown in Table 2 for various formulations. N1 (30 mg) shows a lag time of two hours thus f1 and f2 values were calculated with and without lag time. Calculated f2 value without lag time (48.31) was more close to 50 than with lag time (45.26). N7 (30 mg) has f2 value of 32.32 while rest of the formulations have less than 20. Correlation of f2 to average difference (%) between reference and test profile is non linear where more than 90% similarity in profiles is indicated by f2 value above 50. However, f2 value above 40 indicates that the profiles are more than 80% similar. Further, no tested formulations had f1 value less than 20 except N1 (19.23 without lag time). In addition to these, large difference in Sd values of N1 with and without lag time was observed. However Sd value without lag time for N1 (0.0691) was very close to zero indicated similarity to NIPER formulation.
| Formulation code | Reference formulation | ||
|---|---|---|---|
| f2 | fl | Sd | |
| N1a 30 mg | 45.26 | 27.29 | 0.3881 |
| Nib 30 mg | 48.31 | 19.23 | 0.0691 |
| N2 10 mg | 17.81 | 99.11 | 0.5162 |
| N2 20 mg | 21.16 | 77.34 | 0.2813 |
| N3 20 mg | 18.68 | 95.42 | 0.5489 |
| N4 10 mg | 13.05 | 125.35 | 0.6287 |
| N4 20 mg | 22.95 | 78.82 | 0.4632 |
| N5 10 mg | 10.95 | 138.16 | 0.6784 |
| N5 20 mg | 16.95 | 102.88 | 0.5915 |
| N6 20 mg | 13.04 | 124.81 | 0.6229 |
| N7 10 mg | 17.93 | 99.11 | 0.5681 |
| N7 20 mg | 19.15 | 94.90 | 0.5477 |
| N7 30 mg | 32.32 | 46.90 | 0.1675 |
| N8 10 mg | 06.49 | 166.95 | 0.7575 |
| N8 20 mg | 09.88 | 143.68 | 0.7069 |
| N9 20 mg | 20.02 | 83.92 | 0.3655 |
f2 and sd are similarity and f1 was dissimilarity factors of marketed NFD formulaions and the refernec NIPER formulation. Release studies were performed in modiÞ ed dissolution medium containing phosphate buffer (ph 6.8) with 1% w/v SLS. awith lag time; bwithout lag time; f2 value more than 50 and Sd equal to zero were considered as similar proÞ les
Table 2: Similarity and dissimilarity factor values for drug release profiles obtained from modified release formulations of nfd marketed in india in comparison to nipper formulation
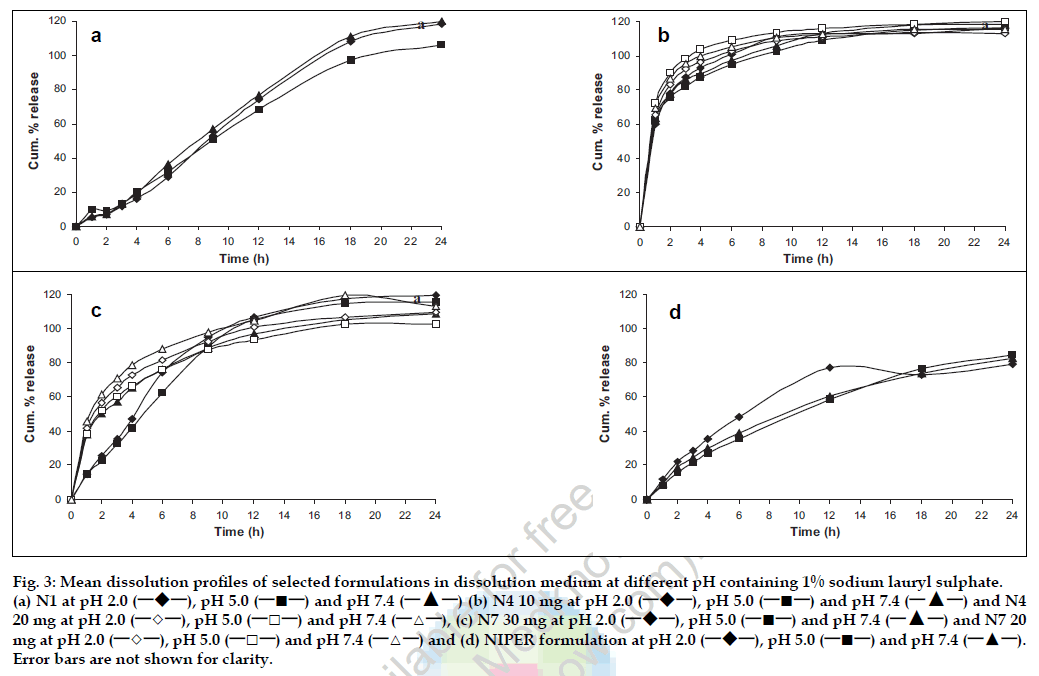
