- *Corresponding Author:
- E. K. Kilari
Department of Pharmacology, Sri Vasavi Institute of Pharmaceutical Sciences, Tadepalligudem, Andhra Pradesh 534101, India
E-mail: ekilari@gmail.com
| Date of Received | 24 June 2023 |
| Date of Revision | 25 July 2023 |
| Date of Acceptance | 05 June 2024 |
| Indian J Pharm Sci 2024;86(3):1110-1116 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The use of herbal supplements, along with antidiabetic drugs, is common for better management of blood glucose levels in type II diabetes. Resveratrol, an herbal isolate, offers numerous benefits for managing diabetes and its complications. Gliclazide is a widely used medication for type II diabetes treatment. The present study was undertaken to determine the potential for interactions between resveratrol and gliclazide based on the relationship between herbal supplements and diabetes. The influence of resveratrol on the activity of gliclazide was determined by conducting single and multiple dose interaction studies in animal models. Blood samples collected at predetermined time intervals from experimental animals were used for the estimation of glucose and insulin levels. Additionally, serum gliclazide levels in rabbits were analysed by high-performance liquid chromatography. Gliclazide alone showed peak reductions in blood glucose levels at 2nd and 8th h after administration in rats and after 3 h in rabbits. In studies involving both single and multiple dose combinations of resveratrol with gliclazide, significant changes were observed in the amount of blood glucose reduction in normal rats, diabetic rats and normal rabbits. Additionally, this combination significantly altered the levels of insulin in the blood. Resveratrol affected the pharmacokinetics of gliclazide in rabbits. Overall, the effects of resveratrol on gliclazide were both related to pharmacokinetic and pharmacodynamic effects. Therefore, when prescribing gliclazide to patients who are using herbal preparations containing resveratrol, it is important to monitor glucose levels closely and adjust the dosage as needed.
Keywords
Resveratrol, gliclazide, herbal formulations, pharmacokinetics, pharmacodynamics
People are utilizing herbal formulations or supplements in conjunction with their medications for chronic conditions[1,2]. Herbal formulations typically contain concentrated products of herbs that have a high percentage of active ingredients within standard limits[3]. These active ingredients may have the potential to interact with allopathic drugs, which are commonly prescribed to treat chronic diseases. Consequently, the pharmacological activity of the allopathic drugs may be altered by the active ingredients, resulting in changes in pharmacodynamics or pharmacokinetics[4]. Many patients with diabetes rely on complementary therapies, with usage varying from country to country. In the United Kingdom, around 17 % of patients turn to these therapies, while in the United States of America, the number rises significantly to 72 %. Notably, herbal medicines stand out as one of the most popular choices, with Saudi Arabia at 68 %, Mexico at 62 %, Iran at 88.4 %, Ethiopia at 58 % and Sudan at 62 %. In India, naturopathy and Ayurveda are preferred by 67 % of diabetic patients[5]. A concerning trend, however, is that the majority of these patients fail to disclose their use of herbal medicine to their doctors, potentially hindering comprehensive treatment approaches[6]. Herbal formulations offer benefits such as antioxidant, hypoglycemic and anti-aging properties, particularly for individuals with diabetes. However, when used in combination with antidiabetic drugs, the active ingredients present in these formulations may interact with the individual's regular medication, potentially leading to toxicity such as hypoglycemia or therapeutic failure.
Resveratrol, a polyphenolic compound found in various plants and foods such as blueberries, blackberries, peanuts, cranberries, grapes, cocoa, dark chocolate, peanuts, pistachios and red wine, is also present in the Polygonum cuspidatum herb used in traditional medicine. Concentrated herbal products containing resveratrol are available on the market and are commonly used for their immune-boosting, anti-aging, cardioprotective and antioxidant properties. They are also used as herbal supplements for diabetics[7,8]. The literature review on resveratrol suggests that there is a possibility of interaction with diabetic pathogenesis and the pharmacodynamics and pharmacokinetics of gliclazide through various mechanisms, which could potentially impact gliclazide.
