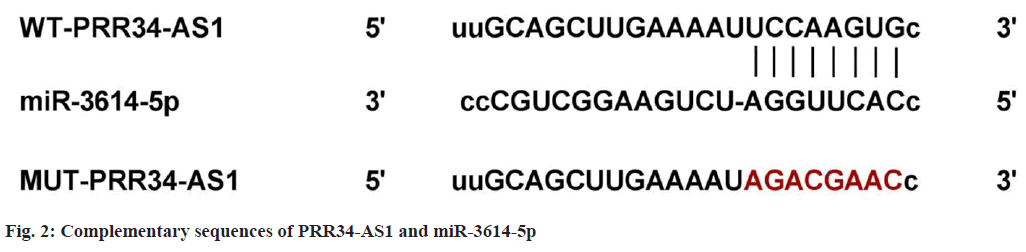- *Corresponding Author:
- Q. Sun
Department of Gastroenterology, Shandong Provincial Third Hospital, Shandong University, Jinan, Shandong 250031, China
E-mail: sunqiang20231214@126.com
| Date of Received | 05 January 2023 |
| Date of Revision | 11 July 2023 |
| Date of Acceptance | 10 November 2023 |
| Indian J Pharm Sci 2023;85(6):1685-1691 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This research explored the possible mechanism of Tripterygium wilfordii in suppressing cancer progression. Cancer coli-2 cells were exposed to Tripterygium wilfordii treatment (0, 20, 40, 80 μmol/l) for 24 h. Cell proliferation, clone formation, and apoptosis were monitored using cell counting kit-8 reagent, plate clone formation, and flow cytometry experiments. PRR34-antisense ribonucleic acid 1, miR-3614-5p, and cleaved-caspase-3 contents were assessed using real-time quantitative reverse transcription assay or Western blot. Dual-luciferase reporter system confirmed the targeting between PRR34-antisense ribonucleic acid 1 and miR-3614-5p. After treatment with various doses of Tripterygium wilfordii, cancer coli-2 cell proliferation, inhibition rate, apoptosis rate, cleaved-caspase-3 protein level and miR-3614-5p expression were increased, and clone formation number as well as PRR34-antisense ribonucleic acid 1 expression were decreased in a concentration-dependent manner. PRR34-antisense ribonucleic acid 1 directly targeted miR-3614-5p. Upregulated PRR34-antisense ribonucleic acid 1 partly abolished Tripterygium wilfordii-induced cancer coli-2 cell proliferation repression and apoptosis promotion via targeting miR-3614-5p. Tripterygium wilfordii might hinder cancer coli-2 cell proliferation by regulating PRR34-antisense ribonucleic acid 1/miR-3614-5p.
Keywords
Colorectal cancer, Tripterygium wilfordii, long noncoding ribonucleic acid, PRR34-antisense ribonucleic acid 1, miR-3614-5p, cell proliferation, apoptosis
As a commonly diagnosed malignancy of the digestive system in China, Colorectal Cancer (CRC) has been recognized as a major challenge that seriously threatens the life of patients[1]. Despite significant advances in surgery and classic adjuvant chemotherapy, patients continue to experience recurrence, metastatic disease, and ultimately experience death[2]. During the past decades, Traditional Chinese medicine (TCM), especially natural products derived from Chinese herbal medicines, have been identified with emerging anti-cancer activities in different human cancers[3]. In fact, TCM has been reported to prevent and treat CRC progression through modulating multiple targets or pathways[4,5]. As a natural anti-inflammatory phytomedicine, Tripterygium wilfordii has the functions of activating blood circulation, clearing collaterals, reducing swelling, and relieving pain. Interestingly, convincing evidence has indicated that multi-glycoside of Tripterygium wilfordii (GTW), extracted from the toot of Tripterygium wilfordii, might promote the apoptosis of epithelial ovarian cancer cells and enhance their sensitivity to cisplatin[6]. However, the specific mechanism of GTW on CRC cell biological behaviors has not been clarified.
