- *Corresponding Author:
- Li Zhang
Department of Urology, Central HospitaL, Yingkou Economic and Technological Development Zone, Yingkou, Liaoning Province 115007, China
E-mail: Zhl10193520@163.com
| Date of Submission | 17 May 2021 |
| Date of Revision | 29 November 2021 |
| Date of Acceptance | 16 July 2022 |
| Indian J Pharm Sci 2022;84(4):874-882 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To evaluate the effects of polyphyllin D on the proliferation and apoptosis of human colon cancer SW480 cell lines and to reveal whether this process is mediated by the micro ribonucleic acid-21/phosphatase and tensin homolog deleted on chromosome 10 pathway. Human colon cancer SW480 cells were transfected with micro ribonucleic acid-21-mimic or mimic-negative control and treated with polyphyllin D at different concentrations (control, 0.25, 0.5, 1 and 2 μM) for 24 h or 48 h. Compared with control polyphyllin D group, the viability of human colon cancer SW480 cells significantly declined but the apoptosis rate significantly rose in 0.5 μM polyphyllin D group and the colony number of SW480 cells significantly decreased in 0.25 μM polyphyllin D group (p<0.05). Compared with control group, 2 μM polyphyllin D group had significantly increased protein expressions of Bcl-2 associated X protein and cleaved caspase-3, but decreased protein expression of B-cell lymphoma-2 in SW480 cells (p<0.001). The protein levels of micro ribonucleic acid-21 and phosphorylated-protein kinase B in SW480 cells were significantly lower, while the phosphatase and tensin homolog deleted on chromosome 10 messenger ribonucleic acid and protein levels were higher in 2 μM polyphyllin D group than those in control group (p<0.001). However, the protein expression of protein kinase B was not changed in 2 μM polyphyllin D group (p>0.05). After transfection with micro ribonucleic acid-21-mimic, the level of micro ribonucleic acid-21 in SW480 cells was significantly higher, while the phosphatase and tensin homolog deleted on chromosome 10 messenger ribonucleic acid and protein levels were significantly lower than those in control group (p<0.001). Compared with control group, the antiproliferative and pro-apoptotic effects of polyphyllin D on SW480 cells were reversed by transfection with micro ribonucleic acid-2-mimic (p<0.001). Polyphyllin D inhibits proliferation and induces apoptosis of human colon cancer SW480 cells via the micro ribonucleic acid-21/phosphatase and tensin homolog deleted on chromosome 10 pathway.
Keywords
Polyphyllin D, colon cancer, SW480 cells, mortality, proliferation, apoptosis
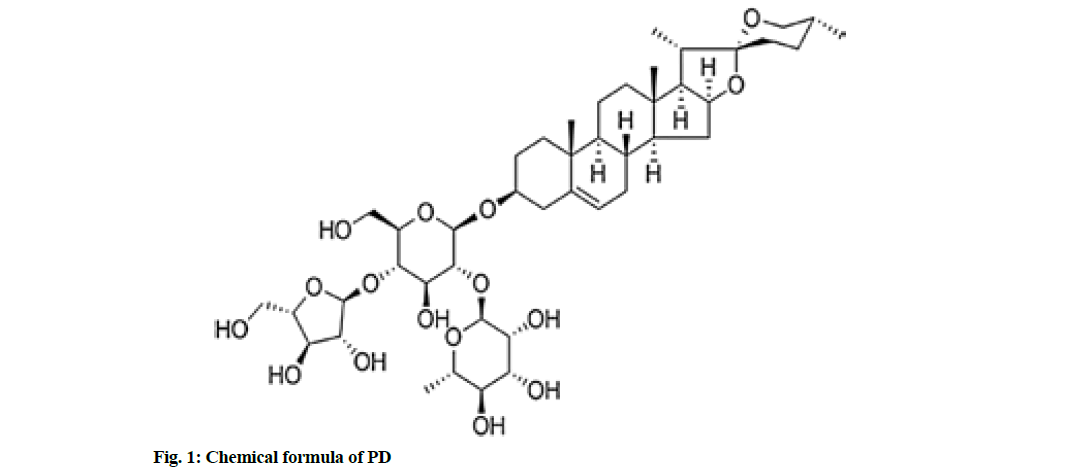
Colon cancer is a kind of common cancer with a high mortality rate globally[1]. There are 1.2 million newlydiagnosed cases and about 600 000 deaths of colon cancer every year in the world[2]. The 5 y survival rate of colon cancer patients is 40 %-60 %[3]. Considering the high incidence and poor prognosis of colon cancer, it is urgently needed to clarify the mechanism of its occurrence and development and develop new therapeutic drugs. Paris polyphylla, a traditional Chinese medicine of Liliaceae, has anti-tumor effects on bladder cancer, pancreatic cancer, breast cancer, liver cancer, lung cancer, etc.[4-7]. Polyphyllin D (PD), C44H70O16), one of steroidal saponins, is the main effective component of Paris polyphylla, with a molecular weight of 855.02 Da. Its chemical formula is shown in fig. 1. According to several studies, PD exerts anti-cancer effects on neuroblastoma, leukemia and pancreatic cancer[8]. In addition, PD can induce apoptosis of colorectal cancer cells through the Janus Kinase (JAK)/Signal Transducer and Activator of Transcription 3 (STAT3) pathway[9]. However, the anticancer effect of PD in colon cancer and its mechanism remains unclear. Micro Ribonucleic Acids (MiRNAs) can regulate gene expression through directly targeting messenger Ribonucleic Acid (mRNAs) of a variety of oncogenes or anti-oncogenes, such as Phosphatase and Tensin homolog Deleted on Chromosome 10 (PTEN), leading to translational suppression or instability[10,11]. There is increasing evidence that miRNAs are targets and markers for cancer diagnosis. In the present study, therefore, the inhibitory effect of PD on the proliferation of human colon cancer SW480 cell lines was explored and the role of miR-21/PTEN pathway in this process was investigated.