In an extensive review of both in vivo and in vitro studies on resveratrol, it was found that resveratrol inhibited Alpha-glucosidase (α-glucosidase) in vitro and, in diabetic rats, significantly lowered blood glucose, triglycerides and Low-Density Lipoprotein-Cholesterol (LDL-C) levels while increasing insulin levels[9]. Resveratrol exhibited antioxidant and anti-inflammatory properties, safeguarding pancreatic Beta (β) cells and delicate cells such as the vascular endothelium[10]. Male rats also showed improved glucose tolerance and protected pancreatic β cells. Additionally, another study revealed that resveratrol's antidiabetic activity involved multiple mechanisms, including the translocation and activation of the Glucose Transporter Type 4 (GLUT4), which triggered certain intracellular insulin-signaling components[11]. These combined mechanisms may enhance the hypoglycemic property of gliclazide.
Resveratrol, a natural compound with potential health benefits, has been studied for its pharmacokinetic properties. Human trials have revealed that resveratrol's metabolites have a 50 % plasma protein binding ability, while resveratrol itself binds to plasma proteins at an impressive rate of 98.3 % in humans[12]. This high plasma protein binding indicates that resveratrol may displace other drugs, such as gliclazide, which is also highly protein-bound (85 %-97 %)[13]. This raises concerns about the possibility of resveratrol displacing gliclazide from protein binding sites. Additionally, in vitro studies on liver microsomes have demonstrated that resveratrol inhibits Cytochrome P450 1A2 (CYP1A2) and Cytochrome P450 family 2 subfamily C member 9 (CYP2C9), with the latter being the main pathway for gliclazide metabolism[14]. Consequently, resveratrol's inhibition of CYP2C9 may potentially affect the metabolism of gliclazide[15]. These combined mechanisms could lead to an increased bioavailability of gliclazide.
Materials and Methods
Drugs and chemicals:
Gliclazide was obtained as a gift sample from Wockhardt Pharmaceuticals, Aurangabad, and resveratrol from Laila Impex, Vijayawada, Andhra Pradesh. Streptozotocin was purchased from Sigma Aldrich. All reagents and chemicals used in the study were of analytical grade.
Animals:
The research utilized inbred adult male albino Wistar rats, aged 8-9 w, with weights ranging between 170-250 g. Additionally, albino rabbits, of either gender, aged 3 m and weighing between 1-1.5 kg, were also included in the study. These animals were procured from Mahaveer Enterprises, Hyderabad, India and were housed under standard laboratory conditions. The housing conditions for the subjects involved a consistent ambient temperature of 25°±2°, relative humidity maintained at 50 %±15 % and a balanced 12 h light and 12 h dark cycle. They were fed a commercial pellet diet from Rayan’s Biotechnologies Pvt. Ltd., Hyderabad, India, and had unrestricted access to water. The experimental protocol was approved by the Institutional Animal Ethics Committee (registration number 516/01/A/CPCSEA). Before the experiment began, the animals underwent an 18 h fasting period with access to water. During the experiment, both food and water were withheld from the animals.
Experimental design:
The resveratrol was suspended in a 0.5 % carboxymethylcellulose and gliclazide solution that was made by dissolving it in a small amount of 0.1 N NaOH. The study design is as follows as separate groups of rats and rabbits were orally treated with different doses of resveratrol, namely 50 mg/kg, 100 mg/kg and 200 mg/kg p.o based on their body weight. Among these doses, 100 mg/kg p.o. of resveratrol demonstrated the most optimal hypoglycemic activity and thus, this dose was chosen for the interaction studies. Similarly, for interaction studies in rats and rabbits, gliclazide doses of 1 mg/kg and 5.6 mg/kg p.o. respectively, were selected. Pharmacodynamic interaction studies were conducted in normal rats (phase 1) and diabetic rats (phase 2), followed by both pharmacodynamic and pharmacokinetic interaction studies in normal rabbits (phase 3).
6 rats were used in the phase 1 experiment, and each treatment was followed by a 1 w washout period. Initially, the rats received an oral treatment of 1 mg/kg of gliclazide. After the washout period, the rats were orally treated with either resveratrol alone or a combination of both resveratrol and gliclazide, using the same doses as mentioned before. Blood samples were collected at predetermined intervals after each treatment.