Currently, some researchers have suggested that Long Non-Coding Ribonucleic Acids (lncRNAs), a class of evolutionarily conserved non-coding RNAs with lengths exceeding 200 nucleotides, were aberrantly expressed in various tumors, and acted as the competitive endogenous RNA (ceRNA) of microRNAs (miRNAs) to control the occurrence and development of tumors[7]. Notably, some recent literature has implied that LncRNA expression, PRR34-Antisense RNA 1 (PRR34- AS1) was obviously upregulated in Hepatocellular Carcinoma (HCC) cell lines, and its upregulation might boost tumor cell growth and metastasis via regulating several miRNAs[8,9]. Here, based on StarBase analysis, there are some binding sites between PRR34-AS1 and microRNA (miR)-3614- 5p. Beyond that, miR-3614-5p has been confirmed as an underlying novel biomarker for CRC and participates in the modulation of cell migration[10,11]. Therefore, the purpose of this study is to validate the role of GTW block CRC progression via the PRR34-AS1/miR-3614-5p axis.
Materials and Methods
Reagents:
Human CRC cell line Cancer coli-2 (Caco-2) was provided by HonSun Biological (Shanghai, China). GTW (10 mg×100 tablets/bottle) was purchased from Meitong Pharmaceutical (Jiangsu, China). Invitrogen (Carlsbad, California, USA) provided the Total RNA Isolation Reagent (TRIzol) reagent and Lipofectamine™ 2000. Reverse transcription and fluorescent quantitative Polymerase Chain Reaction (PCR) reagents were acquired from Tiangen (Beijing, China). GenePharma (Shanghai, China) offered miR-Negative Control (NC), mimics of miR-3614-5p, small interfering (si) NC, si-PRR34-AS1, plasmid cloning Deoxyribonucleic Acid (pcDNA), and pcDNA-PRR34-AS1. Solarbio (Beijing, China) supplied Cell Counting Kit-8 (CCK-8) reagent, cell apoptosis detection kit, and dual-luciferase activity detection reagent. Promega Corporation (Madison, WI, USA) provided dual- luciferase technology (pmirGLO) gene vectors. Cell Signaling Technology (CST) (Danvers, Massachusetts, USA) offered rabbit anti-human cleaved-caspase-3 antibody and Horseradish Peroxidase (HRP) labeled goat anti-rabbit Immunoglobulin G (IgG) secondary antibodies.
Method:
Cell treatment: According to the previous description[12], 1×104 Caco-2 cells were exposed to GTW at various doses for 24 h, recorded at 0, 20, 40 and 80 μmol/l and was considered to be GTW group. For cell transfection, which included lipofectamine method, si-NC or si-PRR34-AS1 were introduced into Caco-2 cells respectively and was named as si-NC or si-PRR34-AS1 group. Simultaneously, the research conducted transfection of pcDNA or pcDNA-PRR34-AS1 in Caco-2 cells, followed by treatment with 80 μmol/l GTW for 24 h, generating GTW+pcDNA or GTW+pcDNA-PRR34-AS1 group.
CCK-8 assay:
After being treated and collected in 96-well plates, 3×103 Caco-2 cells were mixed with 10 μl CCK-8 solution at 37° for 2 h. Finally, the samples from different groups were monitored under a microplate reader at 450 nm.
Clone formation assay:
500 Caco-2 cells were cultured in 6-well plates in an incubator for 14 d. Then, cells were subjected to 400 μl of 1 % paraformaldehyde fixing agent. The sample number from each group was assessed using a microscope by staining with 1 % crystal violet staining solution (≥50 cells were regarded as 1 clone).
Flow cytometry:
After being processed with 0.25 % trypsin, Caco- 2 cells were subjected to centrifugation at 3 000 r/min for 6 min, re-suspending in binding buffer, and dual-staining with AnnexinV Fluorescein Isothiocyante (FITC) and Propidium Iodide (PI). After 15 min, apoptotic cells were determined using FACS Calibur flow cytometry within an hour.