Materials and Methods
Cell culture:
Human colon cancer SW480 cell lines were purchased from American Type Culture Collection (ATCC) and cultured with Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, USA) containing 10 % Fetal Bovine Serum (FBS), 100 U/ml penicillin and 100 μg/ ml streptomycin under 5 % Carbon dioxide, (CO2) at 37°.
Cell transfection and treatment with PD:
MiR-21-mimic and mimic-Negative Control (NC) were purchased from Shanghai GenePharma Co., Ltd. SW480 cells (5×105) were inoculated into a 96- well plate until reaching 70 % confluence. 1 μl of Lipofectamine™ 2000 (Invitrogen, USA) was diluted with 50 μl of antibiotic-free RPMI 1640 and 20 μM miR-21-mimic and mimic-NC were diluted with 50 μl of antibiotic-free RPMI 1640. Then miR-21-mimic or mimic-NC was mixed with Lipofectamine™ 2000 diluent in equal volumes. 100 μl of the above mixture was added into SW480 cells for culture for 6 h and then it was replaced with the complete medium, followed by culture for another 48 h. The control cells were cultured normally.
After transfection, SW480 cells were treated with PD at different concentrations (control, 0.25, 0.5, 1 and 2 μM) for 24 h or 48 h. PD (batch no. CFN90255, purity >98 %) was purchased from Wuhan ChemFaces Co., Ltd.
Cell viability assay:
The cell viability was detected by 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. After treatment with PD, SW480 cells were added with 20 μl of MTT (Sigma, United States of America (USA), 5 mg/ml) in each well, and incubated at 37° for 4 h. Then the cells were added with 150 μl of dimethyl sulfoxide in each well and shaken for 10 min. Finally, the Optical Density at 570 nm (OD570) was measured using the Varioskan™ LUX multifunctional microplate reader (Thermo Science, USA), based on which the relative cell viability was calculated.
Colony formation assay:
SW480 cells were inoculated into a 6-well plate (500 cells/well), cultured with RPMI 1640 medium containing PD at different concentrations (control, 0.25, 0.5, 1 and 2 μM), 10 % FBS, 100 U/ml penicillin and 100 μg/ml streptomycin under 5 % CO2 at 37° for 2 w, fixed with 90 % ethanol for 30 min and stained with 0.1 % crystal violet. The number of colonies with more than 50 cells was counted.
Apoptosis assay:
Apoptosis was analyzed by Annexin V-Fluorescein Isothiocyanate (FITC) and Propidium Iodide (PI) staining using the kits purchased from Beyotime Institute of Biotechnology. After treatment with PD, SW480 cells were collected, washed with ice-cold PBS 3 times, resuspended with 200 μl of binding buffer containing 5 μl of Annexin V-FITC and incubated for 10 min in the dark, followed by centrifugation at 1000 rpm and 4° for 10 min. Then the cells were resuspended with 200 μl of binding buffer containing 10 μl of PI and analyzed using the EPICS® XL flow cytometer (Beckman Coulter, USA).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) :
The total mRNA was extracted from SW480 cells with TRIzol reagent (Invitrogen, USA), and reversely transcribed using the PrimeScript RT kits (Takara, Japan). PCR was performed on the 7900 HT fluorescence quantitative PCR instrument (Applied Biosystems, USA) using the SYBR Prime Script RT-PCR kits (Takara, Japan). The primer sequences were as follows: miR-21 stem-loop: 5'-CTGGTGT AGTCT GAGGCGGTA AGGCAGTC AACA TC-3', F: 5'-CCGGG TAGACTTAAA CGTAC TATTC-3', R: 5'-CTGTC CATTG TGTAT AGAG TCAGA-3'; PTEN F: 5'-AGTTG GTGAGGT ATGG GC3TCGT ACACA-3', R: 5'-AGC TGTGTAA GCATC GGC GGC TCCA-3'; U6 F: 5'-ATC CCTTC TAA GCT TCAGC ACG CGA-3', R: 5'-GACG CTTAATGCCA TGTCAC TGTG CAA-3'; Beta (β)-actin F: 5'-CGGCGT CAAC AGAA CTG GTA-3', R: 5'-TATC TCGAA CGTTG GGTA CCCT-3'. The levels of miR-21 and PTEN were normalized into U6 and β-actin, respectively. The relative expression level was determined using 2-ΔΔCt method.
Western blotting:
The cells were lysed with Radioimmunoprecipitation Assay (RIPA) lysis buffer (Jiangsu KeyGEN BioTECH Co., Ltd.) and the protein concentration was detected using Bicinchoninic Acid (BCA) kits (Jiangsu KeyGEN BioTECH Co., Ltd.). The protein was separated by 10 % Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE), transferred onto a Polyvinylidene Difluoride (PVDF) membrane (Millipore, USA), blocked with 5 % skim milk for 1 h and incubated with primary antibodies (1:1000) to PTEN, B-cell lymphoma-2 (Bcl-2), Bcl- 2 associated X protein (BAX), cleaved caspase-3, phosphorylated-protein kinase B (p-Akt), Akt and β-actin at 4° overnight. Then the protein was incubated again with Horseradish Peroxidase (HRP)-labeled secondary antibodies (1:500) at room temperature for 1 h. All antibodies were purchased from Abcam (USA). Finally, the image was developed using Enhanced Chemiluminescence (ECL) reagent (Beyotime Institute of Biotechnology).
Statistical analysis:
Statistical Package for the Social Sciences (SPSS) 22.0 software was used for statistical analysis and the results were expressed as mean±standard deviation. The test and control values were compared by student's t test and one-way analysis of variance. p<0.05 was considered to be statistically significant.