In the Phase 2 experiment, streptozotocin (65 mg/kg IP) was used to induce diabetes in rats[18]. Diabetic rats with blood glucose levels exceeding 250 mg/dl were utilized in this study. These rats underwent the same treatment protocols as outlined in phase 1.
For the Phase 3 experiment, 6 rabbits were used. The rabbits were administered gliclazide orally at a dose of 5.6 mg/kg of body weight, and blood samples were collected at predetermined time points. Following a 1 w washout period between treatments, the same procedure was repeated using either orally administered resveratrol or a combination of resveratrol and gliclazide, both at the same doses mentioned earlier. Following the single-dose interaction study, the rabbits received daily treatments with resveratrol for 7 consecutive days while being fed regularly. On 8th d, the rabbits fasted for 12 h before receiving another dose of resveratrol. After 30 min, gliclazide was orally administered to the rabbits at a dose of 5.6 mg/kg body weight. Blood samples were collected at predetermined intervals following each treatment, including gliclazide, resveratrol, or combination treatments for both single and multiple administrations.
Blood sampling:
Blood samples were collected from rats and rabbits at specific time points; 0, 1, 2, 4, 6, 8, 12, 18, and 24 h for rats, and 0, 1, 2, 4, 6, 8, 12, 16, 20, and 24 h for rabbits. The blood samples obtained from both rats and rabbits were then analyzed for blood glucose levels using the Glucose Oxidase-Peroxidase Coupled Method (GOD-POD) method in a semi-auto analyzer[19]. Additionally, the rabbit blood samples were subjected to analysis for serum gliclazide using the High-Performance Liquid Chromatography (HPLC) method[16-19]. To estimate serum insulin levels, rabbit blood samples collected at 0, 1, 4, 8, 16, and 24 h were utilized, and the Enzyme-Linked Immunosorbent Assay (ELISA) method was employed for the analysis[20].
Data and statistical analysis:
Pharmacokinetic parameters were calculated using Ramkin software. The data are presented as mean±standard error of mean values. For statistical analysis, Student's paired t-test was employed, and p<0.05 was considered statistically significant.
Results and Discussion
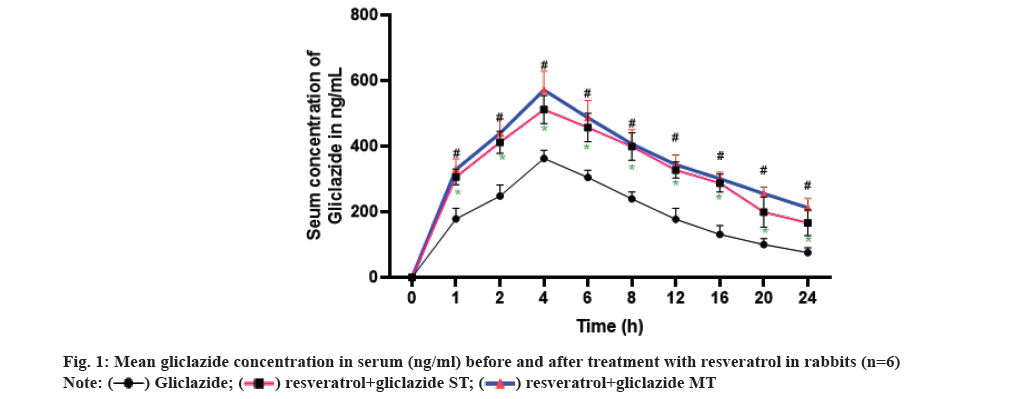
The optimal blood glucose reduction was observed with a dose of 1 mg/kg of gliclazide when compared to doses of 0.5 mg/kg and 2 mg/kg in different treatment groups. Therefore, 1 mg/kg of gliclazide was chosen as the standard for all interaction studies in both normal and diabetic rats. In the case of rabbits, the dose of 5.6 mg/kg of gliclazide was selected as the standard from the groups treated with 2.8 mg/kg, 5.6 mg/kg, and 11.2 mg/kg. Similarly, for resveratrol, the standard dose of 100 mg/kg was chosen for both rats and rabbits from the groups treated with 50 mg/kg, 100 mg/kg, and 200 mg/kg (Table 1 and fig. 1).