Thus, based on similarity and dissimilarity factors, N1 and N7 were selected to study the effect of pH on drug release since modified release formulations should possess pH independent release property. In addition, N4 was also selected for further study because of similarity in dosage form with NIPER formulation as well as availability of biostudy data, which would facilitate meaningful comparison. Formulations thus selected were evaluated for their drug release performance at different pH (fig. 3). All selected formulations showed pH independent release indicating the acceptable quality as modified release formulations. Further dissolution data of selected formulations was fitted to basic release models. From the linear portion of the curves slope, intercept and correlation coefficient were calculated and data is summarized in the Table 3. N1 best fitted to the zero order kinetics as well first order kinetics with a release rate of 1.3925 mg/h and 0.1266 h-1, respectively, with high correlation coefficient, where N4 showed very less correlation for any release kinetic model. In case of N7 the dissolution data followed a first order fit with a release rate and correlation coefficient of 0.3267 and 0.9222, respectively. Similar to N1, NIPER formulation fitted best to first order kinetics.
Figure 3: Mean dissolution profiles of selected formulations in dissolution medium at different pH containing 1% sodium lauryl sulphate. (a) N1 at pH 2.0 (─!─), pH 5.0 (─■─) and pH 7.4 (─▲─) (b) N4 10 mg at pH 2.0 (─!─), pH 5.0 (─■─) and pH 7.4 (─▲─) and N4 20 mg at pH 2.0 (─◇─), pH 5.0 (─□─) and pH 7.4 (─△─), (c) N7 30 mg at pH 2.0 (─!─), pH 5.0 (─■─) and pH 7.4 (─▲─) and N7 20 mg at pH 2.0 (─◇─), pH 5.0 (─□─) and pH 7.4 (─△─) and (d) NIPER formulation at pH 2.0 (─!─), pH 5.0 (─■─) and pH 7.4 (─▲─). Error bars are not shown for clarity.
| Release model | N1 | N4 | N7 | NIPER |
|---|---|---|---|---|
| ZERO ORDER | ||||
| k | 1.3925 | 0.5098 | 0.9477 | 1.3469 |
| R2 | 0.9861 | 0.6642 | 0.8878 | 0.9344 |
| FIRST ORDER | ||||
| k | 0.1266 | 0.1847 | 0.3267 | 0.0715 |
| R2 | 0.9838 | 0.7904 | 0.9222 | 0.9982 |
| HIGUCHI | ||||
| k | 24.1762 | 17.2239 | 26.2288 | 18.2802 |
| R2 | 0.9711 | 0.7214 | 0.9646 | 0.9904 |
| HIXSON-CROWELL | ||||
| K | 0.2131 | 0.1281 | 0.3200 | 0.0894 |
| R2 | 0.9816 | 0.7332 | 0.9790 | 0.9874 |
| BAKER-LONSDALE | ||||
| K | 0.0151 | 0.0176 | 0.0349 | 0.0088 |
| R2 | 0.9598 | 0.7925 | 0.9049 | 0.9876 |
| KORSMEYER-PEPPAS | ||||
| k | -# | 0.5890 | 0.1465 | 0.1095 |
| R2 | - | 0.8790 | 0.9726 | 0.9878 |
| n | - | 0.19 | 0.70 | 0.67 |
K is release rate constant with units mg/h, h-1, %/(h)1/2 and (%)1/3/h for psuedo zero order, first order and Baker-Lonsdale, Higuchi and Hixson-Crowell models respectively; n, release exponent; R2, correlation coefficient. #an osmotic system where and Korsmeyer- Peppas equations are not applicable.
