- *Corresponding Author:
- Jing Hu
Faculty of Laboratorial Medicine, Key Laboratory of Medical Diagnostics Designated by the Ministry of Education, Chongqing Medical University, Chongqing 400016, China
E-mail: jessie805@qq.com
| Date of Received | 30 June 2022 |
| Date of Revision | 10 March 2023 |
| Date of Acceptance | 03 June 2023 |
| Indian J Pharm Sci 2023;85(3):621-631 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Ouabain has shown powerful anti-proliferation activities in various cancers, but its effect on imatinibresistant chronic myeloid leukemia and toxicity on normal mice has not been investigated. Cell Counting Kit-8 assay was used to detect cytotoxicity and reversal effect of ouabain with different concentration (0.01 μM, 0.1 μM, 1.0 μM, 10 μM) on drug resistance of imatinib-resistant cell line of chronic myeloid leukemia (K562/G01 cell line). Flow cytometry was used to detect the apoptosis effect and cell cycle arrest. Hematological examination, biochemical examination and histological examination were used to detect sub-chronic toxicity of ouabain on healthy mice. In our present study, ouabain showed greatly inhibitory effect and significantly reduced half minimal inhibitory concentration of imatinib in K562/ G01 cells, an imatinib-resistant cell line of chronic myeloid leukemia, in a dose- and time- dependent manner, which implied that ouabain increased cell sensitivity to imatinib. Ouabain enhanced apoptosis induced by imatinib in K562/G01 cells not through cell cycle arrest. Animal experiments showed that there were no significant variances in hematological, liver function, kidney function parameters and organ histopathology of all mice groups. These data suggested that ouabain could be a potential agent to treat imatinib-resistant chronic myeloid leukemia for its powerful cytotoxicity as well as reversal effect, but further study is needed to find out its specific mechanism.
Keywords
Chronic Myeloid Leukemia (CML) is a clonal hematopoietic stem cell malignant tumor featured by Philadelphia chromosome, with an estimate of 34 179 cases and 24 054 deaths reported in 2017[1]. BCR-ABL1, a tyrosine kinase with aberration is identified as the key driver in CML, resulting from the fusion of the regulatory regions of BCR and the enzymatic regions of ABL1[2]. Although Tyrosine Kinase Inhibitors (TKIs) such as Imatinib Mesylate (IM) bring a remarkable improvement on the survival rate of early-stage patients, about 40 % of patients with chronic phase have to give up IM because of failure and/or intolerance[3], and the drug resistance associated with IM can’t prevent the recurrence and progression of the disease, which has become one of the most important causes of death in patients with CML. Therefore, new drugs that are capable of reversing IM resistance are particularly needed.
Ouabain (fig. 1a) is a rapid-acting Cardiac Glycoside (CG) obtained from seeds of Strophanthus gratus[4]. The controversy over the safety of CGs has existed for a long period chiefly because of its narrow therapeutic window and cardiac toxicity in clinical research[5], but the clinical security of ouabain is less studied. Ouabain increases intracellular Ca2+ concentration by inhibiting Na+/K+ ATPase, leading to the improvement of myocardial contractility. In addition to its traditional effects, recent researches have proved that ouabain possess abundant healthpromoting properties such as anti-inflammatory effect[6,7], anti-coronavirus effect[8], anti-cancer effect[9,10], and reversal effect on drug resistance[11,12].
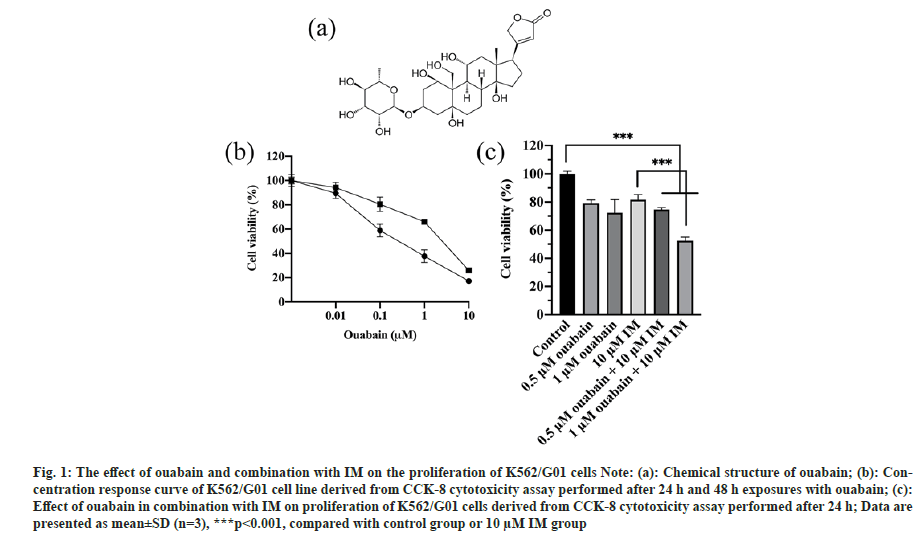
Fig. 1: The effect of ouabain and combination with IM on the proliferation of K562/G01 cells
Note: (a): Chemical structure of ouabain; (b): Concentration
response curve of K562/G01 cell line derived from CCK-8 cytotoxicity assay performed after 24 h and 48 h exposures with ouabain; (c):
Effect of ouabain in combination with IM on proliferation of K562/G01 cells derived from CCK-8 cytotoxicity assay performed after 24 h; Data are
presented as mean±SD (n=3), ***p<0.001, compared with control group or 10 μM IM group
Ouabain was previously reported to be able to target human leukemia[13], including strongly interfering intracellular redox homeostasis in lymphocytic leukemia cells[14] and regulating activities of immune cells in vivo[15]. Nevertheless, its specific effect on imatinib-resistant CML has not been detected.