Results and Discussion
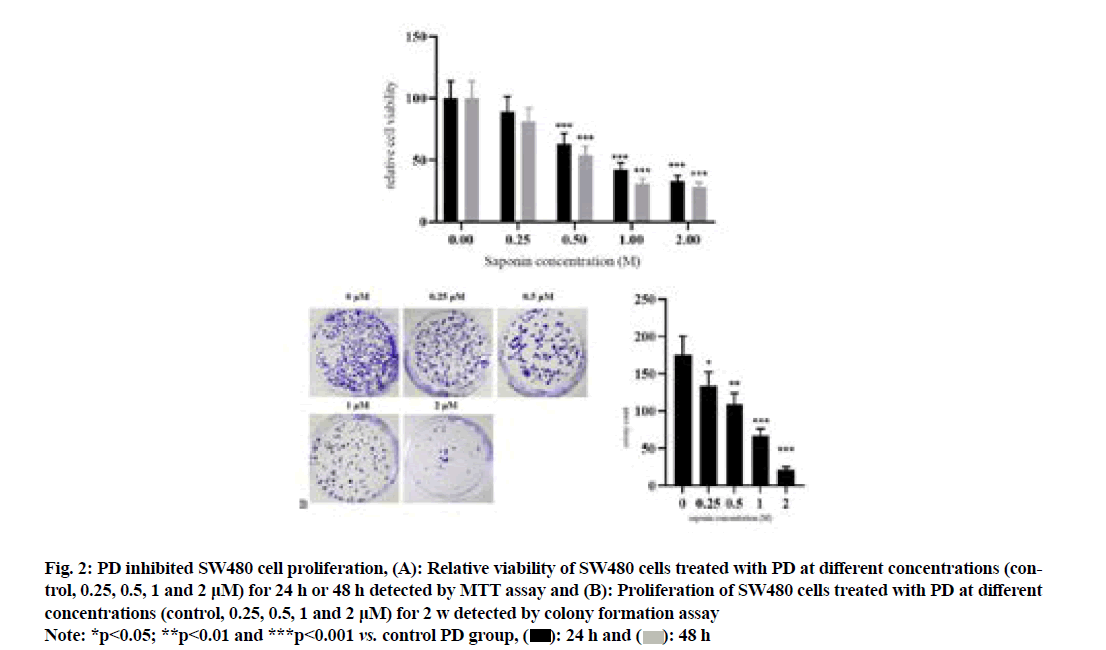
The results of MTT assay (fig. 2A) showed that compared with that in control PD group, the relative viability of SW480 cells significantly declined in 0.5 μM PD group in a concentration-dependent manner (p<0.001). The results of colony formation assay (fig. 2B) revealed that compared with that in control group, the colony number of SW480 cells was significantly decreased in 0.25 μM PD group in a concentrationdependent manner (p<0.05).
Fig. 2: PD inhibited SW480 cell proliferation, (A): Relative viability of SW480 cells treated with PD at different concentrations (control, 0.25, 0.5, 1 and 2 μM) for 24 h or 48 h detected by MTT assay and (B): Proliferation of SW480 cells treated with PD at different concentrations (control, 0.25, 0.5, 1 and 2 μM) for 2 w detected by colony formation assay
Note: *p<0.05; **p<0.01 and ***p<0.001 vs. control PD group, 
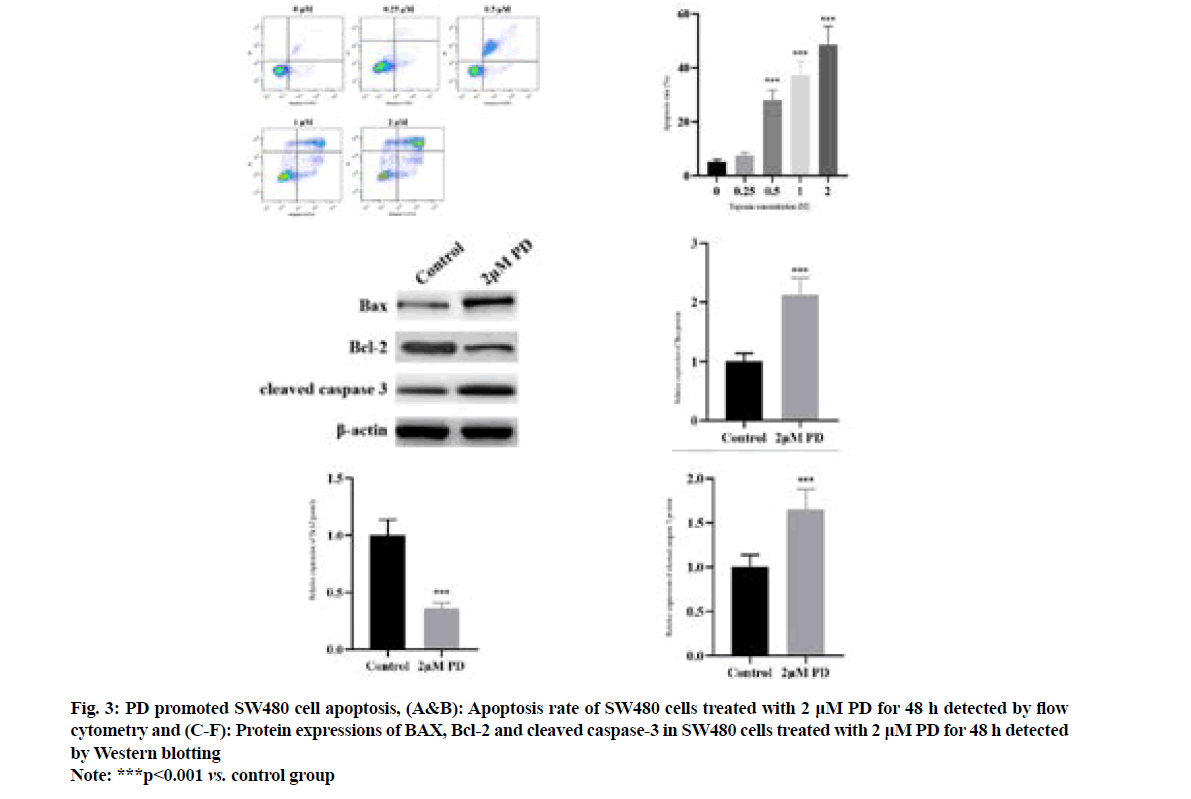
It was found by flow cytometry (fig. 3A and fig. 3B) that compared with that in control PD group, the apoptosis rate of SW480 cells significantly rose in 0.5 μM PD group in a concentration-dependent manner (p<0.001). The results of Western blotting (fig. 3C-fig. 3F) revealed that 2 μM PD significantly increased the protein expressions of BAX and cleaved caspase-3, but decreased the protein expression of Bcl-2 in SW480 cells compared with control group (p<0.001).