Real-Time quantitative Reverse Transcription (qRT)-PCR assay:
Using TRIzol reagent, the total RNA from Caco-2 cells was extracted. Then, 2 μg RNA was reverse transcribed to complementary DNA (cDNA), which was used to carry out qRT-PCR reaction. Finally, 2-??Ct method analyzed the obtained data. Dual-luciferase reporter assay: At first, based on StarBase analysis, base complementarity between PRR34-AS1 and miR-3614-5p was acquired. Subsequently, Wild-Type (WT)-PRR34-AS1 and its corresponding Mutant (MUT) were synthesized and respectively inserted into pmirGLO vectors. Then, Caco-2 cell transfection was implemented with these vectors and miR-Negative Control (NC) or miR-3614-5p for 48 h, followed by analysis of cellular luciferase activity.
Western blotting analysis: After the addition of 400 μl Radioimmunoprecipitation Assay (RIPA) buffer, Caco-2 cell proteins were acquired and denatured in boiling water for 10 min. Then, these samples were subjected to Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE), transmembrane, and blocked for 2 h. After incubation with primary antibodies of cleaved-caspase3 (1:1000) and Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) (1:3000), the gray value was analyzed using Image J software before adding secondary antibodies (1:5000) to membranes.
Statistical analysis:
In this research, results were analyzed according to Statistical Package for Social Sciences (SPSS) 21.0 version. p<0.05 was considered to be the significant difference. Meanwhile, measured data were presented as (x? ±s) and compared with t-test and One-Way Analysis of Variance (ANOVA).
Results and Discussion
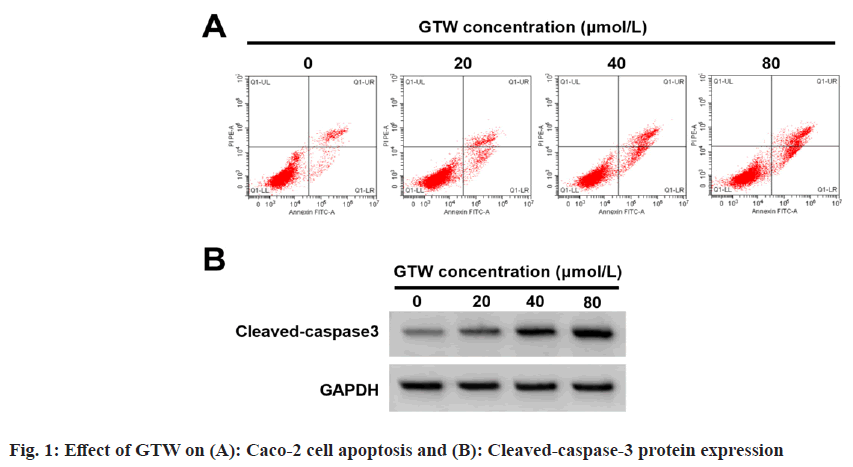
The effects of GTW treatment on Caco-2 cell proliferation and apoptosis was studied. Based on the data displayed in fig. 1A, fig. 1B and Table 1, cell proliferation, inhibition rates, apoptosis rate, and cleaved-caspase-3 protein level were obviously improved with increased GTW dose (p<0.05), and cell colony number was apparently reduced (p<0.05).
| GTW concentration (µmol/l) | Inhibition rate | Apoptosis rate | Colony number | Cleaved-caspase-3 |
|---|---|---|---|---|
| 0 | 0.00±0.00 | 7.81±0.57 | 121.78±7.16 | 0.15±0.02 |
| 20 | 15.35±1.28* | 12.90±0.84* | 100.11±4.15* | 0.28±0.03* |
| 40 | 37.25±2.01*# | 18.11±0.93*# | 78.22±4.34*# | 0.51±0.05*# |
| 80 | 56.31±1.72*#& | 24.02±1.60*#& | 51.78±3.88*#& | 0.74±0.05*#& |
| F | 2540.633 | 390.867 | 315.92 | 386.667 |
| p | <0.05 | <0.05 | <0.05 | <0.05 |
Note: *p<0.05, #p<0.05, and &p<0.05, vs. 0, 20, and 40 μmol/l of GTW concentration respectively
Table 1: Effect of GTW on Caco-2 Cell Proliferation, Inhibition Rates And Apoptosis (X?±s, n=9)
Further, the effects of GTW treatment on PRR34- AS1 and miR-3614-5p expression in Caco-2 cells were also studied. The results from Table 2 exhibited that PRR34-AS1 content gradually decreased in Caco-2 cells after GTW treatment in concentration-dependent ways (p<0.05), whereas miR-3614-5p expression was found to be reinforced (p<0.05).
