- Corresponding Author:
- P. T. Tayade
Pharmaceutical Division, Mumbai University Institute of Chemical Technology (MUICT), Nathlal Parikh Marg, Matunga, Mumbai-400 019, India.
E-mail: pralhad_tayade@rediffmail.com
| Date of Submission | 19 January 2005 |
| Date of Revision | 25 June 2005 |
| Date of Acceptance | 20 February 2006 |
| Indian J Pharm Sci, 2006, 68 (2): 164-170 |
Abstract
The inclusion behavior of hydroxypropyl b-cyclodextrin and natural b-cyclodextrin was studied toward ketoprofen, in order to develop a new oral dosage form with enhanced dissolution rate and bioavailability, and to study the oral pharmacokinetics of ketoprofen in humans, following cyclodextrin complexation. Drug-cyclodextrin solid systems were prepared by kneading, co-evaporation, and freeze-drying. The formation of inclusion complexes with b-cyclodextrin and hydroxypropyl b-cyclodextrin in the solid state, were confirmed by differential scanning calorimetry, Fourier transform infrared spectroscopy, powder X-ray diffractometry, scanning electron microscopy studies, and comparative studies on the in vitro dissolution and in vivo absorption of ketoprofen in humans volunteers, were carried out. The initial dissolution rate of ketoprofen in the inclusion complexes was 15 fold higher, than that of plain drug powder. The maximal plasma concentration of ketoprofen after the oral administration of inclusion complexes to human volunteers increased about 1.5 fold (7.15 vs 4.65 mg/ml), and there was no significant increase in area under concentration-time curve, AUC0-5 (10.35 vs. 9.35 mg. hr/ml), compared to those of ketoprofen powder alone.
Ketoprofen (KTPF) α-(3-benzoyl phenyl) propionic acid is a nonsteroidal antiinflammatory drug with analgesic and antipyretic activity. It is used in a wide variety of acute and chronic inflammatory diseases [1] . However, it has poor aqueous solubility, and peak plasma concentrations occur about 0.5-2.0 h after an oral dose. When ketoprofen is given with food, its bioavailability is not altered, but food intake reduces Cmax by approximately one half, and increases the mean time to peak concentration. The fluctuation of plasma peaks may also be influenced by circadian changes in the absorption process [2]. The enhancement of bioavailability is generally based on increase in aqueous solubility and dissolution rate of ketoprofen [3,4]. The cyclodextrins are water-soluble, nonreducing, and macrocyclic polymers, which have glucose units joined by an α-1,4-linkage. Molecules with suitable size and shape can be held within the cavity of cyclodextrin to form inclusion compounds [5]. It is well known, that cyclodextrin forms inclusion complexes with various nonsteroidal anti-inflammatory drugs. However, it is known that the application of β-cyclodextrin (βCD) in the pharmaceutical field, is limited by its rather low aqueous low solubility, which led to a search for more soluble derivatives of cyclodextrins [6,7]. For example, hydroxypropyl derivatives of cyclodextrins have the advantages of enhanced solubility, as well as lower hemolytic activity and reduced nephrotoxicity [8,9].
Studies on dissolution behavior of tablets with fluctuation dried complexes of ketoprofen with β-cyclodextrin, and study of ketoprofen-cyclodextrin systems for the improvement of oral bioavailability of drug in rats, has already been reported [10,11]. In the present work, inclusion complexes of ketoprofen with βCD and its 2- hydoxypropyl derivative (HPβCD), were prepared using the method of kneading, coprecipitation, and freeze drying. The formation of inclusion complexes with βCD and HPβCD in the solid state, were confirmed by infrared spectroscopy, differential scanning calorimetry, scanning electron microscopy and powder X-ray diffractometry, and their dissolution rates were determined using the USP paddle method. The effect of cyclodextrin on various pharmacokinetic parameters of ketoprofen in humans, was also investigated.
Materials and methods
Ketoprofen was a gift sample from Rhone-Poulenc Pharma, Mumbai, India. β-cyclodextrin was kindly provided by the American Maize Product Company, Amaizo (USA), and 2-hydroxypropyl-β-cyclodextrin was generously donated by Nihon Shokuhin Kako Company, Japan. All reagents and solvents used were of analytical grade.