Fig. 3: PD promoted SW480 cell apoptosis, (A&B): Apoptosis rate of SW480 cells treated with 2 μM PD for 48 h detected by flow cytometry and (C-F): Protein expressions of BAX, Bcl-2 and cleaved caspase-3 in SW480 cells treated with 2 μM PD for 48 h detected by Western blotting
Note: ***p<0.001 vs. control group
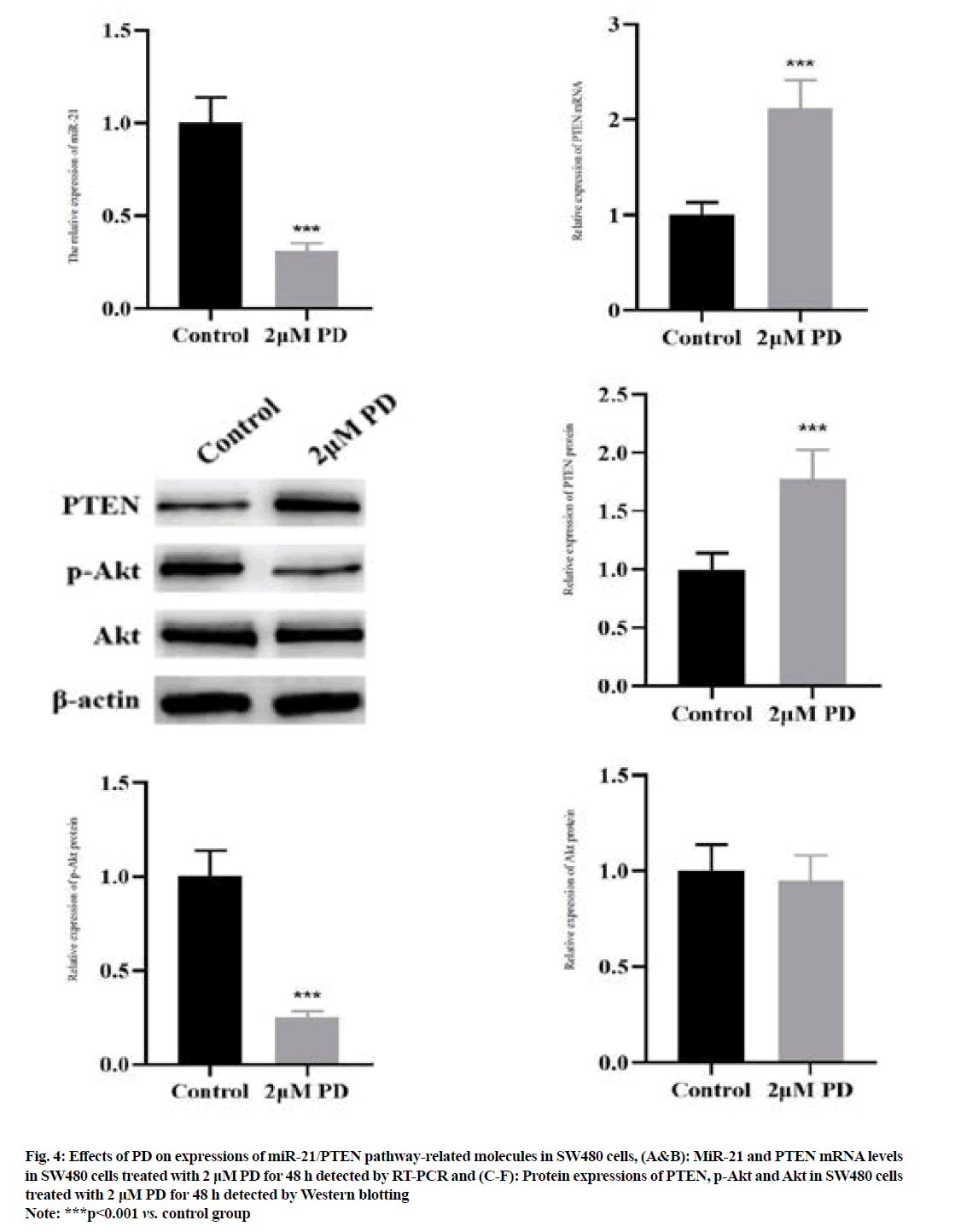
The results of RT-PCR (fig. 4A and fig. 4B) manifested that 2 μM PD significantly reduced the miR-21 level, but increased the PTEN mRNA level in SW480 cells compared with control group (p<0.001). The results of Western blotting (fig. 4C-fig. 4F) manifested that the protein expression of PTEN was significantly higher, while the protein expression of p-Akt was lower in SW480 cells in 2 μM PD group than those in control group (p<0.001). However, the protein expression of Akt was not changed in 2 μM PD group (p>0.05).