In the present study, we explored the effect of ouabain in CML imatinib-resistant K562/G01 cells, an IMresistant cell line of CML, as well as its possible mechanisms, and explored its toxic effect on normal mice, in order to provide more choices for clinical therapy.
Materials and Methods
Preparation of drug stock solution:
The stock solution of IM (Sigma, USA) was prepared by dissolving 10 mg IM in 1.6958 ml Dimethyl Sulfoxide (DMSO) (Solarbio, China) and 15.2622 ml triple steamed water to give 1 mM of stock solution. Ouabain was dissolved in DMSO and prepared into stock solution with a concentration of 10 mM. After filtration and sterilization, store them at -20°.
Cell lines and cell culture:
K562/G01 cells, preserved by our laboratory (Chongqing, China), were cultured in Roswell Park Memorial Institute-1640 medium (Gibco, U.S.A.) containing 10 % fetal bovine serum (Gibco, U.S.A.), 100 μg/ml streptomycin (Hyclone, U.S.A.) and 100 U/ml penicillin (Hyclone, U.S.A.), and placed in 37°, 5 % CO2 as well as saturated humidity incubator. Cells growing well and in the logarithmic growth period were selected for experiments.
Cytotoxicity assay:
About 5×103 cells per well were seeded in 96-well plates and incubated with different concentrations of ouabain or IM for 24 h or 48 h and the control group was treated with an equal amount of medium. 10 μl Cell Counting Kit-8 (CCK-8) solution (Taosu, China) was added into each well and the plates were incubated at 37° for another 3 h. The absorbance was measured at the wavelength of 450 nm. Calculated the cell variety (%)=[(OD sample– OD blank)/(OD control–OD sample)]×100 %. The IC50 of IM was estimated by Prism 5 (GraphPad Software, U.S.A). The drug resistance Reversal Fold (RF) values were calculated by dividing the IC50 with IM alone by those with IM and ouabain in K562/G01 cells.
Cell apoptosis assay by flow cytometry:
About 5×105 cells per well were seeded in 6-well plates, and cultured with IM (10 μM) or ouabain (0.5 μM, 1 μM) or combination of both medicines. Cells were washed twice with Phosphate Buffered Saline (PBS) after 24 h, and incubated with 5 μl of Annexin-V Fluorescein Isothiocyante (FITC) labeling solution as well as Propidiumiodide (PI) for 15 min as manufacturer’s instruction. The flow cytometry was used to analyze cell apoptosis.
Cell cycle assay by flow cytometry:
About 5×105 cells per well were seeded in 6-well plates, and cultured with IM (10 μM) or ouabain (0.5 μM, 1 μM) or combination of both medicines. After 24 h, cells were washed twice with PBS and then the cell suspension was added into 75 % cold ethanol and stored at 4° overnight. After cells were washed with PBS, RNase as well as PI stain solution was added. The samples were tested by flow cytometry.
Experimental animals:
Healthy Kunming (KM) mice (n=24), 6 w old, 18-22 g in weight, specific pathogen free grade, with certificate No. SCXK (YU) 2018-0003, were purchased and kept at the Experimental Animal Center of Chongqing Medical University. Mice were reared in cages according to gender (3 per cage) and adapted for 1 w. Room temperature and humidity were strictly controlled and food and acidified water were freely available. All procedures of animals involved in this experiment were in compliance with the Regulations for the Administration of Affairs Concerning Experimental Animals and were approved by the Animal Ethics Committee of Chongqing Medical University .
Animal grouping and treatment:
Mice (n=12 female; 12 male) were randomly divided into four groups (n=3/sex/group), highdose group (1.0 mg/kg ouabain), medium-dose group (0.5 mg/kg ouabain), low-dose group (0.1 mg/kg ouabain) and control group (given an equal amount of saline). Mice were injected (i.p.) every other day for 30 d. After administration, mice were anaesthetized and hematological examination, biochemical examination and histological examination were taken to detect toxicity of ouabain on normal mice.
Observation of general physical signs of mice:
Observed and recorded the daily activities, mental states, food intakes and defecation of mice every day, and weighed them every 2 d, expressing it as weight ratio (weight ratio=weight/initial weight).
Routine blood test:
After administration, blood was adopted by cutting the tails and mixed with the dilution liquid to measure erythrocytes, Mean Corpuscular Hemoglobin (MCH), leukocytes, Mean Corpuscular Volume (MCV), MCH Concentration (MCHC), hemoglobin and Platelet (PLT) with an automatic blood analyzer.
Alanine Aminotransferase (ALT) and Creatinine (CREA) test:
After administration, serum of mice was collected by centrifugation at 3000 r/min for 20 min, and the ALT and CREA were analyzed with kits (Jiancheng, China).
Relative organ/body weight ratio and histological examination:
After euthanasia, necropsy was performed immediately. The heart, liver, kidney, lung and spleen were harvested, and were expressed as relative organ/body weight ratio after measuring. Organs were fixed in 4 % paraformaldehyde for 24 h, then dehydration, paraffin embedding and slicing were performed. Finally, Paraffinembedded slices were stained with hematoxylin and eosin for further histological examination.
Statistical analysis:
All data were tested by IBM SPSS 26.0 software and represented by mean±Standard Deviation (SD). Comparisons between two groups were performed by Student-t test while comparisons between multiple groups were one-way analysis of variance. p<0.05 was defined as statistical significance.