| GTW concentration (μmol/l) | PRR34-AS1 | miR-3614-5p |
|---|---|---|
| 0 | 1.00±0.00 | 1.00±0.00 |
| 20 | 0.76±0.06* | 1.53±0.08* |
| 40 | 0.51±0.05*# | 2.23±0.10*# |
| 80 | 0.26±0.03*#& | 3.62±0.16*#& |
| F | 522.985 | 1103.457 |
| p | <0.05 | <0.05 |
Note: *p<0.05, #p<0.05, and &p<0.05, with respect to 0, 20, and 40 μmol/l of GTW concentration
Table 2: Effect of GTW on PRR34-AS1 expression and miR-3614-5p expression (X?±s, n=9)
It can be observed that PRR34-AS1 directly targeted miR-3614-5p as it has binding sites with miR-3614-5p (fig. 2). In the experiment of cells co-transfected with WT-PRR34-AS1, cell luciferase activity in miR-3614-5p group was clearly reduced (p<0.05), as shown in Table 3. These results indicated that PRR34-AS1 had a targeted regulation with miR-3614-5p.
| Groups | WT-PRR34-AS1 | MUT-PRR34-AS1 |
|---|---|---|
| miR-NC | 0.93±0.08 | 0.96±0.09 |
| miR-3614-5p | 0.36±0.04* | 1.01±0.12 |
| t | 19.118 | 1 |
| p | <0.05 | 0.332 |
Note: *p<0.05, in comparison with miR-NC group
Table 3: Dual Luciferase Experimental Activity (X?±s, n=9)
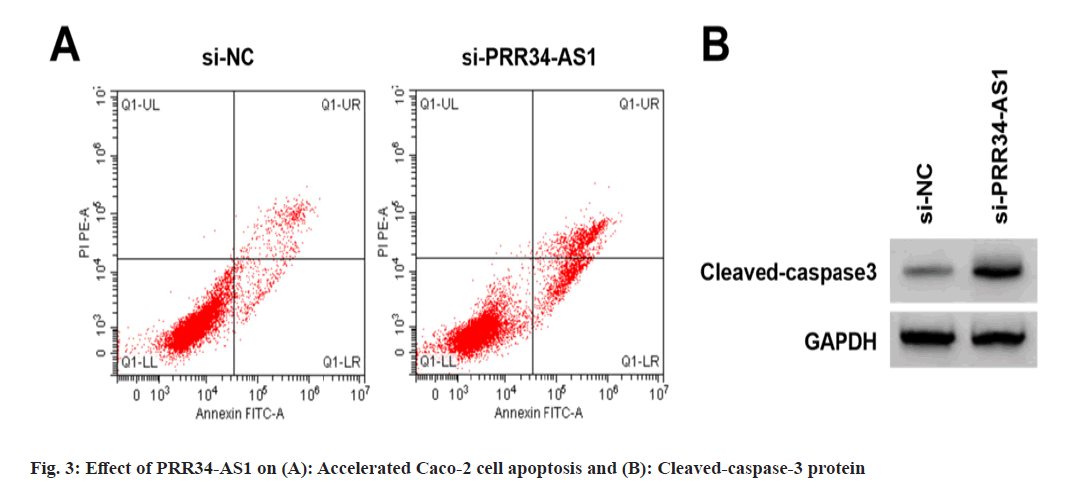
Effects of PRR34-AS1 downregulation on Caco- 2 cell proliferation and apoptosis was explored. According to the data illustrated in fig. 3A, fig. 3B and Table 4, miR-3614-5p expression, proliferation inhibition rates, apoptosis rate, and cleaved-caspase-3 protein were increased in the si- PRR34-AS1 group, and cell colony number was decreased (p<0.05).