Fig. 4: Effects of PD on expressions of miR-21/PTEN pathway-related molecules in SW480 cells, (A&B): MiR-21 and PTEN mRNA levels in SW480 cells treated with 2 μM PD for 48 h detected by RT-PCR and (C-F): Protein expressions of PTEN, p-Akt and Akt in SW480 cells treated with 2 μM PD for 48 h detected by Western blotting
Note: ***p<0.001 vs. control group
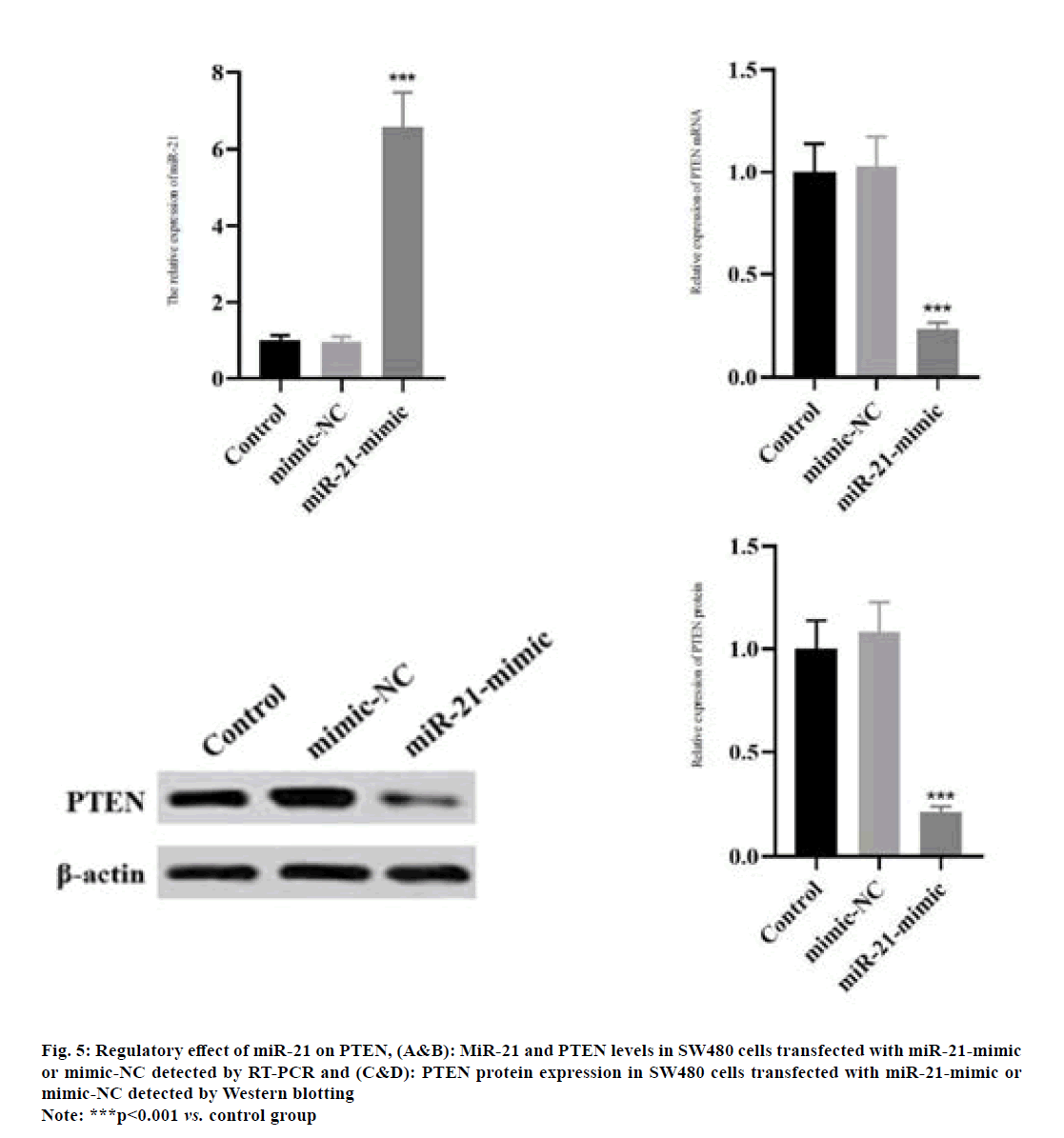
The miR-21 level in SW480 cells was significantly increased in miR-21-mimic group compared with that in control group as shown in fig. 5A (p<0.001). Compared with those in control group, the mRNA and protein expressions of PTEN in SW480 cells significantly declined in miR-21-mimic group (fig. 5B-fig. 5D) (p<0.001).