Results and Discussion
We estimated the cytotoxicity of ouabain to K562/ G01 cells by performing CCK-8 assays. After cells were treated with ouabain at concentrations of 0.01, 0.1, 1, 10 μM respectively for 24 and 48 h, the results confirmed that ouabain obviously decreased the proliferation of K562/G01 cells in a dose- and time- dependent manner (fig. 1b).
In order to assess the effect of ouabain on IMsensitivity of K562/G01 cells, cells were treated with IM alone or combination for 24 h, then we tested cell proliferation by CCK-8 assays. The combination of ouabain with 1 μM and IM showed significant difference on cell viability as compared with group only treated with IM alone, revealing that high concentration of ouabain could definitely promote sensitivity of IM to K562/G01 cells, while inhibitory effect on growth of cells which were incubated with IM and ouabain at the concentration of 0.5 μM revealed no statistically significance (fig. 1c). Besides, the IC50 of IM in the K562/G01 cells decreased with increasing concentration of ouabain as is shown in Table 1. Ouabain greatly reduced the IC50 of IM in K562/G01 cells from 94.92 to 69.56 μM at the lowest concentration (0.01 μM), allowing RF value to achieve 1.36. Moreover, a 131.83-fold increase was induced when cells were treated with 10 μM ouabain. These results demonstrated that ouabain was potential to reverse IM resistance in K562/G01 cells.
| Group | IC50 of IM (μM)±SD | RF |
|---|---|---|
| K562/G01+0 μM ouabain | 94.92±2.20 | - |
| K562/G01+0.01 μM ouabain | 69.56±2.57 | 1.36 |
| K562/G01+0.1 μM ouabain | 61.08±8.56 | 1.55 |
| K562/G01+1.0 μM ouabain | 13.32±1.58 | 7.13 |
| K562/G01+10 μM ouabain | 0.72±0.33 | 131.83 |
Table 1: OUABAIN Modulated K562/G01 Cells Sensitivity
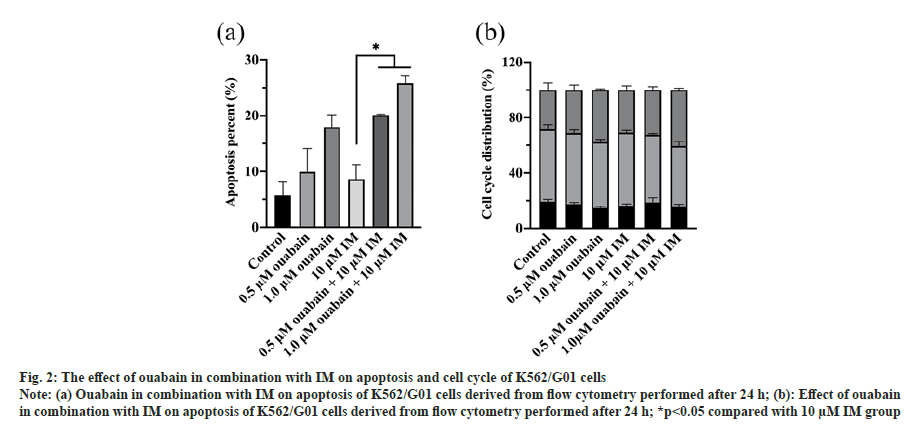
In order to assess effect of ouabain in combination with IM on apoptosis of K562/G01 cells, cells were treated with single drug or combination for 24 h, then tested cell apoptosis by Annexin V-FITC/PI double staining. Our flow cytometry results demonstrated that ouabain potentiated IM-induced apoptosis. As shown in fig. 2a, 0.5 or 1 μM ouabain or 10 μM IM slightly enhanced the apoptosis of K562/G01 cells with the rates of 9.94±4.18 %, 17.97±2.2 %, 8.64±2.59 %, respectively. In contrast, the apoptotic percent was 20.11±0.16 % and 25.81±1.4 % when 10 μM IM was combined with 0.5 or 1 μM ouabain.
In order to identify if the growth inhibitory effect of ouabain was triggered by cell cycle dysfunction, cell cycle distribution was detected by the PI-based technique. We found that the cells in G0/G1, S or G2/M phase had no statistically difference whether treated with single drug or combination, even though the G2/M phase increased in experimental groups (fig. 2b).
Fig. 2: The effect of ouabain in combination with IM on apoptosis and cell cycle of K562/G01 cells
Note: (a) Ouabain in combination with IM on apoptosis of K562/G01 cells derived from flow cytometry performed after 24 h; (b): Effect of ouabain
in combination with IM on apoptosis of K562/G01 cells derived from flow cytometry performed after 24 h; *p<0.05 compared with 10 μM IM group
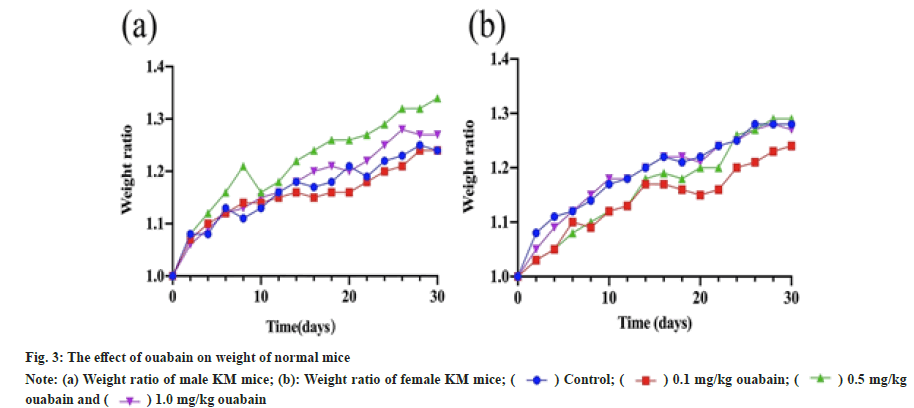
It was observed that the daily activities, mental states, food intakes and defecation of mice in all groups were normal during the medication. At concentration of 0.5 mg/kg, weight of both female and male mice showed maximum increment, while growth of those that handled with 0.1 mg/kg ouabain seemed to be the slowest (fig. 3). However, none of the treatment groups showed statistically difference versus control group (p>0.05) (Table 2), and the growth trend of all groups was generally uniform (fig. 3).