| Groups | PRR34-AS1 | miR-3614-5p | Proliferation inhibition rate | Apoptosis rate | The number of cell colonies | Cleaved-caspase3 |
|---|---|---|---|---|---|---|
| si-NC | 1.00±0.00 | 1.00±0.00 | 0.00±0.00 | 7.79±0.57 | 122.33±7.09 | 0.16±0.02 |
| si-PRR34-AS1 | 0.36±0.03* | 3.31±0.08* | 52.29±2.72* | 21.57±1.03* | 65.56±2.27* | 0.58±0.05* |
| t | 64 | 86.625 | 57.673 | 35.117 | 22.877 | 23.398 |
| p | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Note: *p<0.05, vs. si-NC group
Table 4: Effect of PRR34-AS1 Downregulation on Caco-2 Cell Proliferation and Apoptosis (X?±s, n=9)
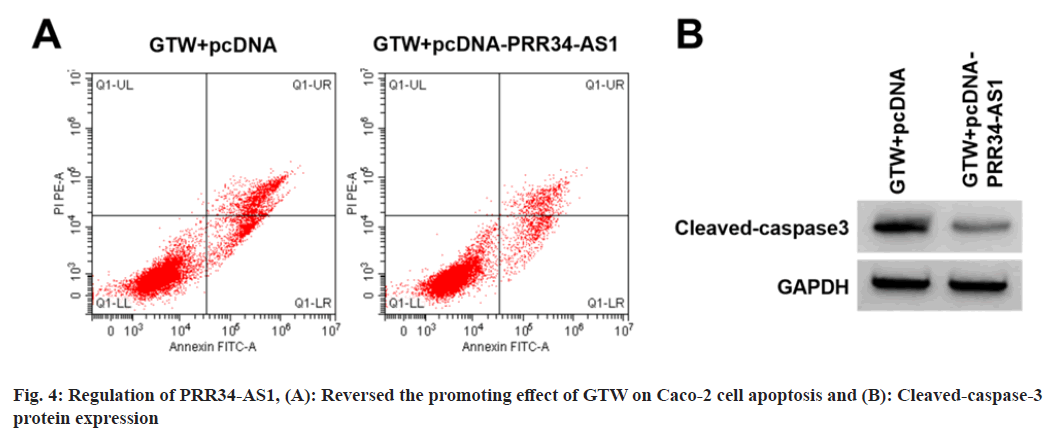
PRR34-AS1 regulated GTW-mediated Caco-2 cell proliferation and apoptosis was analyzed.
Based on the results displayed in fig. 4A, fig. 4B and Table 5, relative to the GTW+pcDNA group, miR- 3614-5p content, proliferation inhibition rates, apoptosis rate, and cleaved-caspase-3 level were elevated in the si-PRR34-AS1 group (p<0.05), cell colony number was reduced (p<0.05).
| Groups | PRR34-AS1 | miR-3614-5p | Inhibition rate | Apoptosis rate | Cell colony number | Cleaved-caspase3 |
|---|---|---|---|---|---|---|
| GTW+pcDNA | 1.00±0.00 | 1.00±0.00 | 56.07±2.88 | 24.15±1.49 | 53.44±1.95 | 0.73±0.07 |
| GTW+pcDNA-PRR34-AS1 | 2.76±0.11* | 0.39±0.04* | 23.42±1.45* | 13.79±1.08* | 91.78±4.78* | 0.34±0.04* |
| t | 48 | 45.75 | 30.378 | 16.889 | 22.28 | 14.512 |
| p | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Note: *p<0.05, relative to GTW+pcDNA group
Table 5: PRR34-AS1 Regulated GTW-Mediated Caco-2 Cell Proliferation and Apoptosis (X?±s, n=9)
With the advancement of medical technology, molecular targeted therapy has become the focus of research, but the morbidity and mortality of CRC are still increasing annually[13]. Convincing evidence shows that the active ingredients extracted from TCM can inhibit tumor cell proliferation and metastasis at different stages of tumorigenesis[14]. Interestingly, many laboratory works have found that lncRNAs-miRNA-messenger RNA (mRNA) regulatory networks could be involved of the progression and development in different human tumors, containing CRC[15]. However, whether lncRNAs might be a potential target of TCM for CRC treatment needs to be further explored.