Fig. 5: Regulatory effect of miR-21 on PTEN, (A&B): MiR-21 and PTEN levels in SW480 cells transfected with miR-21-mimic or mimic-NC detected by RT-PCR and (C&D): PTEN protein expression in SW480 cells transfected with miR-21-mimic or mimic-NC detected by Western blotting
Note: ***p<0.001 vs. control group
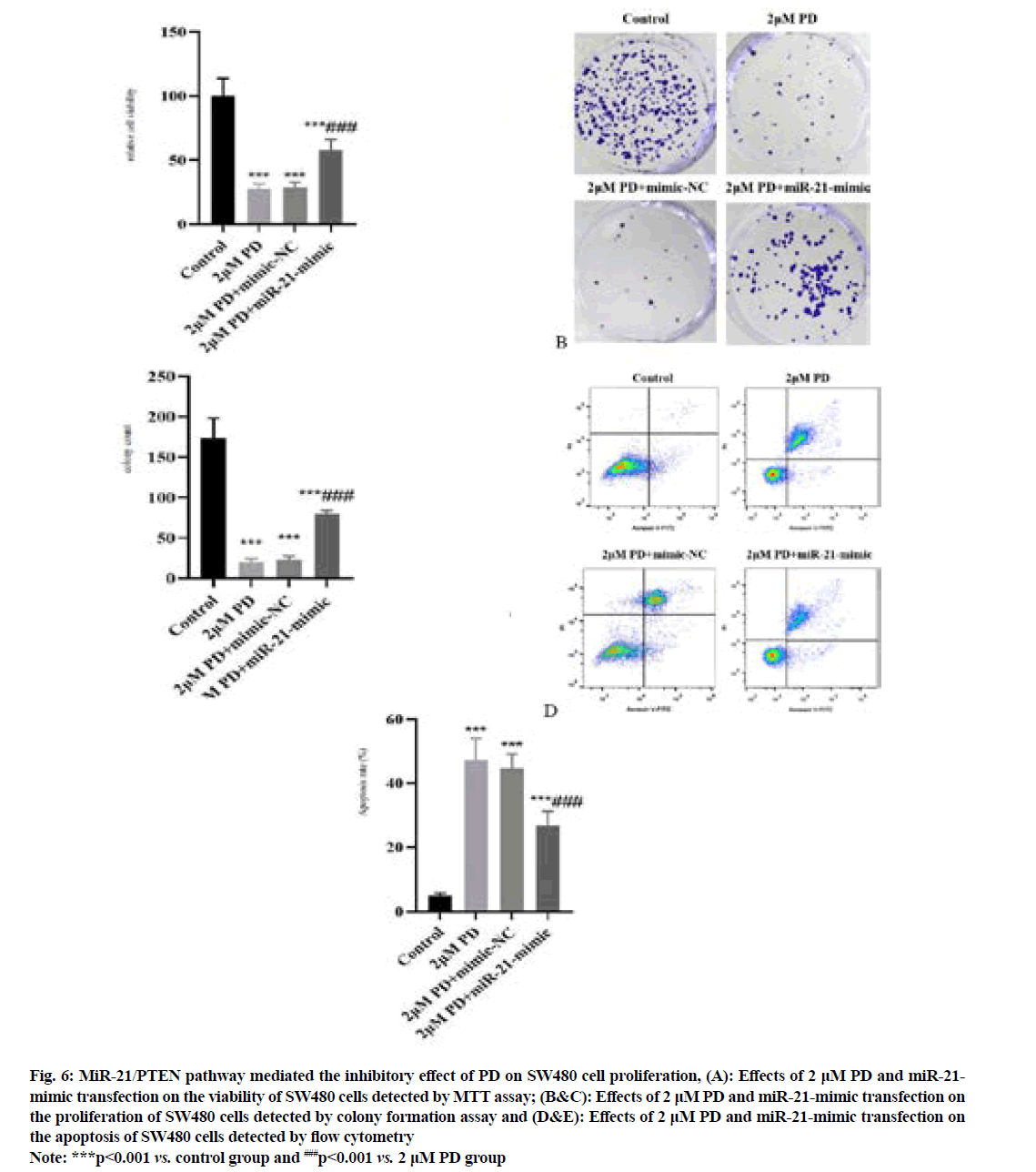
The results of MTT assay and colony formation assay (fig. 6A-fig. 6C) manifested that the proliferation of SW480 cells was significantly inhibited in 2 μM PD group compared with that in control group, while it was significantly enhanced in miR-21-mimic group (p<0.001). In addition, the results of flow cytometry (fig. 6D and fig. 6E) showed that the apoptosis of SW480 cells was significantly enhanced in 2 μM PD group compared with that in control group, while it was significantly weakened in miR-21-mimic group (p<0.001).
Fig. 6: MiR-21/PTEN pathway mediated the inhibitory effect of PD on SW480 cell proliferation, (A): Effects of 2 μM PD and miR-21- mimic transfection on the viability of SW480 cells detected by MTT assay; (B&C): Effects of 2 μM PD and miR-21-mimic transfection on the proliferation of SW480 cells detected by colony formation assay and (D&E): Effects of 2 μM PD and miR-21-mimic transfection on the apoptosis of SW480 cells detected by flow cytometry
Note: ***p<0.001 vs. control group and ###p<0.001 vs. 2 μM PD group
Colon cancer is a common malignancy and also a multistep multi-stage multi-gene cytogenetic disease. It has been proved that colon cancer is related to many factors, including mutations of protooncogenes, inactivation of cancer suppressor genes, microsatellite instability, mismatch repair gene mutation, apoptotic mechanism disorders, gene overexpression, signal transduction disorders, infiltration and metastasis-related molecular changes, epigenetic modification and environment[12, 13]. However, the exact pathogenesis and molecular mechanism of colon cancer have not been fully clear. Therefore, it has been a research hotspot in the field of oncology to deeply study and explore the pathogenesis of colon cancer and develop new therapeutic drugs and targets. It is reported that PD, a steroidal saponin in the traditional Chinese medicine Paris polyphylla, can induce apoptosis of colorectal cancer cell through the JAK/STAT3 pathway[9]. Some scholars have revealed the anti-cancer effect of PD on neuroblastoma, leukemia and pancreatic cancer[8]. However, the anticancer effect of PD on colon cancer and its mechanism are still unclear.
In the present study, human colon cancer SW480 cell lines were treated with PD at different concentrations (control, 0.25, 0.5, 1 and 2 μM). The proliferation of SW480 cells was detected by MTT assay and colony formation assay. The results manifested that PD could suppress the proliferation of colon cancer cells in a dose-dependent manner. PD may be a potential natural anti-cancer drug.