| Time (d) | Sex | Control group | 0.1 mg/kg Ouabain | 0.5 mg/kg Ouabain | 1.0 mg/kg Ouabain |
|---|---|---|---|---|---|
| 0 d | female | 30.70±0.53 | 29.13±1.56 | 33.63±2.05 | 29.63±0.32 |
| male | 32.30±1.25 | 32.60±0.10 | 31.07±2.31 | 33.13±1.97 | |
| 2 d | female | 33.27±1.87 | 30.03±1.89 | 34.63±3.26 | 31.00±2.26 |
| male | 35.13±1.30 | 34.97±0.12 | 33.63±2.51 | 35.07±1.68 | |
| 4 d | female | 33.93±2.40 | 30.67±1.16 | 35.47±2.51 | 32.40±1.31 |
| male | 35.03±1.66 | 35.97±0.06 | 34.93±2.42 | 36.07±1.59 | |
| 6 d | female | 34.33±2.66 | 32.07±1.39 | 36.43±3.32 | 33.10±1.23 |
| male | 36.40±1.71 | 36.37±0.71 | 35.97±2.11 | 37.03±1.46 | |
| 8 d | female | 35.03±3.37 | 31.78±1.95 | 36.83±3.10 | 34.20±1.35 |
| male | 36.60±0.95 | 37.33±1.00 | 36.00±1.21 | 38.13±1.70 | |
| 10 d | female | 35.93±3.37 | 32.67±1.70 | 37.83±2.20 | 34.93±1.69 |
| male | 36.60±0.95 | 37.33±1.00 | 36.00±1.21 | 38.13±1.70 | |
| 12 d | female | 36.17±3.07 | 33.03±1.72 | 38.10±2.81 | 35.07±2.41 |
| male | 37.37±1.00 | 37.63±0.76 | 36.57±1.03 | 38.30±1.71 | |
| 14 d | female | 36.73±2.58 | 33.97±2.29 | 39.73±3.11 | 35.63±1.45 |
| male | 38.17±0.75 | 37.83±1.01 | 38.07±0.91 | 39.13±1.56 | |
| 16 d | female | 37.43±2.74 | 34.10±1.92 | 39.93±2.98 | 36.17±1.94 |
| male | 38.00±0.62 | 37.80±0.89 | 39.20±1.22 | 39.83±1.78 | |
| 18 d | female | 37.20±3.41 | 33.78±2.31 | 39.80±2.69 | 36.20±0.91 |
| male | 38.10±0.52 | 37.87±0.76 | 39.27±1.21 | 40.03±1.84 | |
| 20 d | female | 37.40±3.70 | 33.43±2.00 | 40.43±2.46 | 36.00±1.56 |
| male | 38.00±0.62 | 37.80±0.89 | 39.20±1.22 | 39.83±1.78 | |
| 22 d | female | 37.93±3.10 | 33.67±1.61 | 40.33±2.93 | 36.83±2.02 |
| male | 38.47±0.50 | 38.50±1.32 | 39.33±1.15 | 40.50±1.32 | |
| 24 d | female | 38.40±3.82 | 34.87±1.85 | 42.33±2.51 | 37.20±1.42 |
| male | 39.47±0.64 | 39.13±0.87 | 40.23±2.14 | 41.53±1.85 | |
| 26 d | female | 39.33±2.80 | 35.23±1.29 | 42.80±3.48 | 36.67±1.95 |