| Time (h) | Resveratrol | Gliclazide | Gliclazide+resveratrol |
|---|---|---|---|
| 1 | 14.12±0.60 | 26.26±0.49 | 32.33±0.82* |
| 2 | 20.89±0.71 | 35.97±0.48 | 41.74±0.67* |
| 4 | 27.75±0.62 | 31.40±0.80 | 44.44±1.09* |
| 6 | 21.87±0.49 | 28.38±0.41 | 41.69±0.71* |
| 8 | 16.01±0.63 | 33.12±0.96 | 40.70±1.07* |
| 12 | 11.03±0.56 | 26.28±0.77 | 33.00±1.37* |
| 18 | 7.55±0.46 | 19.53±1.42 | 27.27±1.72* |
| 24 | 3.76±0.56 | 14.80±1.35 | 20.85±1.11* |
Note: Data expressed as mean±standard deviation; *: Statistically significant when compared with gliclazide control
Table 1: Mean percent blood glucose reduction of gliclazide in presence and absence of resveratrol in normal rats (n=6).
Resveratrol alone resulted in reductions in blood glucose levels, with peak reductions of 27.75 %±0.62 % in non-diabetic rats and 44.65 %±0.80 % in diabetic rats at the 4th h. Gliclazide alone induced biphasic hypoglycemia in both normal and diabetic rats, with hypoglycemic effects observed at the 2nd and 8th h. When combined with gliclazide, resveratrol significantly enhanced the mean percent blood glucose reduction at the 4th h in both normal (44.44 %±1.09 %) and diabetic rats (52.67 %±0.32 %) without biphasic hypoglycemia. This indicates the existence of interaction (Table 2).
| Time (h) | Resveratrol | Gliclazide | Gliclazide+resveratrol |
|---|---|---|---|
| 1 | 31.75±1.23 | 30.20±0.45 | 41.13±0.74* |
| 2 | 39.61±1.11 | 41.18±0.65 | 51.28±0.60* |
| 4 | 44.65±0.80 | 36.98±1.36 | 52.67±0.32* |
| 6 | 40.76±1.07 | 37.22±1.27 | 52.39±0.97* |
| 8 | 35.72±1.04 | 42.63±1.16 | 49.45±0.92* |
| 12 | 32.00±1.15 | 35.42±1.17 | 45.90±1.09* |
| 18 | 28.62±1.01 | 30.50±1.24 | 42.05±1.29* |
| 24 | 24.38±0.74 | 26.24±1.08 | 38.28±1.37* |
Note: Data expressed as mean±standard deviation; *: statistically significant when compared withgliclazide control
Table 2: Mean percent blood glucose reduction of gliclazide in presence and absence of resveratrol in diabetic rats (n=6).
In normal rabbits, a single dose of gliclazide and resveratrol produced peak hypoglycemia of 30.37 %±0.58 % and 38.23 %±0.62 %, respectively, at the 4th h, accompanied by corresponding increases in insulin levels of 11.32±0.15 μIU/ml and 15.91±0.19 μIU/ml at the same time point. In the multiple dose combination study, a significant increase in hypoglycemia, serum insulin, and serum gliclazide were also observed (Table 3).
| Time (h) | Resveratrol | Gliclazide | Gliclazide+resveratrol SDT | Gliclazide+resveratrol MDT | ||
|---|---|---|---|---|---|---|
| 1 | 17.35±0.75 | 26.99±0.82 | 35.48±0.77* | 38.55±0.78* | ||
| 2 | 23.73±0.45 | 33.08±0.79 | 44.52±0.75* | 47.83±0.72* | ||
| 4 | 30.37±0.58 | 38.23±0.62 | 48.54±0.39* | 51.31±0.71* | ||
| 6 | 25.90±0.75 | 34.20±0.77 | 48.24±0.74* | 52.97±0.84* | ||
| 8 | 20.84±0.81 | 30.45±0.84 | 44.85±1.05* | 53.33±0.58* | ||
| 12 | 15.71±0.92 | 26.09±0.83 | 39.56±1.24* | 52.28±1.29* | ||
| 16 | 11.85±0.62 | 21.60±1.03 | 34.35±1.72* | 47.90±1.76* | ||
| 20 | 8.97±0.76 | 17.44±0.62 | 29.54±1.58* | 43.02±1.89* | ||
| 24 | 5.63±0.83 | 12.06±0.69 | 24.84±1.42 | 38.49±1.94* | ||
Note: Data expressed as mean±standard deviation; *: Statistically significant when compared with gliclazide control
Table 3: Mean percent blood glucose reduction of gliclazide in presence and absence of resveratrol in rabbits (n=6).