Cell apoptosis and proliferation jointly keep homeostasis and the occurrence of malignancies is closely related to apoptotic disorders[14]. In this study, it was found that PD could induce apoptosis of SW480 cells in a dose-dependent manner. Moreover, it greatly increased the protein expressions of BAX and cleaved caspase-3, but decreased the protein expression of Bcl- 2 in SW480 cells. As known to all, caspase-3 can be activated and caspase cascade can be triggered in cells upon endogenous or exogenous stimulus, thus inducing apoptosis[15]. Bcl-2, an anti-apoptotic protein, mainly acts on the mitochondrial membrane, which is able to inhibit the activation of caspase-3. In contrast, BAX, a pro-apoptotic protein, can activate caspase-3 and induce apoptosis[16]. Therefore, the pro-apoptotic effect of PD on SW480 cells may be achieved by regulating Bcl-2, BAX and caspase-3.
MiRNAs are involved in almost all biological processes, which regulate not only the function and behavior of normal cells, but also the biological behavior of tumor cells throughout the process of tumor formation, including proliferation, differentiation, angiogenesis, apoptosis, drug resistance, metastasis and invasion[17]. Therefore, miRNAs have close correlations with the occurrence and development of cancers. Moreover, miRNAs can inhibit the translation of target gene mRNAs or directly degrade them. Many miRNAs are important players in the occurrence and development of colon cancer. It is reported that miR-29a inhibits the growth of colon cancer[18] and miR-27a inhibits the occurrence and development of colon cancer through targeting the expressions of Sphingosine- Phosphate Phosphatase 1 (SGPP1) and SMAD2[19]. MiR-139-5p suppresses the proliferation and invasion of colon cancer cells by inhibiting the activity of nuclear factor-kappa B[20]. MiR-21 is up-regulated in most human malignancies and involved in every stage of cancer development and progression, including transformation, neoplasia, invasion, metastasis and drug resistance[21]. In addition, the overexpression of miR-21 is correlated with the poor prognosis of 5-FU chemotherapy, including lymph node metastasis and liver metastasis[22]. It is also reported that miR-21 serves as a potential biomarker for early detection of colon cancer[23]. Targeted inhibition on miR-21 can enhance the sensitivity of human colon cancer HT-29 cells to chemoradiotherapy[24]. In the present study, PD obviously inhibited the level of miR-21 in SW480 cells. PTEN is a widely-studied cancer suppressor gene and there is a direct targeted regulatory relation between miR-21 and PTEN. Liu et al. found that miR-21 had an increased expression in ovarian cancer cells and inhibiting miR-21 could enhance PTEN expression and inhibit PI3K/AKT activity, thereby enhancing cell apoptosis and weakening cell proliferation[25]. Liu et al. showed that curcumol suppressed the proliferation of colorectal cancer by targeting miR-21 and regulating the PTEN/PI3K/Akt pathway[26]. Besides, Cao et al. confirmed that exosomal miR-21 promoted the growth of hepatocellular carcinoma through regulating the TETs/PTENp1/PTEN pathway[27]. In this study, it was also proved that miR-21 had targeted inhibition on the expression of PTEN. PD not only inhibited the expression of miR-21, but also promoted the expression of PTEN in SW480 cells. To further validate whether the anti-proliferative and pro-apoptotic effects of PD on SW480 cells are mediated by the miR-21/PTEN pathway, SW480 cells were transfected with miR-21- mimic to up-regulate the expression of miR-21. The results showed that the anti-proliferative and proapoptotic effects of PD on SW480 cells were reversed by up-regulation of miR-21.
In conclusion, PD inhibits proliferation and induces apoptosis of human colon cancer SW480 cell lines through the miR-21/PTEN pathway, which may be an important potential anti-cancer drug.
Authors’ contributions:
Yongjun Zhu and Haijun Hu have contributed equally to this study.
Funding:
This study was financially supported by Xiamen City Project (No: 3502Z20214ZD1089).
Conflict of interests:
The authors declared no conflict of interests.
References
- Qu JJ, Qu XY, Zhou DZ. miR?4262 inhibits colon cancer cell proliferation via targeting of GALNT4. Mol Med Rep 2017;16(4): 3731-6.
[Crossref] [Google Scholar] [Pub Med]
- Zhao J, Chen Y, Liu F. Overexpression of miRNA-143 inhibits colon cancer cell proliferation by inhibiting glucose uptake. Arch Med Res 2018;49(7):497-503.
[Crossref] [Google Scholar] [Pub Med]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57(1):43-66.
[Crossref] [Google Scholar] [Pub Med]
- Guo Y, Liu Z, Li K, Cao G, Sun C, Cheng G, et al. Paris polyphylla-derived saponins inhibit growth of bladder cancer cells by inducing mutant P53 degradation while up-regulating CDKN1A expression. Curr Urol 2018;11(3):131-8.
[Crossref] [Google Scholar] [Pub Med]
- Lin LT, Uen WC, Choong CY, Shi YC, Lee BH, Tai CJ, et al. Paris polyphylla inhibits colorectal cancer cells via inducing autophagy and enhancing the efficacy of chemotherapeutic drug doxorubicin. Molecules 2019;24(11):2102.
[Crossref] [Google Scholar] [Pub Med]
- Negi SJ, Bisht KV, Bhandari KA, Bhatt PV, Singh P, Singh N. Paris polyphylla: Chemical and biological prospectives. Anticancer Agents Med Chem 2014;14(6):833-9.
[Crossref] [Google Scholar] [Pub Med]
- Zhang D, Li K, Sun C, Cao G, Qi Y, Lin Z, et al. Anti-cancer effects of Paris polyphylla ethanol extract by inducing cancer cell apoptosis and cycle arrest in prostate cancer cells. Curr Urol 2018;11(3):144-50.