| male | 39.67±0.83 | 39.60±1.61 | 41.13±1.53 | 42.37±1.46* | |
| 28 d | female | 39.20±3.18 | 35.90±1.22 | 43.40±3.15 | 37.87±1.80 |
| male | 40.53±0.59 | 40.30±1.31 | 41.17±2.44 | 42.13±1.17 | |
| 30 d | female | 39.43±3.59 | 36.00±1.23 | 43.27±3.21 | 37.67±0.98 |
| male | 40.13±0.75 | 40.50±1.00 | 41.57±2.28 | 42.13±1.16 |
Note: Values are expressed as means±SD (n=6); *p<0.05 compared with control group
Table 2: The Effect of OUABAIN on Weight of KM Mice (g)
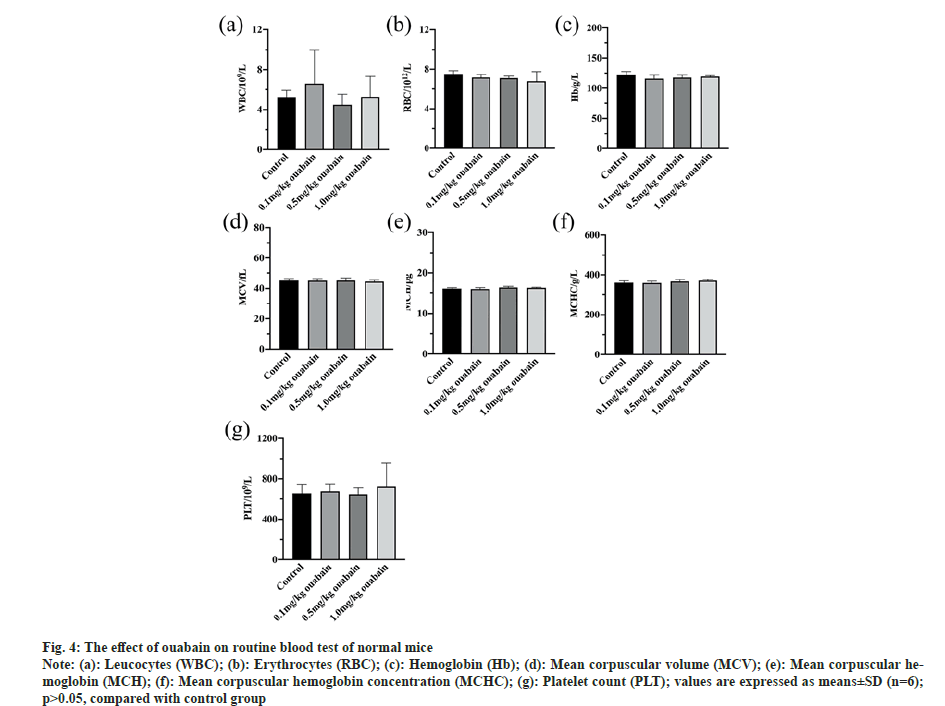
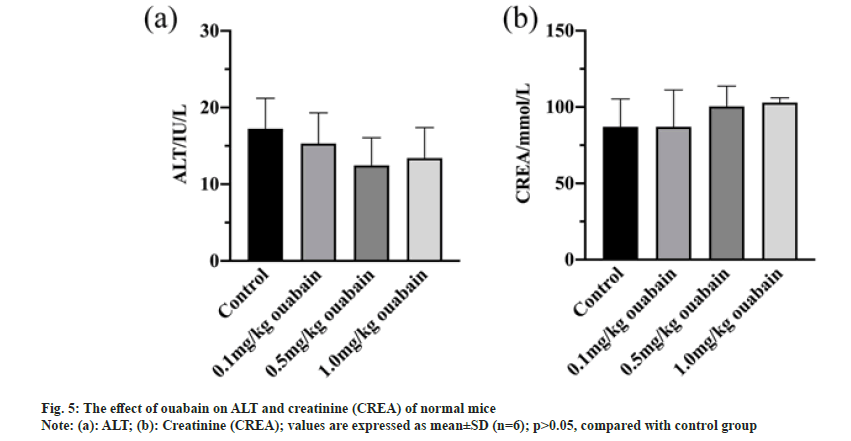
Blood tests were conducted after administration. In terms of the routine blood test, all of items of mice that were administrated with ouabain at low (0.1 mg/kg), middle (0.5 mg/kg) and high dose (1.0 mg/kg), were little different from those of control group (p>0.05) (fig. 4). ALT and creatinine CREA, which are sensitive liver and kidney damage markers respectively, were used to estimate effects of ouabain on liver function and kidney function of KM mice. Whether ALT or CREA, there were no evident variances between treatment and control groups (p>0.05) (fig. 5).
Fig. 4: The effect of ouabain on routine blood test of normal mice
Note: (a): Leucocytes (WBC); (b): Erythrocytes (RBC); (c): Hemoglobin (Hb); (d): Mean corpuscular volume (MCV); (e): Mean corpuscular hemoglobin
(MCH); (f): Mean corpuscular hemoglobin concentration (MCHC); (g): Platelet count (PLT); values are expressed as means±SD (n=6);
p>0.05, compared with control group
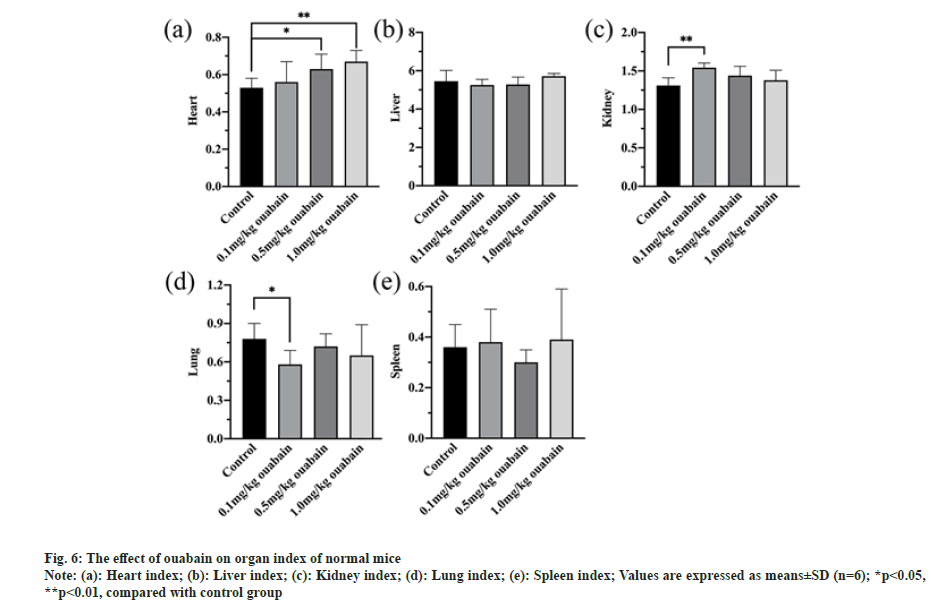
Relative organ/body weight ratio is a common indicator in toxicology study to assess the effect on the organ. The heart/body weight ratio increased when the dosage increased, and that of both middle-dose group (p<0.05) and high-dose group (p<0.01) were much more than control group (fig. 6a), revealing that hearts of those mice may develop hyperemia or hypertrophy .