Drug interactions are frequently observed in clinical practice, and animal models are commonly utilized to evaluate the mechanisms of these interactions[21]. The study investigated the impact of resveratrol on the pharmacodynamics of gliclazide in both normal and diabetic rats, along with normal rabbits. Additionally, the pharmacokinetics of gliclazide was examined specifically in normal rabbits. The normal rat model served to validate the observed responses under the conditions of drug usage, particularly in type II diabetes, while the rabbit model provided insights from a different species.
Resveratrol is commercially available as an immune booster and dietary supplement in various formulations, offering numerous health benefits, particularly for diabetics[22]. It has been shown to reduce blood glucose levels in both normal and diabetic rats, likely by increasing insulin sensitivity. Similarly, gliclazide also stimulates insulin production and leads to hypoglycemia[23]. In rats, both resveratrol and gliclazide induced hypoglycemia. In a single-dose combination study, resveratrol significantly augmented the hypoglycemic effect of gliclazide in normal and diabetic rats, indicating a potential interaction. Furthermore, experiments in rabbits demonstrated that both resveratrol and gliclazide independently caused hypoglycemia, accompanied by increased insulin levels, indicating their insulin-releasing properties (Table 4). In a single-dose interaction study in rabbits, significant interactions were observed, resulting in elevated peak hypoglycemia, peak serum insulin, and peak serum gliclazide levels at the 4th h. These interactions persisted in the multiple dose study as well.
| Time (h) | Resveratrol | Gliclazide | Gliclazide+resveratrol SDT | Gliclazide+resveratrol MDT |
|---|---|---|---|---|
| 0 | 9.12±0.08 | 9.63±0.21 | 9.58±0.15ns | 9.75±0.18ns |
| 1 | 9.96±0.14 | 11.98±0.12 | 13.00±0.10* | 12.73±0.18* |
| 4 | 11.32±0.15 | 15.91±0.19 | 14.85±0.16ns | 15.64±0.16* |
| 8 | 10.29±0.10 | 15.22±0.16 | 13.84±0.26ns | 16.55±0.15* |
| 16 | 9.54±0.18 | 14.93±0.27 | 12.42±0.10* | 14.68±0.15* |
| 24 | 9.15±0.18 | 13.54±0.28 | 11.18±0.18ns | 13.10±0.16* |
Note: Data expressed as mean±standard deviation. *Statistically significant when compared with gliclazide control; MDT: Multiple-Dose Treatment; SDT:Single-Dose Treatment
Table 4: Mean change in serum insulin levels (μIU/ml) of gliclazide in presence and absence of resveratrolin rabbits (n=6).
In both single and multiple dose combinations, co-administration of gliclazide with resveratrol resulted in significant increases in AUC, Cmax, and t1/2, while reducing Clearance(CL), Vd, and Vdss compared to gliclazide alone. These findings strongly support the notion that resveratrol enhances the bioavailability of gliclazide. This effect aligns with previous studies demonstrating resveratrol's inhibition of CYP2C9, an enzyme involved in gliclazide metabolism, as well as its ability to displace drugs from plasma proteins[12-15]. Importantly, this increased bioavailability is not due to enhanced absorption, as the Ka and Tmax remained constant throughout the study (Table 5). The competitive inhibition of gliclazide metabolism by resveratrol, as shown in previous studies, can lead to reduced CL and increased systemic exposure to gliclazide. Moreover, the displacement of gliclazide from plasma proteins by resveratrol can further contribute to its increased bioavailability, allowing a larger fraction of gliclazide to remain unbound and pharmacologically active in the bloodstream.