[Crossref] [Google Scholar] [Pub Med]
- Watanabe S, Suzuki T, Hara F, Yasui T, Uga N. Polyphyllin D, a steroidal saponin in Paris polyphylla, induces apoptosis and necroptosis cell death of neuroblastoma cells. Pediatr Surg Int 2017;33(6):713-9.
[Crossref] [Google Scholar] [Pub Med]
- Yang C, Cai H, Meng X. Polyphyllin D induces apoptosis and differentiation in K562 human leukemia cells. Int Immunopharmacol 2016;36:17-22.
[Crossref] [Google Scholar] [Pub Med]
- Ni WJ, Leng XM. miRNA-dependent activation of mRNA translation. MicroRNA 2016;5(2):83-6.
[Crossref] [Google Scholar] [Pub Med]
- Wang D, Xin L, Lin JH. Identifying miRNA-mRNA regulation network of chronic pancreatitis based on the significant functional expression. Medicine (Baltimore) 2017;96(21):e6668.
[Crossref] [Google Scholar] [Pub Med]
- Wang D, Xin L, Lin JH, Liao Z, Ji JT, Du TT, et al. Metabolic profiling of formalin-fixed paraffin-embedded tissues discriminates normal colon from colorectal cancer. Mol Cancer Res 2020;18(6):883-90.
[Crossref] [Google Scholar] [Pub Med]
- Chaouch MA, Dougaz MW, Dziri C. Laparoscopic vs. open complete mesocolon excision in right colon cancer: A systematic review and meta-analysis. World J Surg 2019;43(12):3179-90.
[Crossref] [Google Scholar] [Pub Med]
- Renault TT, Chipuk JE. Death upon a kiss: Mitochondrial outer membrane composition and organelle communication govern sensitivity to BAK/BAX-dependent apoptosis. Chem Biol 2014;21(1):114-23.
[Crossref] [Google Scholar] [Pub Med]
- Shakeri R, Kheirollahi A, Davoodi J. Apaf-1: Regulation and function in cell death. Biochimie 2017;135:111-25.
[Crossref] [Google Scholar] [Pub Med]
- Song X, Wang Z, Liang H, Zhang W, Ye Y, Li H, et al. Dioscin induces gallbladder cancer apoptosis by inhibiting ROS-mediated PI3K/AKT signalling. Int J Biol Sci 2017;13(6):782-93.
[Crossref] [Google Scholar] [Pub Med]
- Nomair AM, Issa NM, Madkour MA. The clinical significance of serum miRNA-224 expression in hepatocellular carcinoma. Clin Exp Hepatol 2020;6(1):20-7.
[Crossref] [Google Scholar] [Pub Med]
- Han X, Zheng J, Wang Y. miRNA-29a inhibits colon cancer growth by regulation of the PTEN/Akt/GSK3β and Wnt/β-catenin signaling pathways. Oncol Lett 2018;16(2):2638-44.
[Crossref] [Google Scholar] [Pub Med]
- Bao Y, Chen Z, Guo Y, Feng Y, Li Z, Han W, et al. Tumor suppressor microRNA-27a in colorectal carcinogenesis and progression by targeting SGPP1 and Smad2. PLoS One 2014;9(8):e105991.
[Crossref] [Google Scholar] [Pub Med]
- Chen BS, Li CW. Constructing an integrated genetic and epigenetic cellular network for whole cellular mechanism using high-throughput next-generation sequencing data. BMC Syst Biol 2016;10:18.
[Crossref] [Google Scholar] [Pub Med]
- Shi Z, Zhang J, Qian X, Han L, Zhang K, Chen L, et al. AC1MMYR2, an inhibitor of dicer-mediated biogenesis of Oncomir miR-21, reverses epithelial-mesenchymal transition and suppresses tumor growth and progression. Cancer Res 2013;73(17):5519-31.
[Crossref] [Google Scholar] [Pub Med]
- Tomimaru Y, Eguchi H, Nagano H, Wada H, Tomokuni A, Kobayashi S, et al. MicroRNA-21 induces resistance to the anti-tumour effect of interferon-α/5-fluorouracil in hepatocellular carcinoma cells. Br J Cancer 2010;103(10):1617-26.
- Dehghan F, Boozarpour S, Torabizadeh Z. miR-21: A promising biomarker for the early detection of colon cancer. Onco Targets Ther 2019;12:5601-7.
[Crossref] [Google Scholar] [Pub Med]
- Deng J, Lei W, Fu JC, Zhang L, Li JH, Xiong JP. Targeting miR-21 enhances the sensitivity of human colon cancer HT-29 cells to chemoradiotherapy in vitro. Biochem Biophys Res Commun 2014;443(3):789-95.
[Crossref] [Google Scholar] [Pub Med]
- Liu HY, Zhang YY, Zhu BL, Feng FZ, Yan H, Zhang HY, et al. miR-21 regulates the proliferation and apoptosis of ovarian cancer cells through PTEN/PI3K/AKT. Eur Rev Med Pharmacol Sci 2019;23(10):4149-55.
[Crossref] [Google Scholar] [Pub Med]
- Liu H, Wang J, Tao Y, Li X, Qin J, Bai Z, et al. Curcumol inhibits colorectal cancer proliferation by targeting miR-21 and modulated PTEN/PI3K/Akt pathways. Life Sci 2019;221:354-61.
[Crossref] [Google Scholar] [Pub Med]
- Cao LQ, Yang XW, Chen YB, Zhang DW, Jiang XF, Xue P. Exosomal miR-21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth. Mol Cancer 2019;18(1):148.
[Crossref] [Google Scholar] [Pub Med]