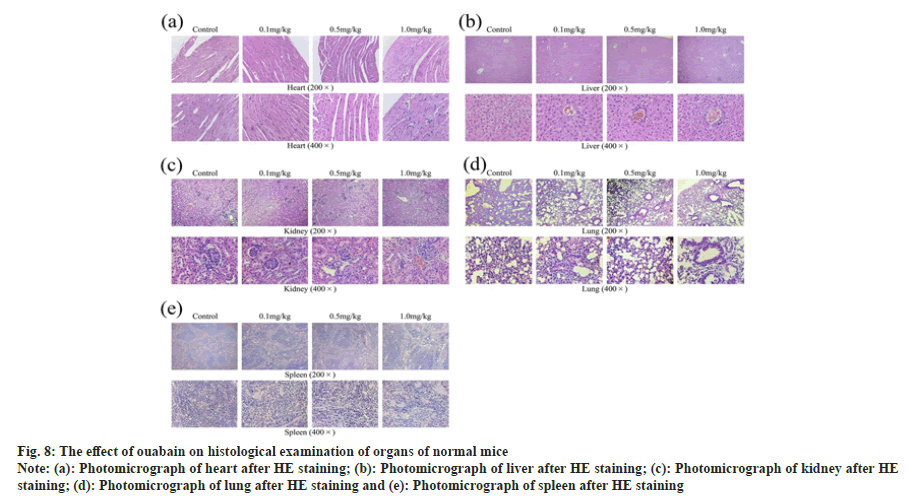
Given that the difference of kidney/body weight ratio (p<0.01) and lung/body weight ratio (p<0.05) only showed in low-dose group (fig. 6c and fig. 6d), it may result from experimental error. In comparison with control group, there were no evident abnormal changes in the organs we tested of treat group whether by naked eyes or under microscope (fig. 7 and fig. 8). No edema, inflammatory infiltration, cell degeneration, necrosis and other pathological reactions were found in heart, liver, spleen, kidney and lung of KM mice (fig. 8).
Fig. 8: The effect of ouabain on histological examination of organs of normal mice
Note: (a): Photomicrograph of heart after HE staining; (b): Photomicrograph of liver after HE staining; (c): Photomicrograph of kidney after HE
staining; (d): Photomicrograph of lung after HE staining and (e): Photomicrograph of spleen after HE staining
Ouabain was formerly reported to play antileukemia efficacy via inducing apoptosis of leukemia cells[16], impacting redox homeostasis[14] and targeting related signaling pathway[17], but the role of ouabain in imatinib-resistant CML remains unclear. Therefore, we assessed its cytotoxicity with different concentrations as well as different treatment duration to investigate the therapeutic potential of ouabain against K562/ G01 cells. Our CCK-8 data demonstrated that ouabain could inhibit cell viability in a timeand dose- dependent manner and even low concentration (0.01 and 0.1 μM) of ouabain was able to produce cytotoxicity effect on K562/ G01 cells, revealing that ouabain was strikingly competent to inhibit the proliferation of K562/ G01 cells.
In order to further clarify whether ouabain could increase the sensitivity of drug-resistant cells to IM, we compared proliferation and apoptosis of K562/G01 cells incubated with single drug or combination. Our data depicted that the inhibitory effect of ouabain with high concentration (1 μM) combined with IM was significantly stronger than the single IM on cell proliferation as well as induction of apoptosis. These results could be connected with the role of ouabain in the regulation of intracellular calcium increase, as the mitochondrial Ca2+ overload was proved to trigger cytochrome c to release, resulting in the activation of caspase 9, which took a crucial part in cell apoptosis[18]. Moreover, the RF increased with the increase of concentration, illustrating that ouabain could definitely inhibit IM resistance in K562/G01 cells in a concentration dependent manner.
As reported, ouabain arrested cell cycle at S and G2/M phase for hepatocellular carcinoma cells[19] and melanoma cells[20], respectively. However, it is worth noting that effect of ouabain against K562/G01 cells was unrelated to cell cycle arrest according to our data, revealing that ouabain increased K562/G01 cells drug sensitivity through other mechanisms probably due to the drug resistance of that cell line.
Previous data exhibited that ouabain could reverse MDR to cisplatin in esophageal carcinoma cells through targeting Wnt/β-catenin and inhibiting the translocation of β-catenin into the nucleus[12], rather than cell cycle arrest, which has been deemed as one of the key signaling pathways of CML resistance towards TKIs[21]. On the one hand, the over activation of Wnt/β-catenin brings about nuclear accumulation of β-catenin and abnormal expression of downstream target genes, rendering granulocyte-macrophage progenitors to show self-renewal capacity, which isn’t likely to be possessed normally, causing arrant proliferation of CML cells[22]. On the other hand, β-catenin keeps Leukemic Stem Cell (LSC) maintenance and survival, which is recognized as a crucial mechanism that induces TKIs resistance[21]. Therefore, it would be tempting to suppose that the inhibitory effect of ouabain on Wnt/β-catenin signaling pathway may be relevant with the potentiation of IMinduced cytotoxicity and we intend to verify that hypothesis in the subsequent study.