| Time (h) | Gliclazide | Gliclazide+resveratrol SDT | Gliclazide+resveratrol MDT |
|---|---|---|---|
| AUC0-24 (µg/ml/h) | 4397.44±198.353 | 7653.821±249.862* | 8353.398±135.229# |
| AUC0-α (µg/ml/h) | 5432.52±301.723 | 10840.620±793.600* | 13727.790±953.684# |
| t1/2(h) | 9.25±0.470 | 12.705±1.186ns | 17.098±2.072ns |
| Ka (h-1) | 1.15±0.000 | 1.153±0.000 | 1.153±0.000 |
| Kel (h-1) | 7.39±0.410 | 5.710±0.559ns | 4.317±0.449# |
| CL (ml/h) | 1572.98±105.181 | 790.001±58.556* | 631.878±44.605# |
| VdSS (ml) | 24186.09±1451.248 | 16182.970±603.409* | 17170.080±603.442# |
| Vdarea (ml) | 20741.10±1059.349 | 14012.980±546.186* | 14978.880±800.951# |
| MRT (h) | 16.44±0.946 | 22.084±2.053ns | 28.970±2.811# |
| Cmax (µg/ml) | 363.03±11.080 | 512.182±17.505* | 513.742±19.416# |
| Tmax (h) | 4.00±0 | 4.000±4.000 | 4.000±0.000 |
Note: Data expressed as mean±standard deviation, AUC: Area Under the concentration time Curve; AUMC: Area Under first Moment Curve; Cmax:Peak serum concentration; Kel: Elimination rate constant; MDT: Multiple-Dose Treatment; MRTLMean Residence Time; SD: Standard Deviation; SDT:Single-Dose Treatment; Tmax:Peak time and t1/2:Terminal half-life
Table 5: Mean pharmacokinetic parameters of gliclazide before and after administration of resveratrolin rabbits (n=6).
Furthermore, both resveratrol and gliclazide possess hypoglycemic properties. Their combined action on blood glucose levels may result in additive or synergistic effects, potentially leading to increased efficacy or an altered pharmacodynamic response. Taken together, these findings indicate that the rise in hypoglycemia of gliclazide in the presence of resveratrol is likely a result of the combined influence of competitive inhibition of metabolism, plasma protein displacement, and the hypoglycemic properties of both drugs. This suggests the involvement of both pharmacodynamic and pharmacokinetic interactions in the observed effects.
The enhancement in gliclazide activity with the selected herbal isolates was statistically significant but not pharmacologically significant. There was a marginal increase in gliclazide response without any hypoglycemia-induced convulsions observed. This indicates the safety of the combinations. Furthermore, since the combinations were found to be safe in two different species and no adverse cases have been reported clinically, they are likely to be safe for clinical use.
Acknowledgements:
The authors thank Wockhardt pharmaceuticals, Aurangabad and RPG Laila Impex, Vijayawada for providing the gift sample of gliclazide and rsveratrol respectively.
Conflict of interests:
The authors declared no conflict of interests.
References
- Furhad S, Bokhari AA. Herbal Supplements. StatPearls 2023.
- Chowdhuri PD, Kundu K. Factors determining choice of complementary and alternative medicine in acute and chronic diseases. J Complement Integr Med 2020;17(3):20190105.
[Crossref] [Google Scholar] [PubMed]
- Monagas M, Brendler T, Brinckmann J, Dentali S, Gafner S, Giancaspro G, et al. Understanding plant to extract ratios in botanical extracts. Front Pharmacol 2022;13:981978.
[Crossref] [Google Scholar] [PubMed]
- Gouws C, Hamman JH. What are the dangers of drug interactions with herbal medicines? Expert Opin Drug Metab Toxicol 2020;16(3):165-7.
[Crossref] [Google Scholar] [PubMed]
- Willcox ML, Elugbaju C, Al-Anbaki M, Lown M, Graz B. Effectiveness of medicinal plants for glycaemic control in type 2 diabetes: An overview of meta-analyses of clinical trials. Front Pharmacol 2021;12:777561.
[Crossref] [Google Scholar] [PubMed]
- Alqathama A, Alluhiabi G, Baghdadi H, Aljahani L, Khan O, Jabal S, et al. Herbal medicine from the perspective of type II diabetic patients and physicians: What is the relationship? BMC Complement Med Ther 2020;20:1-9.