Phase solubility analysis
The solubility measurements of ketoprofen with cyclodextrins, were performed according to Higuchi and Connors. [12] Excess amounts of drug were added to an aqueous solution containing various concentrations of cyclodextrins, and were shaken (300 rpm) at 25±0.5°. At equilibrium after 2 days, the supernatant was filtered (0.45 μm pore size) and spectrophotometrically assayed for drug content at 253 nm, using a Milton Roy UV/Vis Spectrophotometer. Each experiment was carried out in triplicate. The apparent 1:1 binding constants of the KTPF-CD complexes were calculated from the slope and intercept of the straight lines of the phase-solubility diagrams, according to the following equation; Kc = slope/So (1-slope), where Kc is the apparent binding/ stability constant, and So (intercept) is the intrinsic solubility of the compound in absence of complexing agent.
Preparation of solid inclusion complexes
A mixture of KTPF (0.254 g) and βCD (1.135 g) was wetted with water: ethanol (1:1v/v, 3 ml) solution, and kneaded thoroughly for 30 min in a glass mortor. The paste formed, was dried under vacuum at 600 for a day. In another method of complexation by co-precipitation, drug solution (0.254 g in 10 ml ethanol) was added dropwise to aqueous βCD solution (1.135 g in 50 ml of distilled water), with constant stirring. After complete addition, the mixture was maintained at 45° for 2 h with stirring. The co-precipitated mixture was then evaporated on a water bath at 60° for 8 h, and further dried under vacuum at 60° for 24 h.
In the method of freeze-drying, βCDs and drug at 1:1 molar ratio, were dispersed in distilled water. Aqueous ammonia solution (25% w/w ammonia solution, 1.5 ml) was added to dissolve the drug completely. The solution was stirred on a magnetic stirrer at room temperature for 2 h, and then subjected for freeze-drying using a Labconco freeze-drier.
The KTPF-HPβCD complex in the solid state was prepared by the method of freeze-drying only, because the paste produced by the kneading was sticky and difficult to grind, whereas the product produced by coprecipitation was very hygroscopic and difficult to collect. Each solid product was sieved through # 60 sieve, and the same fraction was used for the following tests.
Differential scanning calorimetry (DSC)
A Perkin Elmer DSC model 7 was used for recording DSC thermograms of the ketoprofen raw material, inclusion complexes, and the physical mixtures. Samples (2-8 mg) were accurately weighed and heated in open aluminum cells, at a rate of 10°/min between a temperature range of 30° and 300°, under a nitrogen flow of 40 ml/min. Reproducibility was checked by running the sample in triplicate.
Fourier transform infrared (FTIR) spectroscopy
Fourier transform IR spectra were recorded on a Buck scientific IR spectrophotometer, Model-500. The spectra were recorded for ketoprofen, β-cyclodextrins, its physical mixtures, and solid inclusion complexes. Samples were prepared in KBr disks (2 mg sample in 200 mg KBr). The scanning range was 450-4000 cm-1,and the resolution was 4 cm-1.
X-ray powder diffractometry (XRD)
Powder X-ray diffraction patterns were recorded on a Jeol PW 17291 powder X-ray diffractometer, using Nifiltered, CuKα radiation, a voltage of 40 kV,and a current of 25 mA. The scanning rate employed was 1° min-1 over the angle 2θ range of 0-50°. The XRD patterns of ketoprofen raw material, inclusion complexes, and the physical mixtures were recorded. Scanning electron microscopic (SEM) analysis was carried out using a Cambridge stereoscan-150 scanning microscope. Prior to examination, samples were gold sputter-coated, to render them electrically conductive.
Dissolution rate study
Dissolution rate experiments were performed in 0.1N HCl at 37±0.5°, using USP XXI/XXII apparatus (Electrolab, Mumbai) with paddle stirrer, rotating at 100 rpm. Solid products, each containing 25 mg of drug were subjected for dissolution. At fixed time intervals, samples were withdrawn with a filter syringe (pore size 0.45 μm), and spectrophotometrically assayed for drug content at 253 nm. Each test was performed in triplicate.