In recent years, ouabain has shown powerful anti-cancer effects, but its toxicity should not be overlooked in extremely complex human body as most of researches mainly focused on cellular mechanism. Our preliminary study on toxicity of ouabain on normal mice showed that as compared with the control group, there were no evident variances in hematological parameters, ALT, CREA, and organ histopathology of experimental groups after administration of drug. Though there was a slight fluctuation of growth trend, it was reasonable to consider that ouabain hardly affected weight of mice, given that different eating habits or other non-drug factors may interfere with ultimate results. However, heart/body weight ratio greatly increased at dose of 1.0 mg/kg, suggesting that high dose of ouabain might exert adverse effect on heart of normal mice. In line with our results, some literatures stated that ouabain could cause serious cardiotoxicity including cardiomyocytes apoptosis[23,24] and cardiac remodeling[25]. This attributed to the induction of spontaneous contractile activity, mitochondrial Ca2+ overload and cardiomyocyte production of Reactive Oxygen Species (ROS)[24-26]. As for the difference of lung/body weight ratio and kidney/ body weight ratio, we supposed that they were data deviations in given the limited number of mice and normality of histological examination. Therefore, more experimental samples are required to be included to make more accurate conclusions in further studies. Ouabain was also reported to destroy Spiral Ganglion Neurons (SGNs) in rats, gerbils and mice[27], while it could protect neurons in nanomolar concentration[28] via modulating intracellular Ca2+ level and signal pathways including mTOR pathway[29], Wnt/β- catenin pathway[30] and apoptosis pathways[31]. Furthermore, another research illustrated that ouabain didn’t exert significant toxicity neither on CD34 cells from healthy people nor on AML mice as estimated by weight loss and mortality[17]. Considering the dual effects of ouabain vary substantially across dose and duration of drug treatment, the exact dosage range also needs to be pinned down, which could be the premise of clinical treatment. Anyway, our pre-clinical research suggested that ouabain only produce a degree of cardiac toxicity in a dose dependent manner and might be a safe drug to healthy mice in general.
In conclusion, our results demonstrated the anti-proliferation effect of ouabain on K562/ G01 cells and ouabain in combination with IM results in more remarkable growth inhibition than did IM alone on K562/G01 cells, which pointed to ouabain as a potential agent to increase the sensitivity of drug-resistant cells to IM. Moreover, long-term application of ouabain did not show obvious toxicity on normal mice generally. Nevertheless, further studies are required to clarify its exact mechanism on TKIs resistance as well as clinically appropriate dose.
Author Contributions:
Chang Sun, J. Liu and Shiqi Zhai contributed equally to this work.
Acknowledgements:
This study was supported by the Natural Science Foundation of Chongqing (CSTB2022NSCQMSX0764), and the College Students Innovative Experimental Program of Chongqing Medical University (SRIEP202010).
Conflict of interests:
The authors declare that they have no competing interest.
References
- Lin Q, Mao L, Shao L, Zhu L, Han Q, Zhu H, et al. Global, regional, and national burden of chronic myeloid leukemia, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Front Oncol 2020;10:580759.
[Crossref] [Google Scholar] [PubMed]
- Ross DM, Hughes TP. Treatment-free remission in patients with chronic myeloid leukaemia. Nat Rev Clin Oncol 2020;17(8):493-503.
- Yurttaş NÖ, Eşkazan AE. Novel therapeutic approaches in chronic myeloid leukemia. Leuk Res 2020;91:106337.
[Crossref] [Google Scholar] [PubMed]
- Van Wyk BE. A review of commercially important African medicinal plants. J Ethnopharmacol 2015;176:118-34.
[Crossref] [Google Scholar] [PubMed]
- Whayne TF. Clinical use of digitalis: A state of the art review. Am J Cardiovasc Drugs 2018;18:427-40.
[Crossref] [Google Scholar] [PubMed]
- Cavalcante-Silva LH, Lima ÉD, Carvalho DC, Sales-Neto JM, Alves AK, Galvão JG, et al. Much more than a cardiotonic steroid: Modulation of inflammation by ouabain. Front Physiol 2017;8:895.
[Crossref] [Google Scholar] [PubMed]
- Shao C, Lin S, Liu S, Jin P, Lu W, Li N, et al. HIF1α-induced glycolysis in macrophage is essential for the protective effect of ouabain during endotoxemia. Oxid Med Cell Longev 2019;2019:7136585.
[Crossref] [Google Scholar] [PubMed]
- Islam MT, Sarkar C, El‐Kersh DM, Jamaddar S, Uddin SJ, Shilpi JA, et al. Natural products and their derivatives against coronavirus: A review of the non‐clinical and pre‐clinical data. Phytother Res 2020;34(10):2471-92.
[Crossref] [Google Scholar] [PubMed]
- Lin S, Liu K, Zhang Y, Jiang M, Lu R, Folts CJ, et al. Pharmacological targeting of p38 MAP-Kinase 6 (MAP2K6) inhibits the growth of esophageal adenocarcinoma. Cell Signal 2018;51:222-32.
[Crossref] [Google Scholar] [PubMed]
- Rupaimoole R, Yoon B, Zhang WC, Adams BD, Slack FJ. A high-throughput small molecule screen identifies ouabain as synergistic with miR-34a in killing lung cancer cells. iScience 2020;23(2):100878.
[Crossref] [Google Scholar] [PubMed]
- Guo W, Wei B, Chen T, Xu X, Ruan F, Xiang M. The Na+/K+ ATPase inhibitor ouabain attenuates stemness and chemoresistance of osteosarcoma cells. Med Sci Monit 2019;25:9426.
[Crossref] [Google Scholar] [PubMed]
- Shen Y, Wang Q, Tian Y. Reversal effect of ouabain on multidrug resistance in esophageal carcinoma EC109/CDDP cells by inhibiting the translocation of Wnt/β-catenin into the nucleus. Tumor Biol 2016;37:15937-47.
[Crossref] [Google Scholar] [PubMed]
- Feng Q, Leong WS, Liu L, Chan WI. Peruvoside, a cardiac glycoside, induces primitive myeloid leukemia cell death. Molecules 2016;21(4):534.