[Crossref] [Google Scholar] [PubMed]
- Kemper C, Behnam D, Brothers S, Wahlestedt C, Volmar CH, Bennett D, et al. Safety and pharmacokinetics of a highly bioavailable resveratrol preparation (JOTROLTM). AAPS Open 2022;8(1):11.
[Crossref] [Google Scholar] [PubMed]
- Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, et al. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018;6(3):91.
[Crossref] [Google Scholar] [PubMed]
- Zhang G, Wang X, Ren B, Zhao Q, Zhang F. The effect of resveratrol on blood glucose and blood lipids in rats with gestational diabetes mellitus. Evid Based Complement Alternat Med 2021;2021(1):2956795.
[Crossref] [Google Scholar] [PubMed]
- Xin Y, Zhang H, Jia Z, Ding X, Sun Y, Wang Q, et al. Resveratrol improves uric acid-induced pancreatic β-cells injury and dysfunction through regulation of miR-126. Biomed Pharmacother 2018;102:1120-6.
[Crossref] [Google Scholar] [PubMed]
- Szkudelska K, Deniziak M, Sassek M, Szkudelski I, Noskowiak W, Szkudelski T. Resveratrol affects insulin signaling in type 2 diabetic Goto-Kakizaki rats. Int J Mol Sci 2021;22(5):2469.
[Crossref] [Google Scholar] [PubMed]
- Robinson K, Mock C, Liang D. Pre-formulation studies of resveratrol. Drug Dev Ind Pharm 2015;41(9):1464-9.
[Crossref] [Google Scholar] [PubMed]
- Seedher N, Kanojia M. Reversible binding of antidiabetic drugs, repaglinide and gliclazide, with human serum albumin. Chem Biol Drug Des 2008;72(4):290-6.
[Crossref] [Google Scholar] [PubMed]
- Rastogi H, Patil P, Sharma G, Sharma A, Jana S. Assessment of inhibition of cytochrome p450 by resveratrol through high-throughput in vitro screening: Potential herb-drug interactions. Assessment 2020;6(2)58-66.
- Saberi M, Ramazani Z, Rashidi H, Saberi A. The effect of CYP2C9 genotype variants in type 2 diabetes on the pharmacological effectiveness of sulfonylureas, diabetic retinopathy, and nephropathy. Vasc Health Risk Manag 2020:241-8.
[Crossref] [Google Scholar] [PubMed]
- Vatsavai LK, Kilari EK. Interaction of p-synephrine on the pharmacodynamics and pharmacokinetics of gliclazide in animal models. J Ayurveda Integr Med 2018;9(3):183-9.
[Crossref] [Google Scholar] [PubMed]
- Kumar KE, Mastan S. Influence of efavirenz and nevirapine on the pharmacodynamics and pharmacokinetics of gliclazide in rabbits. J Endocrinol Metab. 2011;1(3):113-24.
- Furman BL. Streptozotocin‐induced diabetic models in mice and rats. Curr Protoc Pharmacol 2015;70(1):5-47.
[Crossref] [Google Scholar] [PubMed]
- Ambade VN, Sharma YV, Somani BL. Methods for estimation of blood glucose: A comparative evaluation. Med J Armed Forces India 1998;54(2):131-3.
[Crossref] [Google Scholar] [PubMed]
- Lequin RM. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin Chem 2005;51(12):2415-8.
[Crossref] [Google Scholar] [PubMed]
- Tornio A, Filppula AM, Niemi M, Backman JT. Clinical studies on drug–drug interactions involving metabolism and transport: methodology, pitfalls, and interpretation. Clin Pharmacol Ther 2019;105(6):1345-61.
[Crossref] [Google Scholar] [PubMed]
- Su M, Zhao W, Xu S, Weng J. Resveratrol in treating diabetes and its cardiovascular complications: A review of its mechanisms of action. Antioxidants 2022;11(6):1085.
[Crossref] [Google Scholar] [PubMed]
- Haeften TW, Veneman TF, Gerich JE, Veen EA. Influence of gliclazide on glucose-stimulated insulin release in man. Metabolism 1991;40(7):751-5.
[Crossref] [Google Scholar] [PubMed]
 .
.