Pharmacokinetic study
A single oral dose (25 mg) pharmacokinetic study, was carried out in a group of six healthy male human volunteers, aged 20–25 years. The protocol of the study was as per the criteria of health, laid down by the Department of Clinical Pharmacology, Grant Medical College, Mumbai. The volunteers were admitted to the study after detailed medical and laboratory examinations. The volunteers were given written information about the aim of the study, and informed consent was obtained from each of them. The volunteers were randomly assigned to the treatment groups. The volunteers were kept on fasting for a period of 12 h, before the start of the study. Blood samples (5 ml) were withdrawn at 0, 0.5, 1.0, 1.5, 2.0, 3.0, and 5.0 h, after administration of the dose, using heparinised disposable syringes. Plasma was separated by immediate centrifugation, and stored at -20° until further analysis by RP HPLC. To 1.0 ml of plasma, 0.1 ml of 0.5N HCl and 5.0 ml of toluene was added, vortexed for 5 min, and centrifuged at 4000 rpm for 15 min. The resulting toluene layer was separated, and the remaining water phase was extracted again with 5.0 ml of toluene. Both the toluene layers were mixed and evaporated to dryness at 60°. The residue was reconstituted with mobile phase, and 20 μl of this sample was subjected for analysis by HPLC, using Merck Finepack 100RP ODS C18 (250 mm x 40 μm, 5μ), injected in the column. The mobile phase consisted of methanol/potassium dihydrogen orthophosphate 0.5 M (55:45). All separations were carried out at a flow rate of 1.0 ml/min. Detection was made at the wavelength of 260 nm.
Results and Discussion
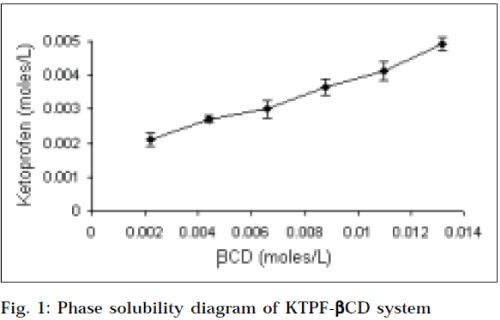
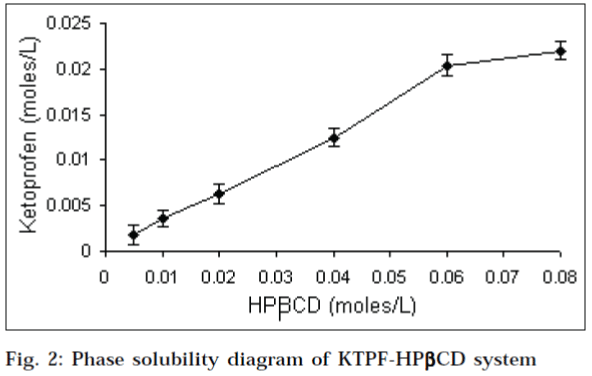
Phase solubility diagrams (Figs.1 and 2) obtained with βcyclodextrins, showed a linear relationship between the amount of KTPF solubilized, and the concentration of cyclodextrin in solution (AL type diagram). According to Higuchi and Connors, this may be attributed to the formation of soluble 1:1 KTPF-CD inclusion complexes. Solubility enhancement was much greater with HPβCD, as compared to that of parent βCD. Stability constant obtained for HPβCD (330 M-1) was higher than that of βCD (241 M-1).
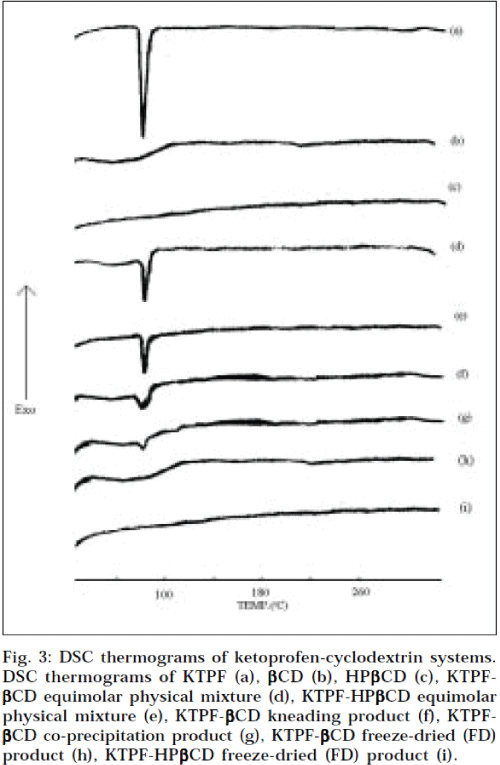
DSC revealed some information on solid-state interactions between drug and cyclodextrin. The DSC thermograms of pure components, and of the different drugcyclodextrin systems are presented in Fig. 3. The DSC curve of KTPF was typical of a crystalline anhydrous substance, with a sharp fusion endotherm (Tpeak =97.3°). Liberation of crystal water from βCD (14.5% as mass fraction) was observed as a broad endothermal peak, at around 100°. HPβCD is an amorphous material, and does not exhibit a melting point, as would be observed for crystalline materials. Characteristic peaks (due to drug melting) were well recognizable in the physical mixtures of drug with both, βCD and HPβCD. However, the characteristic thermal peak of the drug appeared to lower temperatures, but strongly reduced in intensity and somewhat broadened, in the kneaded and co-precipitated products of both, KTPF-βCD and KTPF-HPβCD. In fact, only DSC thermograms of freeze-dried products lacked the drug melting peak corresponding to 97.3°. The disappearance of an endothermic peak may be attributed to an amorphous state, and/or to an inclusion complexation [13].