Notably, recent literature has suggested that GTW, as a Chinese traditional patent medicine, that might enhance the cisplatin sensitivity of ovarian cancer cells by inhibiting the Phosphatidylinositol 3-Kinase/Protein Kinase B/Nuclear Factor kappa B (PI3K/Akt/NF-κB) signaling pathway and thus inducing apoptosis[12]. Herein, our data identified that the CRC cell proliferation inhibition rate was increased and clone formation number was reduced with the increase in the concentration of GTW, implying that GTW might inhibit CRC cell proliferation ability. Beyond that, it has been reported that caspase-3 belongs to the apoptotic execution factor, which might facilitate apoptosis when activated[16,17]. In this work, CRC cell apoptosis rate and cleaved-caspase 3 level were upregulated in GTW-induced CRC cells in a concentration-dependent manner, verifying the promotion of GTW on CRC cell apoptosis. These above findings discovered the anti-tumor role of GTW on CRC development.
Previous literature has revealed that dysregulated PRR34-AS1 might be correlated with many tumor processes. For example, PRR34-AS1 expression is upregulated in acute myeloid leukemia and its expression is associated with poor patient prognosis[18]. Meanwhile, PRR34-AS1 has been reported as an aging-related lncRNA, which might perform the early breast cancer diagnosis and therapeutic target[19]. Furthermore, it has been widely accepted that PRR34-AS1 mainly serves as miRNA sponges to exert diverse biological roles[8,9]. Here, we preliminarily demonstrated that PRR34- AS1 can target binding miR-3614-5p. Some studies have indicated that abnormally expressed miR- 3614-5p might suppress the growth and metastasis of multiple tumors, containing CRC cells[10,11]. Herein, our study’s first step documented that PRR34-AS1 expression was significantly reduced in GTW-triggered CRC cells, and miR-3614-5p content was increased. Simultaneously, functional analysis discovered that down-regulation of PRR34-AS1 might nullify GTW-mediated proliferation inhibition and apoptosis promotion in CRC cells, whereas the phenomenon was partly counteracted by interacting with miR-3614-5p. It is suggested that the anti-cancer effect of GTW on CRC was mediated by PRR34-AS1/miR-3614-5p axis, which provided potential targets of GTW in the treatment of CRC.
In summary, GTW might block CRC cell proliferation and boost apoptosis by inhibiting PRR34-AS1 expression and promoting miR-3614- 5p level. These results suggested that PRR34-AS1 and miR-3614-5p may be potential targets for molecular targeting of CRC, which may lay the experimental foundation for further revealing the molecular mechanism of GTW in CRC.
Acknowledgements:
Fanlu Meng and Yanbo Wang contribute same to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Qu R, Ma Y, Zhang Z, Fu W. Increasing burden of colorectal cancer in China. Lancet Gastroenterol Hepatol 2022;7(8):700.
[Crossref] [Google Scholar] [PubMed]
- Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin 2022;72(4):372-401.
[Crossref] [Google Scholar] [PubMed]
- Zhang X, Qiu H, Li C, Cai P, Qi F. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends 2021;15(5):283-98.
[Crossref] [Google Scholar] [PubMed]
- Chen JF, Wu SW, Shi ZM, Hu B. Traditional Chinese medicine for colorectal cancer treatment: Potential targets and mechanisms of action. Chin Med 2023;18(1):1-31.