Table 3: Kinetic and statistical parameter obtained from drug release data of selected formulations
Fickian diffusion, polymer relaxation and osmotic pressure are the basic drug transport mechanism, which control the release from modified release formulations. Being OROS system, N1 provide controlled release by the mechanism of push-pull osmotic pressure [12]. Mechanism of drug release from matrix-based formulations is described by a simple empirical equation proposed by Peppas [13] where exponent ‘n’ indicates mechanism of drug release and its values lies between 0.45-0.89 for a cylindrical geometry. A value of n = 0.45 indicates Fickian diffusion, 0.45 < n < 0.89 indicates anomalous diffusion (non-Fickian) and n = 0.89 indicates case II relaxation process. N4 showed a fickian diffusion mechanism where N7 and NIPER formulation showed anomalous drug release from the dosage form (Table 4) i.e. combination of both diffusion and relaxation mechanism contributing equally to the overall drug release.
| Formulation code | Dose (mg) | k | Cssmin-Cssmax (ng/ml) | DI | Fluctuations (%) |
|---|---|---|---|---|---|
| N1 | 30 | 1.3925a | 28.2-33.94# | 1.20 | 18.36 |
| N1 | 30 | 0.1269b | 31.3-59.4$ | 1.90 | 61.96 |
| N4 | 20 | 0.1847b | 19.2-49.3 | 2.57 | 87.88 |
| N7 | 30 | 0.3267b | 19.1-97.7 | 5.12 | 134.59 |
| NIPER | 40 | 1.3469a | 27.3-54.6# | 2.00 | 66.67 |
| NIPER | 40 | 0.0715b | 37.4-53.2$ | 1.42 | 34.88 |
#and $-plasma levels calculated using zero and first order release rate constant, amg/h; bh-1. Cssmax and Cssmin, maximum and minimum steady state concentration, respectively; DI, dosage form index, k release rate constant.
Table 4: Predicted steady state plasma concentration level of nifedipine from in vitro drug release profiles and in vivo pharmacokinetic data
Using superposition principle steady state plasma levels were calculated using drug release parameters (Ro- release rate and tdel -time of delivery) for selected formulations7. Further DI and % fluctuation were also calculated, which are indicative of performance of controlled release formulations, from the Cssmin and Cssmax. Predicted steady state plasma drug concentrations for all selected marketed formulations lie with in the therapeutically acceptable range (i.e., 15-75 ng/ml). As can be seen from table 4, DI and % fluctuation was appreciably less in case of N1 and NIPER formulation, where as N7 and N4 showed a highest degree of fluctuation. However the case of N4 should not be considered due to the poor correlation coefficient yielded while fitting to first order release model. The DI and % fluctuation calculated from the predicted plasma levels using first and zero order release rate constant respectively for N1 and NIPER formulation also indicated less fluctuation in the plasma levels of NFD. Thus N1 and NIPER formulation were concluded as superior to any other product in Indian market.
Drug release from majority of marketed modified release formulation was almost immediate and thus the fluctuation in blood level during steadystate conditions may undermine the sole purpose of the delivery system for nifedipine though the therapeutic drug blood levels would be maintained for a prolonged period of time. On the other hand NIPER formulation showed desirable blood levels with least fluctuation and comparable to the sophisticated osmotic delivery system. Thus in nutshell, N1 and NIPER formulation showed therapeutic advantage over other marketed modified release formulations with respect to controlling the fluctuations in plasma levels.
References
- Heart disease and stroke statistics . 2004 update. American heart association, Dallas, USA, 2004
- Gupta, R., J. Hum. Hyperten., 2004, 18, 73.
- Sorkin, E.M., Clussold, S.P. and Brogden, R.N., Drugs, 1985, 30, 182.
- Simon, A. and Levenson, J., Expert Opinion Pharmacotherapy, 2003, 4, 95.
- Sood A. and Panchagnula, R., Int. J. Pharm., 2003, 261, 27.
- Costa, P. and Lobo, J.M.S., Eur. J. Pharm. Sci., 2001, 13, 123.
- Ritschel, W.A., Drug Develop. Ind. Pharm., 1989, 15, 1073.
- Theeuwes, F. and Bayne, W., J. Pharm. Sci., 1977, 66, 1388.
- Shah, V.P., Noory, C., McCullough, B., Clarke, S., Evert, R., Naviasky, H., Srinivasan, B.N., Fortman, D. and Skelly, J.P. Int. J. Pharm., 1995, 125, 99.
- Sood, A., Ashokraj, Y. and Panchagnula, R. Pharm. Technol., 2004 (November), 84.
- Moore, J.W. and Flanner, H.H., Pharm. Technol., 1996 (June), 64.
- Linder, W.D. and Lippold, B.C. Pharm. Res., 1995, 12, 1781.
- Peppas, N.A. Pharm. Acta. Helv., 1985, 60, 110.