Fig. 3: DSC thermograms of ketoprofen-cyclodextrin systems. DSC thermograms of KTPF (a), βCD (b), HPβCD (c), KTPF-βCD equimolar physical mixture (d), KTPF-HPβCD equimolar physical mixture (e), KTPF-βCD kneading product (f), KTPF-βCD co-precipitation product (g), KTPF-βCD freeze-dried (FD) product (h), KTPF-HPβCD freeze-dried (FD) product (i).
No significant change in the IR spectra of both, kneaded and co-precipitated KTPF-CD complexes were observed, except for the broadening of the peaks. The broadening of the peaks was probably due to the restrictions of bending and stretching vibrations of the molecule, due to the cyclodextrin cavity. The FTIR spectra of freeze-dried complexes of KTPF-βCD and KTPF-HPβCD showed significant shift of carbonyl absorption band at 1697 cm-1, assigned to carboxyl carbonyl stretching as shown in Table 1. This may be indicative of the drug monomeric dispersion, as a consequence of the interaction with CDs through hydrogen bonding, which could result in its inclusion into the hydrophobic cavity of the cyclodextrin [14,15].
| Assignment | Plain Drug | PM | KN-β CD | COP-β CD | FD-β CD | FD- HPβ CD |
|---|---|---|---|---|---|---|
| C=O Stretching (cm-1) | 1697.51 | 1697.51 | 1699.44 | 1697.51 | 1660.80 | 1660.80 |
Drug-cyclodextrin solid systems; physical mixture (pm), kneading (kn), co-precipitation (cop) and freeze-dried (fd) products with ßcd and hpßcd.
Table 1: Carboxyl Carbonyl Stretching Bands (Cm-1) Of Ketoprofen And Its Solid Systems With Cyclodextrins
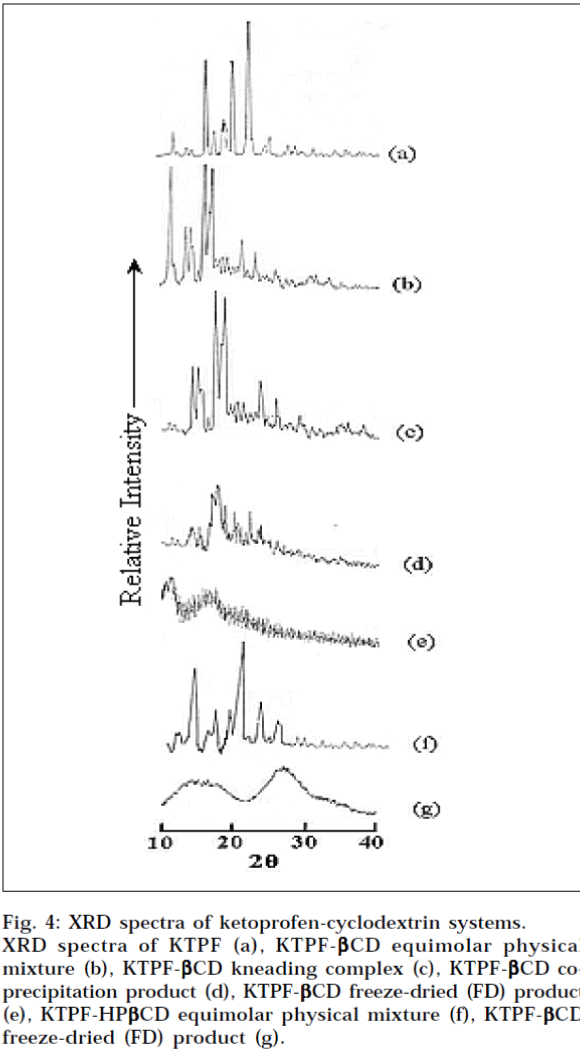
X-Ray powder, diffraction patterns of plain KTPF, drug cyclodextrin physical mixtures, and corresponding solid inclusion complexes with CDs, are shown in Fig. 4. In the X-ray diffractogram of KTPF powder, sharp diffraction peaks, indicating its crystalline state are present. Drug crystallinity peaks were still detectable in the respective physical mixtures with βCD and HPβCD, even if an evident reduction of intensity of crystallinity peaks was observed, particularly in the system with HPβCD. A total amorphization of both drug and cyclodextrin was instead induced by freeze-drying, where X-ray diffraction patterns of KTPF-CD systems were characterized only by large diffraction peaks, in which it is no longer possible to distinguish the characteristic crystallinity peaks of ketoprofen. These results indicate that KTPF is no longer present as a crystalline material, and its CD solid complexes exist in the amorphous state.