[Crossref] [Google Scholar] [PubMed]
- Wang K, Chen Q, Shao Y, Yin S, Liu C, Liu Y, et al. Anticancer activities of TCM and their active components against tumor metastasis. Biomed Pharmacother 2021;133:1-17.
[Crossref] [Google Scholar] [PubMed]
- Liu WC, Tan BZ, Zhan XL. Effect of Tripterygium glycosides on cisplatin-resistant SKOV3/DDP cell line in vitro. Chin J Clin Pharmacol Ther 2017;22(12):1364-70.
- Yuan W, Li X, Liu L, Wei C, Sun D, Peng S, et al. Comprehensive analysis of lncRNA-associated ceRNA network in colorectal cancer. Biochem Biophys Res Commun 2019;508(2):374-9.
[Crossref] [Google Scholar] [PubMed]
- Qin M, Meng Y, Luo C, He S, Qin F, Yin Y, et al. lncRNA PRR34-AS1 promotes HCC development via modulating Wnt/β-catenin pathway by absorbing miR-296-5p and upregulating E2F2 and SOX12. Mol Ther Nucl Acids 2021;25:37-52.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Li Z, Xu B, Yao H, Qi S, Tai J. Long noncoding RNA PRR34-AS1 aggravates the progression of hepatocellular carcinoma by adsorbing microRNA-498 and thereby upregulating FOXO3. Cancer Manag Res 2022;14:2053-4.
[Crossref] [Google Scholar] [PubMed]
- Han L, Sun Y, Lu C, Ma C, Shi J, Sun D. miR-3614-5p is a potential novel biomarker for colorectal cancer. Front Genet 2021;12:1-10.
[Crossref] [Google Scholar] [PubMed]
- Yao H, Zhou X, Zhou A, Chen J, Chen G, Shi X, et al. RFC5, regulated by circ_0038985/miR-3614-5p, functions as an oncogene in the progression of colorectal cancer. Mol Carcinog 2023;62(6):771-85.
[Crossref] [Google Scholar] [PubMed]
- Li T, Zhou HC, Chen J. Effects of Tripterygium glycosides on the proliferation, apoptosis and PI3K/AKT signaling pathway of human oral cancer KB cells. J Guangdong Pharm Univ 2019;35(5):653-7.
- Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin 2022;72(4):372-401.
[Crossref] [Google Scholar] [PubMed]
- Wu KC, Chu PC, Cheng YJ, Li CI, Tian J, Wu HY, et al. Development of a traditional Chinese medicine-based agent for the treatment of cancer cachexia. J Cachexia Sarcopenia Muscle 2022;13(4):2073-87.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Cho KB, Li Y, Tao G, Xie Z, Guo B. Long noncoding RNA (lncRNA)-mediated competing endogenous RNA networks provide novel potential biomarkers and therapeutic targets for colorectal cancer. Int J Mol Sci 2019;20(22):1-26.
[Crossref] [Google Scholar] [PubMed]
- Shi J, Zhong X, Song Y, Wu Z, Gao P, Zhao J, et al. Long non-coding RNA RUNX1-IT1 plays a tumour-suppressive role in colorectal cancer by inhibiting cell proliferation and migration. Cell Biochem Funct 2019;37(1):11-20.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Li Q, Xue B, He R. MALAT1 inhibits the Wnt/β-catenin signaling pathway in colon cancer cells and affects cell proliferation and apoptosis. Bosnian J Basic Med Sci 2020;20(3):357.
[Crossref] [Google Scholar] [PubMed]
- Nan FY, Gu Y, Xu ZJ, Sun GK, Zhou JD, Zhang TJ, et al. Abnormal expression and methylation of PRR34?AS1 are associated with adverse outcomes in acute myeloid leukemia. Cancer Med 2021;10(15):5283-96.
[Crossref] [Google Scholar] [PubMed]
- Liu Z, Ren C, Cai J, Yin B, Yuan J, Ding R, et al. A novel aging-related prognostic lncRNA signature correlated with immune cell infiltration and response to immunotherapy in breast cancer. Molecules 2023;28(8):1-22.
[Crossref] [Google Scholar] [PubMed]