[Crossref] [Google Scholar] [PubMed]
- Yosifov DY, Idler I, Bhattacharya N, Reichenzeller M, Close V, Ezerina D, et al. Oxidative stress as candidate therapeutic target to overcome microenvironmental protection of CLL. Leukemia 2020;34(1):115-27.
- Shih YL, Shang HS, Chen YL, Hsueh SC, Chou HM, Lu HF, et al. Ouabain promotes immune responses in WEHI‐3 cells to generate leukemia mice through enhancing phagocytosis and natural killer cell activities in vivo. Environ Toxicol 2019;34(5):659-65.
[Crossref] [Google Scholar] [PubMed]
- Yin W, Li X, Feng S, Cheng W, Tang B, Shi YL, et al. Plasma membrane depolarization and Na, K-ATPase impairment induced by mitochondrial toxins augment leukemia cell apoptosis via a novel mitochondrial amplification mechanism. Biochem Pharmacol 2009;78(2):191-202.
[Crossref] [Google Scholar] [PubMed]
- Tailler M, Senovilla L, Lainey E, Thepot S, Metivier D, Sebert M, et al. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene 2012;31(30):3536-46.
[Crossref] [Google Scholar] [PubMed]
- Varghese E, Samuel SM, Sadiq Z, Kubatka P, Liskova A, Benacka J, et al. Anti-cancer agents in proliferation and cell death: The calcium connection. Int J Mol Sci 2019;20(12):3017.
[Crossref] [Google Scholar] [PubMed]
- Xu Z, Wang F, Fan F, Gu Y, Shan N, Meng X, et al. Quantitative proteomics reveals that the inhibition of Na+/K+-ATPase activity affects S-phase progression leading to a chromosome segregation disorder by attenuating the Aurora A function in hepatocellular carcinoma cells. J Proteome Res 2015;14(11):4594-602.
[Crossref] [Google Scholar] [PubMed]
- Wang L, Cai W, Han B, Zhang J, Yu B, Chen M. Ouabain exhibited strong anticancer effects in melanoma cells via induction of apoptosis, G2/M phase arrest, and migration inhibition. Onco Targets Ther 2021;14:1261.
[Crossref] [Google Scholar] [PubMed]
- Staal FJ, Famili F, Garcia Perez L, Pike-Overzet K. Aberrant Wnt signaling in leukemia. Cancers 2016;8(9):78.
[Crossref] [Google Scholar] [PubMed]
- Pehlivan M, Çalışkan C, Yüce Z, Sercan HO. Secreted Wnt antagonists in leukemia: A road yet to be paved. Leuk Res 2018;69:24-30.
[Crossref] [Google Scholar] [PubMed]
- Botelho AF, Pierezan F, Soto-Blanco B, Melo MM. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019;158:63-8.
[Crossref] [Google Scholar] [PubMed]
- Ramirez-Ortega M, Zarco G, Maldonado V, Carrillo JF, Ramos P, Ceballos G, et al. Is digitalis compound-induced cardiotoxicity, mediated through guinea-pig cardiomyocytes apoptosis? Eur J Pharmacol 2007;566(1-3):34-42.
[Crossref] [Google Scholar] [PubMed]
- Botelho AF, Miranda AL, Freitas TG, Milani PF, Barreto T, Cruz JS, et al. Comparative cardiotoxicity of low doses of digoxin, ouabain, and oleandrin. Cardiovasc Toxicol 2020;20:539-47.
[Crossref] [Google Scholar] [PubMed]
- Gonano LA, Sepúlveda M, Rico Y, Kaetzel M, Valverde CA, Dedman J, et al. Calcium-calmodulin kinase II mediates digitalis-induced arrhythmias. Circ Arrhythm Electrophysiol 2011;4(6):947-57.
[Crossref] [Google Scholar] [PubMed]
- Fu Y, Ding D, Jiang H, Salvi R. Ouabain-induced cochlear degeneration in rat. Neurotox Res 2012;22:158-69.
[Crossref] [Google Scholar] [PubMed]
- Kinoshita PF, Orellana AM, Nakao VW, de Souza Port's NM, Quintas LE, Kawamoto EM, et al. The Janus face of ouabain in Na+/K+‐ATPase and calcium signalling in neurons. Br J Pharmacol 2022;179(8):1512-24.
[Crossref] [Google Scholar] [PubMed]
- Song HL, Demirev AV, Kim NY, Kim DH, Yoon SY. Ouabain activates transcription factor EB and exerts neuroprotection in models of Alzheimer's disease. Mol Cell Neurosci 2019;95:13-24.
[Crossref] [Google Scholar] [PubMed]
- Orellana AM, Leite JA, Kinoshita PF, Vasconcelos AR, Andreotti DZ, de Sá Lima L, et al. Ouabain increases neuronal branching in hippocampus and improves spatial memory. Neuropharmacol 2018;140:260-74.
- Ivanova MA, Kokorina AD, Timofeeva PD, Karelina TV, Abushik PA, Stepanenko JD, et al. Calcium export from neurons and multi-kinase signaling cascades contribute to ouabain neuroprotection in hyperhomocysteinemia. Biomolecules 2020;10(8):1104.
[Crossref] [Google Scholar] [PubMed]
 ) Control; (
) Control; ( ) 0.1 mg/kg ouabain; (
) 0.1 mg/kg ouabain; ( ) 0.5 mg/kg
ouabain and (
) 0.5 mg/kg
ouabain and ( ) 1.0 mg/kg ouabain
) 1.0 mg/kg ouabain