Fig. 4: XRD spectra of ketoprofen-cyclodextrin systems.
XRD spectra of KTPF (a), KTPF-βCD equimolar physical mixture (b), KTPF- βCD kneading complex (c), KTPF- βCD co- precipitation product (d), KTPF- βCD freeze-dried (FD) product (e), KTPF-HP βCD equimolar physical mixture (f), KTPF- βCD freeze-dried (FD) product (g).
The particle morphology of KTPF, β-cyclodextrins, its physical mixtures, and solid complexes, can be seen in SEM photographs presented in Fig. 5. KTPF appeared as plate- like crystals, tending to form aggregates. HPβCD consisted of shrunken, cylindrical particles, whereas βCD appeared as irregularly shaped crystals. The physical mixtures showed particles of HPβCD and βCD embedded with KTPF particles, and a comparable morphology with pure compounds, taken separately. In contrast, a drastic change in the morphology and change in crystalline nature was observed in 1:1 freeze-dried, coprecipitated, and kneaded products of both, HPβCD and βCD, revealing an apparent interaction in the solid state.
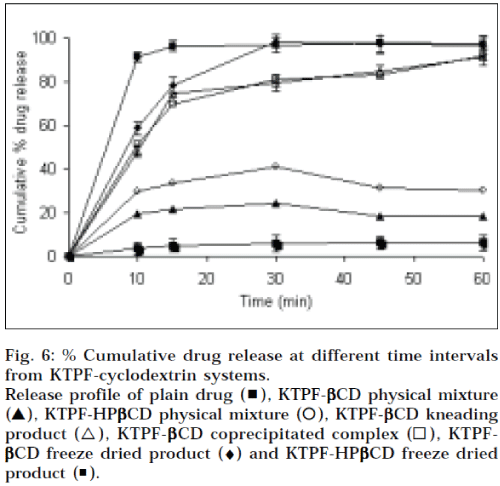
The dissolution curves of KTPF from the binary systems with βCD and HPβCD, are presented in Fig. 6. As for the increase in dissolution rate observed for physical mixtures with cyclodextrins, it can be assumed that in the early phase of the dissolution, its, dissolving more rapidly than the drug, can act on the hydrodynamic layer surrounding the particles of drug, resulting into in situ inclusion process, giving an increase of the amount of drug dissolved. It is evident that KTPF dissolves faster from cyclodextrin complexes than from physical mixtures, but the improvement obtained by freeze-drying is clearly more evident in combinations with cyclodextrins. Initial dissolution rate of ketoprofen in the inclusion complexes increased markedly (about 10 fold) within 5 min, compared to powder alone. This enhancement in the drug dissolution rate may be attributed to the amorphous or nearly amorphous state of such systems, as confirmed by DSC, XRD studies, generally occurring during freeze-drying (i.e. a decrease in crystallinity), and to reduction in the particle size, resulting in an increase of the surface area of the drug, according to the Noyes-Whitney equation [16], dC/dt = D/h x S (Cs – Ct), where dC/dt is the dissolution rate, D is the diffusion coefficient of the drug, h is the thickness of the diffusion layer, S is the surface area of the dissolving solid, Cs and Ct are the aqueous solubility, and the concentration of the drug in the aqueous medium at time t, respectively.
Fig. 6: % Cumulative drug release at different time intervals from KTPF-cyclodextrin systems. Release profile of plain drug (▖), KTPF-βCD physical mixture (▲), KTPF-HPβCD physical mixture (⚪), KTPF-βCD kneading product (△), KTPF-βββββCD coprecipitated complex (◻), KTPF-βCD freeze dried product (♦) and KTPF-HPβCD freeze dried product (◾).
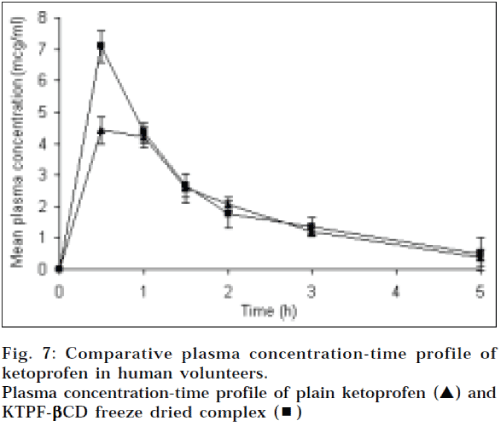
The mean plasma concentration-time profiles of ketoprofen after the oral administration of ketoprofen plain (25 mg) and inclusion complex with βCD equivalent to 25 mg of ketoprofen to human male volunteers, can be seen in Fig. 7. The noncompartmental pharmacokinetic parameters are calculated, based on the observed plasma data and presented in table 2. When the equivalent dose of ketoprofen (25 mg) was administered to human male volunteers, the inclusion complex with βCD attained peak plasma level of 7.15 mg/ml, which was about 1.5 fold higher than that of drug powder alone (4.65 μg/ml). The area under the plasma concentration-time curve (AUC0-5h) of the ketoprofen-βCD complex was increased to 10.35 mg. h/ml, as compared to drug alone (9.35 μg. h/ml). Tmax and Kel of the inclusion complex were decreased, compared to that of powder alone, which relates to rapid dissociation of the complex, following the dissolution in the gastrointestinal tract. This implies that ketoprofencyclodextrin inclusion complexes might improve the absorption characteristics of ketoprofen.
| Product | Cmax (µg/ml) | AUC 0-5 h (µg. hr/ml) | T max (hr) | Kel |
|---|---|---|---|---|
| Ketoprofen plain | 4.65 ± 0.61 | 9.35 ± 0.68 | 0.66 ± 0.37 | 0.56 ± 0.05 |
| Ketoprofen-β CD complex | 7.15 ± 1.26 | 10.35 ± 1.17 | 0.58 ± 0.19 | 0.44 ± 0.07 |
Table 2: Pharmacokinetic Parameters Of Ketoprofen After Oral Administration To Human Male Volunteers
Acknowledgements
The authors are grateful to Indian Institute of Technology (IIT), Mumbai and Tata Institute of Fundamental Research (TIFR), Mumbai for help in performing characterization studies.
References
- Avouac, B., and Teule, M., J. Clin. Pharmacol., 1988, 28, S2-7.
- Physicians Desk Reference, 55th Edn., Medical Economics Company, Inc., NJ, 2001, 3412.
- Nambu, M., Shimoda, Y., Takahashi, H., Ueda, H. and Nagai T., Chem. Pharm. Bull., 1978, 26, 2952.
- Seo, H., Tsuruoka, M., Hashimoto, T., Fuginaka, T., Otagiri, M. and Uekama, K., Chem. Pharm. Bull., 1983, 31, 286.
- Frank, S.G., J. Pharm. Sci., 1975, 64, 585.
- Duchene, D., In; Cyclodextrins and their Industrial Uses, Editions deSante, Paris, 1987.
- Hirayama, F. and Uekama, K., In; Cyclodextrins and their industrial uses, Editions den Sante, Paris, 1987.
- Brewster, M. E. and Estes, K. S., J. Pharm. Sci., 1988, 77, 981.
- Yoshida, Y., Yamamoto, M., Hirayama, F. and Uekama, K., Chem.Pharm.Bull., 1988, 36, 4075.
- Funk, O., Schwabe, L. and Fromming, K. H., Pharmazie, 1993, 48, 745-747.
- Ahn, H. J., Kim, K. M., Choi, J. S. and Kim, C. K. Drug Dev. Ind.Pharm., 1997, 23, 397-401.
- Higuchi, T. and Connors, K.A., Adv. Anal. Chem. Instrum., 1965, 4, 117.
- Kim, K.H., Frank, M.J. and Henderson, N.L., J. Pharm. Sci., 1985, 74, 283.
- Nakai, Y., Yamamoto, K., Terada, K. and Honbe, H., J. Incl.Phenom., 1984, 2, 523.
- Nakai, Y., Yamamoto, K., Terada, K. and Akimoto, K., Chem.Pharm. Bull., 1984, 38, 685.
- Noyes, A. A. and Whitney, W. R., J. Amer. Chem. Soc., 1897, 19, 930.